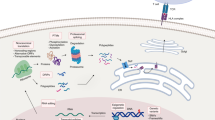
Abstract
Post-translational modification (PTM) of antigens provides an additional source of specificities targeted by immune responses to tumors or pathogens, but identifying antigen PTMs and assessing their role in shaping the immunopeptidome is challenging. Here we describe the Protein Modification Integrated Search Engine (PROMISE), an antigen discovery pipeline that enables the analysis of 29 different PTM combinations from multiple clinical cohorts and cell lines. We expanded the antigen landscape, uncovering human leukocyte antigen class I binding motifs defined by specific PTMs with haplotype-specific binding preferences and revealing disease-specific modified targets, including thousands of new cancer-specific antigens that can be shared between patients and across cancer types. Furthermore, we uncovered a subset of modified peptides that are specific to cancer tissue and driven by post-translational changes that occurred in the tumor proteome. Our findings highlight principles of PTM-driven antigenicity, which may have broad implications for T cell-mediated therapies in cancer and beyond.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
MC38 immunopeptidomics data were deposited in the PRIDE archive with ID PXD017448 and standard MaxQuant95 analysis results. All public data references and accession IDs are listed in the deposited data table in the Supplementary Information.
Code availability
PROMISE is accessible at https://github.com/merbllab/PROMISE.
References
Obara, W. et al. Present status and future perspective of peptide-based vaccine therapy for urological cancer. Cancer Sci. 109, 550–559 (2018).
Jiang, D., Niwa, M., Koong, A. C. & Diego, S. Cancer immunotherapy: moving forward with peptide T cell vaccines. Eur. J. Vasc. Endovasc. Surg. 49, 48–56 (2016).
Xia, A.-L., Wang, X.-C., Lu, Y.-J., Lu, X.-J. & Sun, B. Oncotarget chimeric-antigen receptor T (CAR-T) cell therapy for solid tumors: challenges and opportunities. Oncotarget 8, 90521–90531 (2017).
Finn, O. J. & Rammensee, H. G. Is it possible to develop cancer vaccines to neoantigens, what are the major challenges, and how can these be overcome? Neoantigens: nothing new in spite of the name. Cold Spring Harb. Perspect. Biol. 10, a028829 (2018).
Hsiue, E. H. C. et al. Targeting a neoantigen derived from a common TP53 mutation. Science 371, eabc8697 (2021).
Alpízar, A. et al. A molecular basis for the presentation of phosphorylated peptides by HLA-B antigens. Mol. Cell. Proteomics 16, 181–193 (2017).
Bassani-Sternberg, M. et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 7, 13404 (2016).
Mohammed, F. et al. The antigenic identity of human class I MHC phosphopeptides is critically dependent upon phosphorylation status. Oncotarget 8, 54160–54172 (2017).
Marcilla, M. et al. Increased diversity of the HLA-B40 ligandome by the presentation of peptides phosphorylated at their main anchor residue. Mol. Cell. Proteomics 13, 462–474 (2014).
Marino, F. et al. Arginine (di)methylated human leukocyte antigen class I peptides are favorably presented by HLA-B*07. J. Proteome Res. 16, 34–44 (2017).
Malaker, S. A. et al. Identification of glycopeptides as posttranslationally modified neoantigens in leukemia. Cancer Immunol. Res. 5, 376–384 (2017).
Petersen, J., Purcell, A. W. & Rossjohn, J. Post-translationally modified T cell epitopes: immune recognition and immunotherapy. J. Mol. Med. 87, 1045–1051 (2009).
Ramarathinam, S.H., Croft, N.P., Illing, P.T., Faridi, P. & Purcell, A.W. Employing proteomics in the study of antigen presentation: an update. Expert Rev. Proteomics 15, 637–645 (2018).
Mertins, P. et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534, 55–62 (2016).
Gillette, M. A. et al. Proteogenomic characterization reveals therapeutic vulnerabilities in lung adenocarcinoma. Cell 182, 200–225 (2020).
Karasaki, T. et al. Prediction and prioritization of neoantigens: integration of RNA sequencing data with whole-exome sequencing. Cancer Sci. 108, 170–177 (2017).
Jurtz, V. et al. NetMHCpan-4.0: improved peptide–MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J. Immunol. 199, 3360–3368 (2017).
Abelin, J. G. et al. Mass spectrometry profiling of HLA-associated peptidomes in mono-allelic cells enables more accurate epitope prediction. Immunity 46, 315–326 (2017).
Gfeller, D. et al. The length distribution and multiple specificity of naturally presented HLA-I ligands. J. Immunol. 201, 3705–3716 (2018).
Bulik-Sullivan, B. et al. Deep learning using tumor HLA peptide mass spectrometry datasets improves neoantigen identification. Nat. Biotechnol. 37, 55–71 (2019).
O’Donnell, T. J., Rubinsteyn, A. & Laserson, U. MHCflurry 2.0: improved pan-allele prediction of MHC class I-presented peptides by incorporating antigen processing. Cell Syst. 11, 42–48 (2020).
Ouspenskaia, T. et al. Unannotated proteins expand the MHC-I-restricted immunopeptidome in cancer. Nat. Biotechnol. 40, 209–217 (2022).
Chong, C., Coukos, G. & Bassani-Sternberg, M. Identification of tumor antigens with immunopeptidomics. Nat. Biotechnol. 40, 175–188 (2022).
Yu, F. et al. Identification of modified peptides using localization-aware open search. Nat. Commun. 11, 4065 (2020).
Devabhaktuni, A. et al. TagGraph reveals vast protein modification landscapes from large tandem mass spectrometry datasets. Nat. Biotechnol. 37, 469–479 (2019).
Solntsev, S. K., Shortreed, M. R., Frey, B. L. & Smith, L. M. Enhanced global post-translational modification discovery with metaMorpheus. J. Proteome Res. 17, 1844–1851 (2018).
Zhang, J. et al. PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell. Proteomics 11, M111.010587 (2012).
Geiszler, D.J. et al. PTM-Shepherd: analysis and summarization of post-translational and chemical modifications from open search results. Mol. Cell Proteomics 20, 100018 (2021).
Skinner, O. S. & Kelleher, N. L. Illuminating the dark matter of shotgun proteomics. Nat. Biotechnol. 33, 717–718 (2015).
Laumont, C. M. et al. Global proteogenomic analysis of human MHC class I-associated peptides derived from non-canonical reading frames. Nat. Commun. 7, 10238 (2016).
Starck, S. R. & Shastri, N. Nowhere to hide: unconventional translation yields cryptic peptides for immune surveillance. Immunol. Rev. 272, 8–16 (2016).
Erhard, F., Dölken, L., Schilling, B. & Schlosser, A. Identification of the cryptic HLA-I immunopeptidome. Cancer Immunol. Res. 8, 1018–1026 (2020).
Liepe, J., Sidney, J., Lorenz, F. K. M., Sette, A. & Mishto, M. Mapping the MHC class I-spliced immunopeptidome of cancer cells. Cancer Immunol. Res. 7, 62–76 (2019).
Faridi, P. et al. Comment on “A subset of HLA-I peptides are not genomically templated: evidence for cis- and trans-spliced peptide ligands”. Sci. Immunol. 4, eaaw1622 (2019).
Kong, A. T., Leprevost, F. V., Avtonomov, D. M., Mellacheruvu, D. & Nesvizhskii, A. I. MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry–based proteomics. Nat. Methods 14, 513–520 (2017).
Gurd, F. R. N. et al. Overalkylation of a protein digest with iodoacetamide. Proc. Natl Acad. Sci. U. S. A. 25, 3576–3582 (1991).
Du, Y., Wang, F., May, K., Xu, W. & Liu, H. Determination of deamidation artifacts introduced by sample preparation using 18O-labeling and tandem mass spectrometry analysis. Anal. Chem. 84, 6355–6360 (2012).
Mei, S. et al. Immunopeptidomic analysis reveals that deamidated HLA-bound peptides arise predominantly from deglycosylated precursors. Mol. Cell. Proteomics 19, 1236–1247 (2020).
Bassani-Sternberg, M., Pletscher-Frankild, S., Jensen, L. J. & Mann, M. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol. Cell. Proteomics 14, 658–673 (2015).
Chong, C. et al. High-throughput and sensitive immunopeptidomics platform reveals profound interferonγ-mediated remodeling of the human leukocyte antigen (HLA) ligandome. Mol. Cell. Proteomics 17, 533–548 (2018).
Shraibman, B., Kadosh, D. M., Barnea, E. & Admon, A. Human leukocyte antigen (HLA) peptides derived from tumor antigens induced by inhibition of DNA methylation for development of drug-facilitated immunotherapy. Mol. Cell. Proteom. 15, 3058–3070 (2016).
Ternette, N. et al. Immunopeptidomic profiling of HLA-A2-positive triple negative breast cancer identifies potential immunotherapy target antigens. Proteomics 18, 1700465 (2018).
Ma, K., Vitek, O. & Nesvizhskii, A. I. A statistical model-building perspective to identification of MS/MS spectra with PeptideProphet. BMC Bioinformatics(Suppl 16), S1 (2012).
Bassani-Sternberg, M. et al. Deciphering HLA-I motifs across HLA peptidomes improves neo-antigen predictions and identifies allostery regulating HLA specificity. PLoS Comput. Biol. 13, e1005725 (2017).
Vita, R. et al. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 47, D339–D343 (2019).
Deres, K., Beck, W., Faath, S., Jung, G. & Rammensee, H. G. MHC/peptide binding studies indicate hierarchy of anchor residues. Cell. Immunol. 151, 158–167 (1993).
MacLachlan, B. J. et al. Using X-ray crystallography, biophysics, and functional assays to determine the mechanisms governing T-cell receptor recognition of cancer antigens. J. Vis. Exp. 120, 54991 (2017).
Wang, Y. et al. How an alloreactive T-cell receptor achieves peptide and MHC specificity. Proc. Natl. Acad. Sci. U. S. A. 114, E4792–E4801 (2017).
Sarkizova, S. et al. A large peptidome dataset improves HLA class I epitope prediction across most of the human population. Nat. Biotechnol. 38, 199–209 (2020).
Fogdell-Hahn, A., Ligers, A., Gronning, M., Hillert, J. & Olerup, O. Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens 55, 140–148 (2000).
Wallace, G. R. HLA-B*51 the primary risk in Behçet disease. Proc. Natl. Acad. Sci. 111, 8706–8707 (2014).
Hjalgrim, H. et al. HLA-A alleles and infectious mononucleosis suggest a critical role for cytotoxic T-cell response in EBV-related Hodgkin lymphoma. Proc. Natl Acad. Sci. U. S. A. 107, 6400–6405 (2010).
Sidney, J. et al. Low HLA binding of diabetes-associated CD8+ T-cell epitopes is increased by post translational modifications. BMC Immunol. 19, 12 (2018).
Alpízar, A. et al. A molecular basis for the presentation of phosphorylated peptides by HLA-B antigens. Mol. Cell. Proteomics 16, 181–193 (2016).
Raveh, B., London, N. & Schueler-Furman, O. Sub-angstrom modeling of complexes between flexible peptides and globular proteins. Proteins 78, 2029–2040 (2010).
Borbulevych, O. Y., Baxter, T. K., Yu, Z., Restifo, N. P. & Baker, B. M. Increased immunogenicity of an anchor-modified tumor-associated antigen is due to the enhanced stability of the peptide/MHC complex: implications for vaccine design. J. Immunol. 174, 4812–4820 (2005).
Schuster, H. et al. A tissue-based draft map of the murine MHC class I immunopeptidome. Sci. Data 5, 180157 (2018).
Almeida, L. G. et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic Acids Res. 37, D816–D819 (2009).
Lever, J., Zhao, E. Y., Grewal, J., Jones, M. R. & Jones, S. J. M. CancerMine: a literature-mined resource for drivers, oncogenes and tumor suppressors in cancer. Nat. Methods 16, 505–507 (2019).
Timmerman, L. A. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013).
Tang, X. et al. Cystine addiction of triple-negative breast cancer associated with EMT augmented death signaling. Oncogene 36, 4235–4242 (2017).
Solleder, M. et al. Mass spectrometry based immunopeptidomics leads to robust predictions of phosphorylated HLA class I ligands. Mol. Cell. Proteomics 19, 390–404 (2020).
Singh, S. K. et al. Synthetic uncleavable ubiquitinated proteins dissect proteasome deubiquitination and degradation, and highlight distinctive fate of tetraubiquitin. J. Am. Chem. Soc. 138, 16004–16015 (2016).
Sun, H. et al. Diverse fate of ubiquitin chain moieties: the proximal is degraded with the target, and the distal protects the proximal from removal and recycles. Proc. Natl Acad. Sci. USA 116, 7805–7812 (2019).
Nielsen, M., Andreatta, M., Peters, B. & Buus, S. Immunoinformatics: predicting peptide–MHC binding. Annu. Rev. Biomed. Data Sci. 3, 191–215 (2020).
Hassan, C. et al. Naturally processed non-canonical HLA-A∗02:01 presented peptides. J. Biol. Chem. 290, 2593–2603 (2015).
Bade-Döding, C. et al. The impact of human leukocyte antigen (HLA) micropolymorphism on ligand specificity within the HLA-B*41 allotypic family. Haematologica 96, 110–118 (2011).
Burrows, S. R., Rossjohn, J. & McCluskey, J. Have we cut ourselves too short in mapping CTL epitopes? Trends Immunol. 27, 11–16 (2006).
Ebert, L. M. et al. A long, naturally presented immunodominant epitope from NY-ESO-1 tumor antigen: implications for cancer vaccine design. Cancer Res. 69, 1046–1054 (2009).
Hassan, C. et al. The human leukocyte antigen-presented ligandome of B lymphocytes. Mol. Cell. Proteomics 12, 1829–1843 (2013).
Mommen, G. P. M. et al. Expanding the detectable HLA peptide repertoire using electron-transfer/ higher-energy collision dissociation (EThcD). Proc. Natl Acad. Sci. USA 111, 4507–4512 (2014).
Probst-Kepper, M. et al. An alternative open reading frame of the human macrophage colony-stimulating factor gene is independently translated and codes for an antigenic peptide of 14 amino acids recognized by tumor-infiltrating CD8 T lymphocytes. J. Exp. Med. 193, 1189–1198 (2001).
Bartok, O. et al. Anti-tumour immunity induces aberrant peptide presentation in melanoma. Nature 590, 332–337 (2020).
Cobbold, M. et al. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci. Transl. Med. 5, 203ra125 (2013).
Mohammed, F. et al. Phosphorylation-dependent interaction between antigenic peptides and MHC class I: a molecular basis for presentation of transformed self. Nat. Immunol. 9, 1236–1243 (2009).
Kim, M., Zhong, J. & Pandey, A. Common errors in mass spectrometry-based analysis of posttranslational modifications. Proteomics 16, 700–714 (2017).
Li, Y., Silva, J. C., Skinner, M. E. & Lombard, D. B. Mass spectrometry-based detection of protein acetylation. Methods Mol. Biol. 1077, 81–104 (2013).
Verrastro, I., Pasha, S., Jensen, K. T., Pitt, A. R. & Spickett, C. M. Mass spectrometry-based methods for identifying oxidized proteins in disease: advances and challenges. Biomolecules 5, 378–411 (2015).
Na, S. & Paek, E. Software eyes for protein post-translational modifications. Mass Spectrom. Rev. 34, 133–147 (2015).
Wolf-Levy, H. et al. Revealing the cellular degradome by mass spectrometry analysis of proteasome-cleaved peptides. Nat. Biotechnol. 36, 1110–1116 (2018).
Thomsen, M. C. F. & Nielsen, M. Seq2Logo: a method for construction and visualization of amino acid binding motifs and sequence profiles including sequence weighting, pseudo counts and two-sided representation of amino acid enrichment and depletion. Nucleic Acids Res. 40, W281–W287 (2012).
Vacic, V., Iakoucheva, L. M. & Radivojac, P. Two Sample Logo: a graphical representation of the differences between two sets of sequence alignments. Bioinformatics 22, 1536–1537 (2006).
Alam, N. & Schueler-Furman, O. Modeling peptide-protein structure and binding using Monte Carlo sampling approaches: Rosetta FlexPepDock and FlexPepBind. Methods Mol. Biol. 1561, 139–169 (2017).
London, N., Lamphear, C. L., Hougland, J. L., Fierke, C. A. & Schueler-Furman, O. Identification of a novel class of farnesylation targets by structure-based modeling of binding specificity. PLoS Comput. Biol. 7, e1002170 (2011).
McMurtrey, C. et al. Toxoplasma gondii peptide ligands open the gate of the HLA class I binding groove. elife 5, e12556 (2016).
Liu, J. et al. Cross-allele cytotoxic T lymphocyte responses against 2009 pandemic H1N1 influenza A virus among HLA-A24 and HLA-A3 supertype-positive individuals. J. Virol. 86, 13281–13294 (2012).
Wynn, K. K. et al. Impact of clonal competition for peptide-MHC complexes on the CD8 + T-cell repertoire selection in a persistent viral infection. Blood 111, 4283–4292 (2008).
Kuhlman, B. et al. Design of a novel globular protein fold with atomic-level accuracy. Science 302, 1364–1369 (2003).
Alford, R. F. et al. The Rosetta all-atom energy function for macromolecular modeling and design. J. Chem. Theory Comput. 13, 3031–3048 (2017).
Peters, B. & Sette, A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinformatics 6, 132 (2005).
Alam, N. et al. High-resolution global peptide–protein docking using fragments-based PIPER—FlexPepDock. PLoS Comput. Biol. 13, e1005905 (2017).
Milner, E. et al. The effect of proteasome inhibition on the generation of the human leukocyte antigen (HLA) peptidome. Mol. Cell. Proteomics 12, 1853–1864 (2013).
Paul Zolg, D. et al. ProteomeTools: systematic characterization of 21 post-translational protein modifications by liquid chromatography tandem mass spectrometry (LC-MS/MS) using synthetic peptides. Mol. Cell. Proteomics 17, 1850–1863 (2018).
Li, K., Vaudel, M., Zhang, B., Ren, Y. & Wen, B. PDV: an integrative proteomics data viewer. Bioinformatics 35, 1249–1251 (2019).
Cox, J., Michalski, A. & Mann, M. Software lock mass by two-dimensional minimization of peptide mass errors. J. Am. Soc. Mass. Spectrom. 22, 1373–1380 (2011).
Acknowledgements
We thank the members of the Merbl lab, as well as A. Solomon, O. Bartok, G. Cafri, C. Cohen and M. Besser for scientific discussion and I. Cohen, A. Eisenberg-Lerner, J. DeMartino, C. Putterman, E. Zisman and A. Erez for critical reading of the manuscript. We would like to thank S. Meril for technical help and A. Erez for MC38 cells. Y.M. is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 677748), the I-CORE Program of the Planning and Budgeting Committee, the Israel Science Foundation (grant nos. 1775/12 and 2109/18), the University of Michigan: UM/Israel Research Partnership Weizmann and the Moross Integrated Cancer Center (MICC). This research was partially supported by the Israeli Council for Higher Education (CHE) via the Weizmann Data Science Research Center and by a research grant from Madame Olga Klein—Astrachan. A.I.N. was supported by the US National Institutes of Health grants R01-GM-094231 and U24-CA210967. Y.M. is the incumbent of the Leonard and C. Berall Career Development Chair. A.K. was partially supported by the Israeli Council for Higher Education(CHE) via Weizmann Data Science Research Center and by the research grant from Madam Olga Klein Astrachan.
Author information
Authors and Affiliations
Contributions
A.K. and A.J. led the study and performed all computational analyses unless otherwise mentioned. M.P.K. carried out sampling preparation and experiment design, T.S. performed 3D modeling, D.M. and Y.L. consulted regarding MS analyses and algorithm development. E.B. generated the HLA I peptidomics data and A.K., G.C.T., F.V.L. and F.Y. performed software development. Y.S. consulted regarding assay design, O.S.F., A.A., L.E. and A.I.N. supervised the work of respective group members, A.J., A.K. and Y.M. wrote the manuscript and Y.M. guided and supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Mark Cobbold, Michele Mishto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 PROMISE enrichment in PSM level.
(a) Percentage of novel PSMs with modifications that were identified through PROMISE (reds), on multiple immunopeptidomics datasets, out of the PSMs identified in standard search (gray). Bottom, pie chart of PSMs enriched by PROMISE search. Out of 73,648 modified PSMs identified in the analysis, 60,640 were IDs unique to PROMISE. (dark red) and 13,008 had improved matching score compared to the standard search (light red). (b) Distribution of hyperscores for PSMs which conflicted between Standard (gray) and PROMISE (dark red). Vertical lines mark the average score (c) Examples of 4 spectra that received a better peptide match in PROMSIE (left) compared to the standard search (right).
Extended Data Fig. 2 Monoallelic binding preferences.
(a) Volcano plot showing the site score plotted against the negative log10 transformed p value from the χ2 test with Benjamini-Hochberg multiple comparison correction. Letters indicate the motifs in Fig. 3 labeled by their panels. (b) The counts of peptides containing the indicated modification per haplotype are plotted against the counts of peptides containing unmodified amino acids. The Pearson correlation and p value for the correlation are indicated on each graph. Counts of N - Deamidation are more correlated to its mimic D - unmodified (top) than its source amino acid (N - unmodified). Counts of Q - Deamidation are more correlated to its mimic E - unmodified (bottom) than its source amino acid (Q - unmodified). The haplotypes that have canonical binding motifs that contain an E or D are labeled in pink in the graphs. (c, d) Reanalysis of monoallelic HLA data recapitulates phosphoserine peptides features as described in Adán Alpízar et al. (c) HLA-B*27:05 Phosphoserine position density (top) and the sequence logo (weblogo3) of the peptides carrying phosphoserine in position 4 RRXpS motif (bottom). (d) HLA-B*07:02 Phosphoserine position density (top) and K/RPXpS motif (bottom). (e) Rosetta FlexPepDock structural model of the interaction between the modified peptide KP(ox)LKVIFV (yellow sticks) and the MHC molecule haplotype HLA-A0201 (gray surface \ cartoon). The modified amino acid (green) creates a more stable interaction with the MHC molecule as compared to the unmodified form. The effect of the modified amino acid is shown in detail in the zoom-in picture. The proline hydroxyl group at position 2 forms a stabilizing hydrogen bond with MHC receptor residue E-87 (shown as dashed yellow line, as well as other hydrogen bonds between peptide and receptor). FlexPepDock reweighted score was calculated for the interaction between the MHC and modified or unmodified peptide (n = 5 simulations, box and whiskers indicates mean and quartiles (f) The percentage of peptides in each haplotype with the indicated modification that were not considered binders in NetMHC in their unmodified forms. This indicates that the binding is due to the alteration caused by the PTM. Modifications are sorted by their average percentage of PTM-driven binding and the haplotypes that had the highest percentages are labeled.
Extended Data Fig. 3 Mouse spectra validation.
(a) Similarity score distribution for three types of PSM pairs: (top) two PSMs event taken from the same synthetic peptide in the same sample run (light red, n = 300). We compared the PSM with the highest hyperscore to the PSM with the median hyperscore. (middle) A native PSM taken from HeLa digest standard proteomics compared to a matching synthetic spectrum (dark red, n = 261). (bottom) Similarity score between two randomly chosen PSMs (gray, n = 300). (b, c) Modified HLA peptides, identified in MC38 cell line and not in healthy mouse colon tissue or reported in the IEDB dataset, were synthesized (Peptide 2.0 Inc) and their spectra were captured using mass spectrometry. For each modified peptide, a similarity score was calculated between the synthetic spectrum and the original spectrum using R package OrgMassSpecR. For a similarity score below 80%, manual annotation was done to validate the spectra. (b) summary table (c) spectrum comparison visualization and a similarity score are created by R package OrgMassSpecR, synthesized spectrum (red) in a mirror image of the original spectrum in the dataset (blue). In case manual annotation was done, visualization is created using PDV software94 including a,y,b ions and all potential losses. For the full spectra validation list see Supplementary Information.
Extended Data Fig. 4 Expression of genes encoding for testis antigens identified in PROMISE.
(a) TCGA mRNA expression data of testis genes in four different cancer type from which patient sample or cell lines immunopeptidomics data was analyzed by PROMISE: COAD – HCT116; BRCA – PXD009738, HCC1143 and HCC1937; SKCM – PXD004894; GBM – PXD003790. (b) The expression of the parent gene from 4 modified HLA I-bound peptide identified in PROMISE is shown for TCGA expression data from BRCA primary tumor and normal tissue (Tumor n = 1097, Normal n = 114, box and whiskers indicate mean and quartiles). The parent testis gene is significantly overexpressed in the tumor tissue vs. the normal (Wilcox p values for tumor vs. adjacent abundance indicated in figures).
Extended Data Fig. 5 Human spectra validation.
(a, b) Modified HLA peptides, that were shared across multiple patients, were synthesized (Peptide 2.0 Inc) and their spectra were captured using mass spectrometry. For each modified peptide, a similarity score was calculated between the synthetic spectrum and the original spectrum using R package OrgMassSpecR. For a similarity score below 80%, manual annotation was done to validate the spectra. (a) summary table (b) spectrum comparison visualization and a similarity score are created by R package OrgMassSpecR, synthesized spectrum (red) in a mirror image of the original spectrum in the dataset (blue). In case manual annotation was done, visualization is created using PDV software94 including a,y,b ions and all potential losses. For the full spectra validation list see Supplementary Information.
Supplementary information
Supplementary Information
Supplementary pipeline description, mouse spectra validation, anchor versus middle positions per haplotype table, human spectra validation, reagents table, and deposited data table.
Supplementary Data 1
Modified peptides from PROMISE analysis (210 raw files; tab 1: modified peptides subgroup FDR; tab 2: all peptides, sorted by spectrum counts). Each MS replica is prefixed by its PRIDE dataset identifier and cancer type. For each modified peptide, PROMISE documents the following information: PSM information from MSFragger and Philosopher, and the best spectrum. The intensity value is presented for peptides that passed the FDR threshold in at least one cohort. From the prioritizing stage: the spectrum validation data, PTM localization window and PTM alternative solution, external database matches for the modification site from dbPTM12 and PhosphoSitePlus13, IEDB (ref. 45) match, CancerMine (ref. 59) annotation and testis genes hit (CT Antigens Database).
Supplementary Data 2
Modified peptides from PROMISE analysis (210 raw files) with global FDR cutoff.
Supplementary Data 3
HLA haplotype motifs from NetMHCpan are presented at the top of the page, followed by a histogram of the site distribution for each identified modification type. The histogram represents the modified amino acid frequency in each position (red) compared to the unmodified amino acid background (gray). Each histogram contains positions 1–7 from the N terminus and the C terminus and the preceding position (C-1). Overall, 9-mer epitopes are presented naturally with all their positions, positions 7 and C-1 are identical for 8-mer epitopes and peptides longer than nine residues are truncated accordingly.
Supplementary Data 4
Modified peptides from PROMISE analysis on immunopeptidome data of MC38 cells (PXD017448). Peptides are sorted by spectrum counts. For each modified peptide PROMISE documents the following information: PSM information from MSFragger and Philosopher, the intensity in each replica and the best spectrum. From the prioritizing stage: the spectrum validation data, PTM localization window and PTM alternative solution, external database matches for the modification site from dbPTM12 and PhosphoSitePlus13 and IEDB (ref. 45) match.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kacen, A., Javitt, A., Kramer, M.P. et al. Post-translational modifications reshape the antigenic landscape of the MHC I immunopeptidome in tumors. Nat Biotechnol 41, 239–251 (2023). https://doi.org/10.1038/s41587-022-01464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-022-01464-2
This article is cited by
-
Prediction of tumor-reactive T cell receptors from scRNA-seq data for personalized T cell therapy
Nature Biotechnology (2024)
-
The landscape of T cell antigens for cancer immunotherapy
Nature Cancer (2023)
-
Taking the temperature of lung cancer antigens
Nature Cancer (2023)
-
MSBooster: improving peptide identification rates using deep learning-based features
Nature Communications (2023)
-
The genomics revolution comes to the immunopeptidome
Genes & Immunity (2023)