Abstract
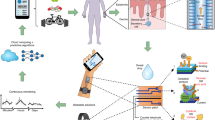
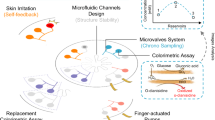
Peripheral biochemical monitoring involves the use of wearable devices for minimally invasive or noninvasive measurement of analytes in biofluids such as interstitial fluid, saliva, tears and sweat. The goal in most cases is to obtain measurements that serve as surrogates for circulating analyte concentrations in blood. Key technological developments to date include continuous glucose monitors, which use an indwelling sensor needle to measure glucose in interstitial fluid, and device-integrated sweat stimulation for continuous access to analytes in sweat. Further development of continuous sensing technologies through new electrochemical sensing modalities will be a major focus of future research. While there has been much investment in wearable technologies to sense analytes, less effort has been directed to understanding the physiology of biofluid secretion. Elucidating the underlying biology is crucial for accelerating technological progress, as the biofluid itself often presents the greatest challenge in terms of sample volumes, secretion rates, filtration, active analyte channels, variable pH and salinity, analyte breakdown and other confounding factors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Heikenfeld, J. et al. Wearable sensors: modalities, challenges, and prospects. Lab Chip 18, 217–248 (2018).
Allied Analytics LLP. Continuous Glucose Monitoring Systems Market by Components, Demographics, and Adult Population, and End User – Global Opportunity Analysis and Industry Forecast, 2016–2024 (Allied Analytics, 2018).
Engler, R., Routh, T. L. & Lucisano, J. Y. Adoption barriers for continuous glucose monitoring and their potential reduction with a fully implanted system: results from patient preference surveys. Clin. Diabetes 36, 50–58 (2018).
Christiansen, M. P. et al. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol. Ther. 20, 197–206 (2018).
Farandos, N. M., Yetisen, A. K., Monteiro, M. J., Lowe, C. R. & Yun, S. H. Contact lens sensors in ocular diagnostics. Adv. Healthc. Mater. 4, 792–810 (2015).
Anderson, J. M. & Van Itallie, C. M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 1, a002584 (2009).
Rana, R., Minhas, L.A. & Mubarik, A. Histological study of human sublingual gland with special emphasis on intercalated and striated ducts. Pak. Armed Forces Med. J. 62, 606–611 (2012).
Zenk, J., Hosemann, W. G. & Iro, H. Diameters of the main excretory ducts of the adult human submandibular and parotid gland: a histologic study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 85, 576–580 (1998).
Hellquist, H. & Skalova, A. Histology. in Histopathology of the Salivary Glands 1–22, https://doi.org/10.1007/978-3-540-46915-5_1 (Springer, Berlin and Heidelberg, Germany, 2014).
Boysen, T. C., Yanagawa, S., Sato, F. & Sato, K. A modified anaerobic method of sweat collection. J. Appl. Physiol. 56, 1302–1307 (1984).
Sonner, Z. et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9, 031301 (2015).
Guyton, A. C. A concept of negative interstitial pressure based on pressures in implanted perforated capsules. Circ. Res. 12, 399–414 (1963).
Fogh-Andersen, N., Altura, B. M., Altura, B. T. & Siggaard-Andersen, O. Composition of interstitial fluid. Clin. Chem. 41, 1522–1525 (1995).
Cohen, J. et al. Measurement of tissue cortisol levels in patients with severe burns: a preliminary investigation. Crit. Care 13, R189 (2009).
Cooke, I. M., Bevill, R. P., Nelson, D. R. & Koritz, G. D. Pharmacokinetics of penicillin G in plasma and interstitial fluid collected with dialysis fiber bundles in sheep. Vet. Res. 27, 147–159 (1996).
Zeitlinger, M. A. et al. Protein binding: do we ever learn? Antimicrob. Agents Chemother. 55, 3067–3074 (2011).
Gottås, A. et al. Levels of heroin and its metabolites in blood and brain extracellular fluid after i.v. heroin administration to freely moving rats. Br. J. Pharmacol. 170, 546–556 (2013).
Vermeer, B. J., Reman, F. C. & van Gent, C. M. The determination of lipids and proteins in suction blister fluid. J. Invest. Dermatol. 73, 303–305 (1979).
Bailey, T., Bode, B. W., Christiansen, M. P., Klaff, L. J. & Alva, S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol. Ther. 17, 787–794 (2015).
Jina, A. et al. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 8, 483–487 (2014).
Cunningham, D.D. Transdermal microfluidic continuous monitoring systems. in In Vivo Glucose Sensing 191–215, https://doi.org/10.1002/9780470567319.ch7 (Wiley, 2010).
Miller, P. R. et al. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun. Biol. 1, 173 (2018).
Ripolin, A. et al. Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 521, 92–101 (2017).
Lewis, D. R. & Chadha, J. S. AlphaWise diabetes survey: ABT’s Libre underappreciated; MDT acceleration likely; price the concern for DXCM. Morgan Stanley Research 1–24 (2018).
Hoss, U., Budiman, E. S., Liu, H. & Christiansen, M. P. Feasibility of factory calibration for subcutaneous glucose sensors in subjects with diabetes. J. Diabetes Sci. Technol. 8, 89–94 (2014).
Arroyo-Currás, N. et al. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl. Acad. Sci. USA 114, 645–650 (2017).
Humphrey, S. P. & Williamson, R. T. A review of saliva: normal composition, flow, and function. J. Prosthet. Dent. 85, 162–169 (2001).
Wong, D. T. Towards a simple, saliva-based test for the detection of oral cancer. Expert Rev. Mol. Diagn. 6, 267–272 (2006).
Ship, J. A. & Fischer, D. J. The relationship between dehydration and parotid salivary gland function in young and older healthy adults. J. Gerontol. A Biol. Sci. Med. Sci. 52, M310–M319 (1997).
Proctor, G. B. & Carpenter, G. H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 133, 3–18 (2007).
Veerman, E. C. I., van den Keybus, P. A., Vissink, A. & Nieuw Amerongen, A. V. Human glandular salivas: their separate collection and analysis. Eur. J. Oral Sci. 104, 346–352 (1996).
Proctor, G. B. The physiology of salivary secretion. Periodontol. 2000 70, 11–25 (2016).
Villiger, M. et al. Evaluation and review of body fluids saliva, sweat and tear compared to biochemical hydration assessment markers within blood and urine. Eur. J. Clin. Nutr. 72, 69–76 (2018).
Makaram, P., Owens, D. & Aceros, J. Trends in nanomaterial-based non-invasive diabetes sensing technologies. Diagnostics (Basel) 4, (27–46 (2014).
Vasconcelos, A. C. U., Soares, M. S. M., Almeida, P. C. & Soares, T. C. Comparative study of the concentration of salivary and blood glucose in type 2 diabetic patients. J. Oral Sci. 52, 293–298 (2010).
Segura, R. et al. A new approach to the assessment of anaerobic metabolism: measurement of lactate in saliva. Br. J. Sports Med. 30, 305–309 (1996).
Oliveira, L. D. S., Oliveira, S. F., Manchado-Gobatto, F. D. B. & Costa, M. D. C. Salivary and blood lactate kinetics in response to maximal workload on cycle ergometer. Revista Brasileira de Cineantropometria & Desempenho Humano 17, 565 (2015).
Kiang, T. K. L. & Ensom, M. H. H. A qualitative review on the pharmacokinetics of antibiotics in saliva: implications on clinical pharmacokinetic monitoring in humans. Clin. Pharmacokinet. 55, 313–358 (2016).
Vining, R. F. & McGinley, R. A. The measurement of hormones in saliva: possibilities and pitfalls. J. Steroid Biochem. 27, 81–94 (1987).
Jaedicke, K. M., Preshaw, P. M. & Taylor, J. J. Salivary cytokines as biomarkers of periodontal diseases. Periodontol. 2000 70, 164–183 (2016).
Korte, D. L. & Kinney, J. Personalized medicine: an update of salivary biomarkers for periodontal diseases. Periodontol. 2000 70, 26–37 (2016).
Sapna, G., Gokul, S. & Bagri-Manjrekar, K. Matrix metalloproteinases and periodontal diseases. Oral Dis. 20, 538–550 (2014).
Slavish, D. C., Graham-Engeland, J. E., Smyth, J. M. & Engeland, C. G. Salivary markers of inflammation in response to acute stress. Brain Behav. Immun. 44, 253–269 (2015).
Myette-Côté, É., Baba, K., Brar, R. & Little, J. P. Detection of salivary insulin following low versus high carbohydrate meals in humans. Nutrients 9, 1204 (2017).
Branson, B. M. FDA approves OraQuick for use in saliva. AIDS Clin. Care 16, 39 (2004).
Foudeh, A. M., Fatanat Didar, T., Veres, T. & Tabrizian, M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 12, 3249–3266 (2012).
Parry, J. V., Perry, K. R. & Mortimer, P. P. Sensitive assays for viral antibodies in saliva: an alternative to tests on serum. Lancet 2, 72–75 (1987).
Granger, D. A. et al. Focus on methodology: salivary bioscience and research on adolescence: an integrated perspective. J. Adolesc. 35, 1081–1095 (2012).
Kim, J. et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 74, 1061–1068 (2015).
Ritzer, J. et al. Diagnosing peri-implant disease using the tongue as a 24/7 detector. Nat. Commun. 8, 264 (2017).
Heikenfeld, J. Non-invasive analyte access and sensing through eccrine sweat: challenges and outlook circa 2016. Electroanalysis 28, 1242–1249 (2016).
Simmers, P., Li, S. K., Kasting, G. & Heikenfeld, J. Prolonged and localized sweat stimulation by iontophoretic delivery of the slowly-metabolized cholinergic agent carbachol. J. Dermatol. Sci. 89, 40–51 (2018).
Sonner, Z., Wilder, E., Gaillard, T., Kasting, G. & Heikenfeld, J. Integrated sudomotor axon reflex sweat stimulation for continuous sweat analyte analysis with individuals at rest. Lab Chip 17, 2550–2560 (2017).
Desax, M.-C., Ammann, R. A., Hammer, J., Schoeni, M. H. & Barben, J. Nanoduct sweat testing for rapid diagnosis in newborns, infants and children with cystic fibrosis. Eur. J. Pediatr. 167, 299–304 (2008).
Bourne, G. H. & Danielli, J. F. International Review of Cytology Vol. 87 (Academic, 1984).
Jajack, A., Brothers, M., Kasting, G. & Heikenfeld, J. Enhancing glucose flux into sweat by increasing paracellular permeability of the sweat gland. PLoS One 13, e0200009 (2018).
Bovell, D. The human eccrine sweat gland: structure, function and disorders. J. Local Glob. Health Sci. 2015, 5 (2015).
Hurley, H. J. & Witkowski, J. Dye clearance and eccrine sweat secretion in human skin. J. Invest. Dermatol. 36, 259–272 (1961).
Hauke, A. et al. Complete validation of a continuous and blood-correlated sweat biosensing device with integrated sweat stimulation. Lab Chip 18, 3750–3759 (2018).
Choi, J. et al. Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab Chip 17, 2572–2580 (2017).
Baker, L. B. Sweating rate and sweat sodium concentration in athletes: a review of methodology and intra/interindividual variability. Sports Med. 47 (Suppl. 1), 111–128 (2017).
Katchman, B., Zhu, M., Cristen, J. B. & Anderson, K. S. Eccrine sweat as a biofluid for profiling immune biomarkers. Proteomics Clin. Appl. 12, e1800010 (2018).
Moyer, J., Wilson, D., Finkelshtein, I., Wong, B. & Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 14, 398–402 (2012).
Ament, W., Huizenga, J. R., Mook, G. A., Gips, C. H. & Verkerke, G. J. Lactate and ammonia concentration in blood and sweat during incremental cycle ergometer exercise. Int. J. Sports Med. 18, 35–39 (1997).
Jia, M., Chew, W. M., Feinstein, Y., Skeath, P. & Sternberg, E. M. Quantification of cortisol in human eccrine sweat by liquid chromatography – tandem mass spectrometry. Analyst 141, 2053–2060 (2016).
La Count, T. D., Jajack, A., Heikenfeld, J. & Kasting, G. B. Modeling glucose transport from systemic circulation to sweat. J. Pharm. Sci. 108, 364–371 (2019).
Steed, E., Balda, M. S. & Matter, K. Dynamics and functions of tight junctions. Trends Cell Biol. 20, 142–149 (2010).
Marques-Deak, A. et al. Measurement of cytokines in sweat patches and plasma in healthy women: validation in a controlled study. J. Immunol. Methods 315, 99–109 (2006).
Cizza, G. et al. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER study. Biol. Psychiatry 64, 907–911 (2008).
Peng, R. et al. A new oil/membrane approach for integrated sweat sampling and sensing: sample volumes reduced from μL’s to nL’s and reduction of analyte contamination from skin. Lab Chip 16, 4415–4423 (2016).
Twine, N. B. et al. Open nanofluidic films with rapid transport and no analyte exchange for ultra-low sample volumes. Lab Chip 18, 2816–2825 (2018).
Jia, W. et al. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 85, 6553–6560 (2013).
Jajack, A. et al. Continuous, quantifiable, and simple osmotic preconcentration and sensing within microfluidic devices. PLoS One 14, e0210286 (2019).
OpenStax. Epithelial tissue. OpenStax CNX http://cnx.org/contents/a16a9513-1ac9-495d-9096-bb8b31905a44@3 (2016).
Kim, Y. C., Park, J. H. & Prausnitz, M. R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 64, 1547–1568 (2012).
Zhang, W., Du, Y. & Wang, M. L. On-chip highly sensitive saliva glucose sensing using multilayer films composed of single-walled carbon nanotubes, gold nanoparticles, and glucose oxidase. Sens. Biosensing Res. 4, 96–102 (2015).
Perogamvros, I., Keevil, B. G., Ray, D. W. & Trainer, P. J. Salivary cortisone is a potential biomarker for serum free cortisol. J. Clin. Endocrinol. Metab. 95, 4951–4958 (2010).
Fullard, R. J. & Snyder, C. Protein levels in nonstimulated and stimulated tears of normal human subjects. Invest. Ophthalmol. Vis. Sci. 31, 1119–1126 (1990).
Runström, G., Mann, A. & Tighe, B. The fall and rise of tear albumin levels: a multifactorial phenomenon. Ocul. Surf. 11, 165–180 (2013).
Sørensen, T. & Jensen, F. T. Tear flow in normal human eyes. Determination by means of radioisotope and gamma camera. Acta Ophthalmol. (Copenh.) 57, 564–581 (1979).
Mishima, S., Gasset, A., Klyce, S. D. Jr. & Baum, J. L. Determination of tear volume and tear flow. Invest. Ophthalmol. 5, 264–276 (1966).
Kallapur, B. et al. Quantitative estimation of sodium, potassium and total protein in saliva of diabetic smokers and nonsmokers: a novel study. J. Nat. Sci. Biol. Med. 4, 341–345 (2013).
Brandtzaeg, P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann. NY Acad. Sci. 1098, 288–311 (2007).
Vairo, D. et al. Towards addressing the body electrolyte environment via sweat analysis: pilocarpine iontophoresis supports assessment of plasma potassium concentration. Sci. Rep. 7, 11801 (2017).
Green, J. M., Bishop, P. A., Muir, I. H., McLester, J. R. Jr. & Heath, H. E. Effects of high and low blood lactate concentrations on sweat lactate response. Int. J. Sports Med. 21, 556–560 (2000).
Derbyshire, P. J., Barr, H., Davis, F. & Higson, S. P. J. Lactate in human sweat: a critical review of research to the present day. J. Physiol. Sci. 62, 429–440 (2012).
Bruen, D., Delaney, C., Florea, L. & Diamond, D. Glucose sensing for diabetes monitoring: recent developments. Sensors (Basel) 17, (1866 (2017).
Jung, K. et al. The sweat of patients with atopic dermatitis contains specific IgE antibodies to inhalant allergens. Clin. Exp. Dermatol. 21, 347–350 (1996).
Acknowledgements
Authors at the University of Cincinnati and Eccrine Systems would like to acknowledge support from the Air Force Research Labs (USAF contract #FA8650-16-C-6760). The University of Cincinnati authors further acknowledge support from the National Science Foundation (EPMD award #ECCS-1608275), the Ohio Federal Research Network (PO FY16-049; WSARC-1077-700), Eccrine Systems and the industrial members of the Center for Advanced Design and Manufacturing of Integrated Microfluidics (NSF I/UCRC award #IIP-1738617).
Author information
Authors and Affiliations
Contributions
J.H. led the organization and revision of the manuscript, and all other others contributed to the writing. A.J led the local structure section; B.F. the ISF section; S. Granger and S. Gaitonde the saliva section; J.H., G.B. and B.K. the sweat section; and J.H. the introduction and discussion sections.
Corresponding author
Ethics declarations
Competing interests
J.H. is the Chief Science Officer and cofounder of Eccrine Systems, Inc., which is commercializing sweat biosensing technology. B.F. is an employee of Abbott Diabetes Care, which produces and markets the FreeStyle Libre Flash Glucose Monitoring System. S. Granger and S. Gaitonde are employees of Salimetrics LLC, which commercializes saliva biosensing devices.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heikenfeld, J., Jajack, A., Feldman, B. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat Biotechnol 37, 407–419 (2019). https://doi.org/10.1038/s41587-019-0040-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-019-0040-3
This article is cited by
-
The challenges and promise of sweat sensing
Nature Biotechnology (2024)
-
Interstitial fluid-based wearable biosensors for minimally invasive healthcare and biomedical applications
Communications Materials (2024)
-
A wearable aptamer nanobiosensor for non-invasive female hormone monitoring
Nature Nanotechnology (2024)
-
Carbon nanotubes: a powerful bridge for conductivity and flexibility in electrochemical glucose sensors
Journal of Nanobiotechnology (2023)
-
Field effect transistor based wearable biosensors for healthcare monitoring
Journal of Nanobiotechnology (2023)