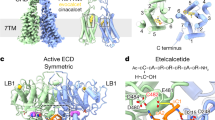
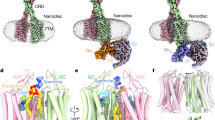
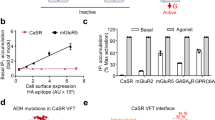
Abstract
The human calcium-sensing receptor (CaSR) detects fluctuations in the extracellular Ca2+ concentration and maintains Ca2+ homeostasis1,2. It also mediates diverse cellular processes not associated with Ca2+ balance3,4,5. The functional pleiotropy of CaSR arises in part from its ability to signal through several G-protein subtypes6. We determined structures of CaSR in complex with G proteins from three different subfamilies: Gq, Gi and Gs. We found that the homodimeric CaSR of each complex couples to a single G protein through a common mode. This involves the C-terminal helix of each Gα subunit binding to a shallow pocket that is formed in one CaSR subunit by all three intracellular loops (ICL1–ICL3), an extended transmembrane helix 3 and an ordered C-terminal region. G-protein binding expands the transmembrane dimer interface, which is further stabilized by phospholipid. The restraint imposed by the receptor dimer, in combination with ICL2, enables G-protein activation by facilitating conformational transition of Gα. We identified a single Gα residue that determines Gq and Gs versus Gi selectivity. The length and flexibility of ICL2 allows CaSR to bind all three Gα subtypes, thereby conferring capacity for promiscuous G-protein coupling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are included in this Article and its Supplementary Information. The cryo-EM density maps, atomic coordinates and raw micrographs have been deposited in the Electron Microscopy Data Bank, RCSB Protein Data Bank and Electron Microscopy Data Bank, respectively. The accession codes for the CaSR–G protein complex structures reported in this paper are EMDB-43811, 9ASB and EMPIAR-11949 (CaSR–miniGisq in nanodiscs); EMDB-43966, 9AXF and EMPIAR-11951 (CaSR–miniGisq in detergent); EMDB-43908, 9AVL and EMPIAR-11950 (CaSR–Gi3 in nanodiscs); EMDB-43990, 9AYF and EMPIAR-11955 (CaSR–miniGi1 in detergent); EMDB-43901, 9AVG and EMPIAR-11956 (CaSR–miniGis in nanodiscs). Source data are provided with this paper.
References
Brown, E. M. et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366, 575–580 (1993).
Hofer, A. M. & Brown, E. M. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 4, 530–538 (2003).
Gray, E. et al. Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: involvement of protein kinases. J. Endocrinol. 190, 703–710 (2006).
Mace, O. J., Schindler, M. & Patel, S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 590, 2917–2936 (2012).
Ruat, M. & Traiffort, E. Roles of the calcium sensing receptor in the central nervous system. Best Pract. Res. Clin. Endocrinol. Metab. 27, 429–442 (2013).
Conigrave, A. D. & Ward, D. T. Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract. Res. Clin. Endocrinol. Metab. 27, 315–331 (2013).
Hendy, G. N., Guarnieri, V. & Canaff, L. Calcium-sensing receptor and associated diseases. Prog. Mol. Biol. Transl. Sci. 89, 31–95 (2009).
de Jesus Ferreira, M. C. et al. Co-expression of a Ca2+-inhibitable adenylyl cyclase and of a Ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney. Inhibition of hormone-dependent cAMP accumulation by extracellular Ca2+. J. Biol. Chem. 273, 15192–15202 (1998).
Kifor, O. et al. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am. J. Physiol. Renal Physiol. 280, F291–F302 (2001).
Mamillapalli, R., VanHouten, J., Zawalich, W. & Wysolmerski, J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J. Biol. Chem. 283, 24435–24447 (2008).
Mamillapalli, R. & Wysolmerski, J. The calcium-sensing receptor couples to Gαs and regulates PTHrP and ACTH secretion in pituitary cells. J. Endocrinol. 204, 287–297 (2010).
Huang, C., Hujer, K. M., Wu, Z. & Miller, R. T. The Ca2+-sensing receptor couples to Gα12/13 to activate phospholipase D in Madin-Darby canine kidney cells. Am. J. Physiol. Cell Physiol. 286, C22–C30 (2004).
Abid, H. A., Inoue, A. & Gorvin, C. M. Heterogeneity of G protein activation by the calcium-sensing receptor. J. Mol. Endocrinol. 67, 41–53 (2021).
Liang, Y. L. et al. Dominant negative G proteins enhance formation and purification of agonist-GPCR-G protein complexes for structure determination. ACS Pharmacol. Transl. Sci. 1, 12–20 (2018).
Nehme, R. et al. Mini-G proteins: novel tools for studying GPCRs in their active conformation. PLoS ONE 12, e0175642 (2017).
Geng, Y. et al. Structural mechanism of ligand activation in human calcium-sensing receptor. eLife 5, e13662 (2016).
Zhang, C. et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2, e1600241 (2016).
Nemeth, E. F. Allosteric modulators of the extracellular calcium receptor. Drug Discov. Today Technol.10, e277–e284 (2013).
Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature 477, 549–555 (2011).
Maeda, S. et al. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat. Commun. 9, 3712 (2018).
Park, J. et al. Symmetric activation and modulation of the human calcium-sensing receptor. Proc. Natl Acad. Sci. USA 118, e2115849118 (2021).
Isberg, V. et al. Generic GPCR residue numbers—aligning topology maps while minding the gaps. Trends Pharmacol. Sci. 36, 22–31 (2015).
Ray, K., Fan, G. F., Goldsmith, P. K. & Spiegel, A. M. The carboxyl terminus of the human calcium receptor. Requirements for cell-surface expression and signal transduction. J. Biol. Chem. 272, 31355–31361 (1997).
Gama, L. & Breitwieser, G. E. A carboxyl-terminal domain controls the cooperativity for extracellular Ca2+ activation of the human calcium sensing receptor. A study with receptor-green fluorescent protein fusions. J. Biol. Chem. 273, 29712–29718 (1998).
Chang, W., Chen, T. H., Pratt, S. & Shoback, D. Amino acids in the second and third intracellular loops of the parathyroid Ca2+-sensing receptor mediate efficient coupling to phospholipase C. J. Biol. Chem. 275, 19955–19963 (2000).
Goolam, M. A. et al. Roles of intraloops-2 and -3 and the proximal C-terminus in signalling pathway selection from the human calcium-sensing receptor. FEBS Lett. 588, 3340–3346 (2014).
Gao, Y. et al. Asymmetric activation of the calcium-sensing receptor homodimer. Nature 595, 455–459 (2021).
Wen, T. et al. Structural basis for activation and allosteric modulation of full-length calcium-sensing receptor. Sci. Adv. 7, eabg1483 (2021).
Ling, S. et al. Structural mechanism of cooperative activation of the human calcium-sensing receptor by Ca2+ ions and L-tryptophan. Cell Res. 31, 383–394 (2021).
Chen, X. et al. Structural insights into the activation of human calcium-sensing receptor. eLife 10, e68578 (2021).
Wall, M. A. et al. The structure of the G protein heterotrimer Giα1β1γ2. Cell 83, 1047–1058 (1995).
Flock, T. et al. Universal allosteric mechanism for Gα activation by GPCRs. Nature 524, 173–179 (2015).
Conklin, B. R., Farfel, Z., Lustig, K. D., Julius, D. & Bourne, H. R. Substitution of three amino acids switches receptor specificity of Gqα to that of Giα. Nature 363, 274–276 (1993).
Conklin, B. R. et al. Carboxyl-terminal mutations of Gq alpha and Gs alpha that alter the fidelity of receptor activation. Mol. Pharmacol. 50, 885–890 (1996).
Bettler, B., Kaupmann, K., Mosbacher, J. & Gassmann, M. Molecular structure and physiological functions of GABAB receptors. Physiol. Rev. 84, 835–867 (2004).
Pollak, M. R. et al. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 75, 1297–1303 (1993).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Gorkhali, R. et al. Extracellular calcium alters calcium-sensing receptor network integrating intracellular calcium-signaling and related key pathway. Sci. Rep. 11, 20576 (2021).
Ritchie, T. K. et al. Chapter 11—Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 (2009).
Reeves, P. J., Callewaert, N., Contreras, R. & Khorana, H. G. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl Acad. Sci. USA 99, 13419–13424 (2002).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Thom, C. et al. Structures of neurokinin 1 receptor in complex with Gq and Gs proteins reveal substance P binding mode and unique activation features. Sci. Adv. 7, eabk2872 (2021).
Qu, X. et al. Structural basis of tethered agonism of the adhesion GPCRs ADGRD1 and ADGRF1. Nature 604, 779–785 (2022).
Cao, C. et al. Structure, function and pharmacology of human itch GPCRs. Nature 600, 170–175 (2021).
Johnson, R. M. et al. Cryo-EM structure of the dual incretin receptor agonist, peptide-19, in complex with the glucagon-like peptide-1 receptor. Biochem. Biophys. Res. Commun. 578, 84–90 (2021).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr. 65, 1074–1080 (2009).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Novotny, M., Madsen, D. & Kleywegt, G. J. Evaluation of protein fold comparison servers. Proteins 54, 260–270 (2004).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Schrage, R. et al. The experimental power of FR900359 to study Gq-regulated biological processes. Nat. Commun. 6, 10156 (2015).
Olsen, R. H. J. et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 16, 841–849 (2020).
Acknowledgements
We thank B. Kobilka for the gift of expression plasmids for the Nb35 nanobody and single-chain variable antibody fragment scFv16; C. Tate for the miniG-protein design and sequences; A. Inoue for the gift of Gαq/11-knockout HEK293 cells; J. DiBerto and B. Roth for the gift of TRUPATH biosensors; J. Wang, C. Hernandez and K. Neselu for assistance with cryo-EM data collection; and R. Biswas for critical reading of the manuscript. Initial screening of cryo-EM grids was performed at the Columbia University Cryo-electron Microscopy Center. Titan Krios data collection was conducted at the Laboratory for Biomolecular Structure at Brookhaven National Laboratory, National Center for Cryo-EM Access and Training and the Simons Electron Microscopy Center located at New York Structural Biology Center. The Laboratory for BioMolecular Structure is supported by the Department of Energy Office of Biological and Environmental Research (KP1607011). The National Center for Cryo-EM Access and Training and Simons Electron Microscopy Center are directed by B. Carragher and C. Potter and supported by the National Institutes of Health Common Fund Transformative High-Resolution Cryo-Electron Microscopy Program (U24 GM129539) and by grants from the Simons Foundation (SF349247) and the New York State Assembly. This work was supported by National Institutes of Health grant no. R35GM141871 (to Q.R.F.) and a grant from the Hope for Depression Research Foundation (to J.A.J.).
Author information
Authors and Affiliations
Contributions
H.Z., J.P., J.Y., A.F. and G.L. generated expression plasmids and performed mutagenesis. H.Z., J.P., A.F. and G.L. cultured insect and mammalian cells. H.Z. and J.P. purified protein and prepared cryo-EM grids. A.F., Z.Z. and R.G. screened cryo-EM grids. A.F., G.H., L.W., J.M. and E.E. collected cryo-EM data. H.Z. and J.P. processed cryo-EM data. Q.R.F. and H.Z. built and refined the models. H.Z., J.P. and Q.R.F. analysed the structures. H.Z. performed the cell-based functional assays. W.A.H. contributed to the structural analysis. J.J.M., W.B.A., J.Y., Z.L., X.L. and J.A.J. contributed to the functional analysis. O.B.C. contributed to cryo-EM image processing. J.P. and H.Z. prepared the figures. Q.R.F., H.Z., A.F. and J.P. wrote the paper; all authors contributed to revisions. H.Z., Q.R.F., A.D.C. and J.A.J. designed the experiments. Q.R.F. conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Reza Vafabakhsh, Qiuyan Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Purification and cryo-EM imaging of CaSR-G-protein complexes.
a-e, Size exclusion chromatography profile (left), SDS-PAGE (middle), and representative 2D class averages (right) of purified CaSR-miniGisq in nanodiscs (a), CaSR-miniGisq in detergent (b), CaSR-Gi3 in nanodiscs (c), CaSR-miniGi1 in detergent (d), and CaSR-miniGis in nanodiscs (e). All samples were assayed on SDS-PAGE under reducing and non-reducing conditions. The presence of dithiothreitol (DTT) reduced the disulfide-linked CaSR homodimer to monomer. Samples of the CaSR-G-protein complex before nanodisc assembly, along with MSP2N2 protein, were included as controls on SDS-PAGE (last two lanes in a, c, and e). For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2 Cryo-EM image processing of CaSR-G-protein complexes.
Schematic of cryo-EM data processing workflow used to generate reconstructions of CaSR-miniGisq in nanodiscs, CaSR-miniGisq in detergent, CaSR-Gi3 in nanodiscs, CaSR-miniGi1 in detergent, and CaSR-miniGis in nanodiscs.
Extended Data Fig. 3 Cryo-EM reconstruction analysis.
a, Locally refined density maps corresponding to the ECD (top), TMD (middle) and G-protein regions (bottom) for each of the five CaSR-G-protein complexes. Maps are colored according to local resolution. b-f, Fourier Shell Correlation (FSC) curves (purple) corrected by high-resolution noise substitution for local refinement maps of ECD (left), TMD (middle), and G protein (right) of CaSR-miniGisq in nanodiscs (b), CaSR-miniGisq in detergent (c), CaSR-Gi3 in nanodiscs (d), CaSR-miniGi1 in detergent (e) and CaSR-miniGis in nanodiscs (f). Curves reaching the FSC cut-off value of 0.143 (blue line) determine the estimated resolution for the corresponding density.
Extended Data Fig. 4 Structural models and cryo-EM density of CaSR-G-protein complexes reconstituted in nanodiscs.
Structural models of helices, loops and ligands within corresponding densities of the CaSR-miniGisq (a-d), CaSR-Gi3 (e-h) and CaSR-miniGis (i-l) complexes in nanodiscs. Residue labels signify the start and end of each helix or loop. CaSRfree (a, e, i) and CaSRG (b, f, j) TMD helices are displayed in blue and cyan, respectively, along with the co-agonist TNCA (gray) and PAM R-568 (yellow) in the corresponding subunit. The C-terminal module of Gα H5 helix (yellow), POPG (green), and CHS (magenta) are presented in (c), (g) and (k). CaSRG intracellular loops (ICL1, 2, 3) are shown in cyan in (d), (h) and (l). An ordered H8 helix is shown in cyan in (h). The density for CaSRfree TMD helices are displayed at contour levels of 0.3-0.5 (a), 0.1 (e), and 0.35 (i), while CaSRG TMD helices are contoured at 0.23-0.5 (b), 0.1 (f), and 0.35 (j). TNCA is contoured at 0.5 (a, b), 0.3 (e, f), and 0.35 (i, j), while R-568 is contoured at 0.18-0.3 (a, b), 0.01 (e, f), and 0.13-0.35 (i, j). POPG is contoured at 0.3 (c), 0.05 (g), and 0.15 (k). CHS is contoured at 0.3 (c), 0.1 (g), and 0.1 (k). The CaSRG ICLs are contoured at 0.15 in all complexes (d, h, l). The CaSRG H8 helix is contoured at 0.15 (h). The C-terminal module of Gα H5 helix is contoured at 0.5 (c), 0.3 (g), and 0.35 (k).
Extended Data Fig. 5 Structural models and cryo-EM density of detergent-solubilized CaSR-G-protein complexes.
a, b, Cryo-EM density map (left) and structural model (right) of CaSR in complex with miniGisq (a), or miniGi1 (b). Each complex is solubilized in detergent and composed of the CaSRfree (blue), CaSRG (cyan), Gα (yellow), Gβ (violet) and Gγ (crimson) subunits. The nanobody Nb35 (light green) and single-chain antibody fragment scFv16 (dark gray) were used to stabilize the heterotrimeric G protein. The bound CaSR ligands include TNCA (gray), Ca2+ (green), PO43− (orange), and R-568 (yellow). An LMNG detergent (lime) is found at the TMD dimer interface. Multiple cholesterol (CLR, pink) and CHS (magenta) molecules surround the TMDs in the CaSR-miniGisq complex. N-linked glycans (NAG, light gray) are attached to the ECD. c-j, Structural models of helices, loops and ligands within corresponding densities of the CaSR-miniGisq (c-f) and CaSR-miniGi1 (g-j) complexes in detergent. Residue labels signify the start and end of each helix or loop. CaSRfree (c, g) and CaSRG (d, h) TMD helices are displayed in blue and cyan, respectively, along with the co-agonist TNCA (gray) and PAM R-568 (yellow) in the corresponding subunit. The C-terminal module of Gα H5 helix (yellow), LMNG (lime), CLR (pink) and CHS (magenta) are presented in (e) and (i). CaSRG intracellular loops (ICL1, 2, 3) and H8 helix are shown in cyan in (f) and (j). The density for CaSRfree TMD helices are displayed at contour levels of 0.2-0.3 (c) and 0.15-0.35 (g), while CaSRG TMD helices are contoured at 0.1-0.3 (d) and 0.15 (h). TNCA is contoured at 0.5 (c, d) and 0.3-0.35 (g, h), while R-568 is contoured at 0.2 (c, d) and 0.1-0.15 (g, h). LMNG is contoured at 0.2 (e) and 0.15 (i). Both CHS and CLR are contoured at 0.2 (e). The CaSRG ICLs are contoured at 0.15 in all complexes (f, j). The CaSRG H8 helix is contoured at 0.25 (f) and 0.15 (j). The C-terminal module of Gα H5 helix is contoured at 0.3 (e) and 0.35 (i).
Extended Data Fig. 6 CaSR dimer arrangement pre- and post-G-protein coupling.
a, Alignment of CaSR ECD (left) and TMD (right) of PAM-bound CaSRpreG (cyan; PDB: 7SIL) and CaSR-Gi3 complex in nanodiscs (lime). b, Alignment of CaSR ECD (left) and TMD (right) of CaSR-miniGisq (orange), CaSR-Gi3 (lime) and CaSR-miniGis (yellow) complexes in nanodiscs. c, TMD dimers of CaSR structures along its activation pathway. Elements at the TMD dimer proximal zone or TMD dimer interface are depicted in color during evolution from the inactive state to the G-protein-bound fully active state as agonist, PAM and finally G protein are added. d, Superposition of one CaSRpreG subunit (cyan; PDB: 7SIL) onto CaSRfree of CaSR-Gi3 complex. Side (left) and top (right) views of the alignment show the S8206.52 side chain (lime) of CaSRG has space to remain adjacent to the inward-flipped F8216.53 of CaSRfree (gray), but would clash with the outward F8216.53 of CaSRpreG (red star). This would prevent TM6 helix of CaSfree from remaining kinked at this position. e, Alignment of CaSRfree (blue) and CaSRG (cyan) VFT modules from the CaSR-Gi3 complex. Red arrows illustrate the pivot of the corresponding CaSRG TMD toward the vertical dimer axis relative to CaSRfree, resulting in a shift of the CaSRG TM6 toward the extracellular membrane. f, The CaSR TM6-TM6 interface of CaSR-Gi3 complex in a full-on side view. The Cα atoms of TM6 residues from CaSRfree (blue) are joined to their counterparts in CaSRG (cyan) by dashed lines to show the relative displacement of CaSRG TM6 toward the extracellular membrane. This persists up until the shallow ECL3 in CaSRG allows the CaSRfree ECL3 to reach a similar elevation. Solid lines for G830ECL3 Cα and N802ICL3 Cα pairs run nearly parallel to the membrane due to their positions being at the same level between the CaSRfree and CaSRG subunits. g, Close-up views of the CaSR ECL2 and ECL3 in the PAM R-568-bound CaSRpreG structure (PDB: 7SIL) alongside CaSR-miniGisq, CaSR-Gi3 and CaSR-miniGis complexes in nanodiscs. Orientations of L756ECL2, E757ECL2 and Y829ECL3 side chains are shown in each structure. Y829ECL3(G) mediates the interaction between ECL3 and ECL2 of CaSRG and may play a role in stabilizing specific loop conformations within this subunit. h, The CaSR-miniGisq, CaSR-Gi3 and CaSR-miniGis complexes, each with its CaSRG TMD and bound G protein duplicated and aligned onto CaSRfree as an outline depiction. The actual G protein (colored) bound to CaSRG yields a clash with its imaginary partner through their Gαs-derived hgh4 regions in the cases of CaSR-miniGisq and CaSR-miniGis. Additional clashes with s6h5, HG and H4 occur in the case of CaSR-miniGis. The hgh4 loops in native Gαq and Gαi are 13 residues shorter than in Gαs. The CaSR-Gi3 complex does not show such clash, consistent with a shorter hgh4 loop.
Extended Data Fig. 7 CaSR interaction with G protein.
a, Functional analysis of CaSR ICL2 deletion mutants lacking three to ten residues near its C-terminal end. Ca2+-induced activation of Gq, Gi3 and Gi1 were measured using BRET assay. b, Un-corrected IP1 accumulation data collected from assay buffer alone or HEK293 T/17 cells transfected with wild-type CaSR or pcDNA3.1. The vertical orange line marks 8 mM Ca2+, which is chosen as the maximum Ca2+ dose in our IP1 experiments. c, Functional analysis of CaSR ICL2 deletion mutants lacking one to ten residues around its turning point or near its C-terminal end. Ca2+-induced IP1 accumulation through wild-type and mutant CaSR was measured in Gαq/11-knockout HEK293 cells co-transfected with Gαq, Gαqi9 or Gαqs5. Data points represent averages ± s.e.m, with the number of independent experiments (n) indicated. Cell surface expression levels are described in the Methods (a-c). d, Alignment of CaSRG TMDs showing relative orientations of the Gα H5 helices in all five of our CaSR-G-protein complexes. The axis of each individual H5 helix is represented by a straight rod and colored to match the corresponding CaSR-G-protein complex. Regions I and II correspond to the C-terminal and N-terminal ends of the H5 helices, respectively. e, Regions I and II in (d), viewed from the C- and N-terminal ends of H5 helices, respectively. Using the Gαi3 H5 helix as the reference, the angle between the axes of each pair of H5 helices is marked for their N-terminal ends in region II. These angles reflect the relative orientations of H5 helix insertion into the receptor among various CaSR-G-protein complexes. The C-terminal ends of the H5 helices are closer together as they converge on the G-protein-binding pocket of CaSR. f, g, Comparison of the depth of Gα H5 helix insertion between nanodisc-reconstituted and detergent-solubilized complexes using the same alignment from (d). The CaSR-miniGisq in nanodiscs manifests deeper penetration of H5 helix than the same complex in detergent (f), and CaSR-Gi3 in nanodiscs similarly shows a deeper insertion of H5 when compared with CaSR-miniGi1 in detergent (g).
Extended Data Fig. 8 CaSR-G-protein interactions.
a, b, Barcode presentations of CaSR (a) and Gα (b) residues that take part in the CaSR-G-protein interfaces of our five complexes. Stars designate residues with functional significance as per our mutational analyses. In (a), filled circles indicate G-protein-binding residues of CaSR that are common to all five complexes (green) or in specific complexes (gray). Empty circles represent CaSR residues not in contact with G protein. Dotted circles mark disordered residues. In (b), G-protein residues that contact CaSR in complexes bearing miniGisq (cyan), Gi3 (green), miniGi1 (green) or miniGis (blue) are interspersed with those that lack contact with the receptor (empty). c, d, Functional analysis of G-protein-binding residues in CaSR. Ca2+ potency (EC50) and maximal response (Emax) of wild-type and mutant receptors are compared for Gq, Gi3 and Gi1 (c) as well as Gq, Gqi9 and Gqs5 (d) using BRET-based G-protein activation and IP1 accumulation assay, respectively. e, Analysis of Ca2+ potency (EC50) and maximal response (Emax) of wild-type CaSR-mediated IP1 accumulation through wild-type or mutant Gαq. Ca2+-stimulated IP1 accumulation was measured in Gαq/11-knockout HEK293 cells co-transfected with either wild-type or mutant Gαq. C-ter, C-terminal tail (a,c,d). Bar segments represent averages ± s.e.m, with the number of independent experiments (n) indicated (c-e). One-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test was used to calculate statistical differences in EC50 and Emax between wild-type and mutant receptors. ND stands for not determined due to an incomplete response curve within the dose concentration range tested. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant. Cell surface expression levels of wild-type and mutant CaSR are described in the Methods (c,d).
Extended Data Fig. 9 Receptor transmembrane dimer interface of various CaSR-G-protein complexes.
a, Structural elements at the receptor TMD dimer interface of CaSR-Gi3 (blue and cyan), CaSR-miniGisq (green) and CaSR-miniGis (yellow) complexes in nanodiscs, as well as those of CaSR-miniGisq (blue) and CaSR-miniGi1 (violet) complexes in detergent. b, Specific TMD dimer interactions between CaSRfree (blue) and CaSRG (cyan) within each of the CaSR-G-protein complexes shown in (a). The TMD dimer interface is divided into three sections (I-III) based on the contacts involving TM6 (I), TM7 (II) and ECL3 (III) of CaSRfree. In the cases of CaSR-miniGis in nanodiscs and CaSR-miniGi1 in detergent, TM1 of CaSRfree contributes to dimer interactions in section II. An additional interaction between ECL2 of both subunits is found in the CaSR-miniGis complex and included as part of section III. F8216.53(G) is part of the dimer interface, while F8216.53(free) (yellow) is directed toward the transmembrane core. Hydrogen bonds are depicted by dashed lines. c, d, Functional analysis of mutations at the receptor TMD dimer interface and phospholipid-binding site. Ca2+ potency (EC50) and maximal response (Emax) of wild-type and mutant receptors are compared for Gq, Gqi9 and Gqs5 (c) as well as Gq and Gi3 (d) using IP1 accumulation and BRET-based G-protein activation assay, respectively. Most of the mutants showed reduced G-protein activation when compared to the wild-type receptor. Bar segments represent averages ± s.e.m, with the number of independent experiments (n) indicated. One-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test was used to calculate statistical differences in EC50 and Emax between wild-type and mutant receptors. ND stands for not determined due to an incomplete response curve within the dose concentration range tested. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant. Cell surface expression levels of wild-type and mutant CaSR are described in in the Methods.
Extended Data Fig. 10 Transmembrane ligands of CaSR-G-protein complexes.
a, Cartoon representation of CaSRfree (blue) and CaSRG (cyan) in CaSR-Gi3 complex showing the locations of various bound molecules, including R-568 (lime), POPG (green), and CHS (magenta). Transparent densities from the 3D reconstruction surround models of POPG and CHS (map contour level 0.15). b, Superposition of POPG molecules from CaSR-G-protein complexes reconstituted in nanodiscs. The complexes are aligned to one another based on the TMDs of their CaSRfree subunits. c, Detailed views of the POPG- and CHS-binding sites in the CaSR-Gi3 (left), CaSR-miniGisq (middle) and CaSR-miniGis (right) complexes in nanodiscs. Residues from CaSRfree and CaSRG that coordinate POPG at the TMD dimer interface are displayed for each complex. d, Electrostatic surface of the CaSR-Gi3 complex showing the binding environment of POPG and CHS molecules. The degree of electrostatic potential is calculated using ChimeraX53. e, Close-up view of CaSRG and CaSRfree ICL3 loops upon alignment of their transmembrane bundles. POPG (green; brought into the alignment with CaSRG) stabilizes CaSRG ICL3, but would clash with the CaSRfree ICL3 conformation (red star). A red arrow illustrates the G-protein-induced conformational differences between the ICL3 loops. f, Detailed views of the LMNG- and CHS-binding sites in CaSR-miniGisq (left) and the LMNG binding site in CaSR-miniGi1 (right). Residues from CaSRfree and CaSRG that coordinate LMNG at the TMD dimer interface are displayed for each complex. Both complexes were solubilized in detergent. g, Superposition of the CaSRpreG (gray; PDB: 7SIL), CaSRfree and CaSRG transmembrane bundles based on their TMD helices, displaying the distinct poses of their respectively bound R-568 molecules as well as the coordinating residues F8216.53 and Y8256.57. h, R-568-binding pockets within CaSRfree (left) and CaSRG (right) subunits of the CaSR-Gi3 complex in nanodiscs. i, R-568-binding sites within the CaSRfree (top) and CaSRG (bottom) belonging to (from left to right) CaSR-miniGisq in nanodiscs, CaSR-miniGis in nanodiscs, CaSR-miniGisq in detergent, and CaSR-miniGi1 in detergent. Hydrogen bonds are depicted by dashed lines (c,f,h,i).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuo, H., Park, J., Frangaj, A. et al. Promiscuous G-protein activation by the calcium-sensing receptor. Nature (2024). https://doi.org/10.1038/s41586-024-07331-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-024-07331-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.