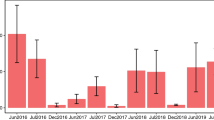
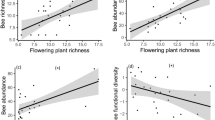
Abstract
Climate change could pose an urgent threat to pollinators, with critical ecological and economic consequences. However, for most insect pollinator species, we lack the long-term data and mechanistic evidence that are necessary to identify climate-driven declines and predict future trends. Here we document 16 years of abundance patterns for a hyper-diverse bee assemblage1 in a warming and drying region2, link bee declines with experimentally determined heat and desiccation tolerances, and use climate sensitivity models to project bee communities into the future. Aridity strongly predicted bee abundance for 71% of 665 bee populations (species × ecosystem combinations). Bee taxa that best tolerated heat and desiccation increased the most over time. Models forecasted declines for 46% of species and predicted more homogeneous communities dominated by drought-tolerant taxa, even while total bee abundance may remain unchanged. Such community reordering could reduce pollination services, because diverse bee assemblages typically maximize pollination for plant communities3. Larger-bodied bees also dominated under intermediate to high aridity, identifying body size as a valuable trait for understanding how climate-driven shifts in bee communities influence pollination4. We provide evidence that climate change directly threatens bee diversity, indicating that bee conservation efforts should account for the stress of aridity on bee physiology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All datasets generated and/or analysed during the current study are publicly available. Long-term bee monitoring data are available via the Environmental Data Initiative (EDI) at https://doi.org/10.6073/pasta/cdc8381b8b2be97188daeffcd6310e9b. Also available via EDI are the SEV-LTER meteorological data (https://doi.org/10.6073/pasta/decdaa0c695cb2070c73f5b684a32e73), plant phenology data (https://doi.org/10.6073/pasta/ceb693495ef57b8b1ba075ca1ee0f7ed), and plant biomass data (https://doi.org/10.6073/pasta/5d6fa085c3d31bc1bc352081ec9e839a). Bee body mass, life history trait, and physiological tolerance data are available via the Open Science Framework (OSF) at https://doi.org/10.17605/OSF.IO/H2YV6. Projected future climate data are available from ClimateNA at https://climatena.ca/.
Code availability
Computer code used in the analyses is available via Zenodo at https://doi.org/10.5281/zenodo.8412361 (ref. 92).
References
Kazenel, M. R. et al. Predicting changes in bee assemblages following state transitions at North American dryland ecotones. Sci. Rep. 10, 708 (2020).
Rudgers, J. A. et al. Climate sensitivity functions and net primary production: a framework for incorporating climate mean and variability. Ecology 99, 576–582 (2018).
Gagic, V. et al. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B 282, 20142620 (2015).
Garibaldi, L. A. et al. Trait matching of flower visitors and crops predicts fruit set better than trait diversity. J. Appl. Ecol. 52, 1436–1444 (2015).
Didham, R. K. et al. Interpreting insect declines: seven challenges and a way forward. Insect Conserv. Divers. 13, 103–114 (2020).
Goulson, D., Nicholls, E., Botías, C. & Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 (2015).
Willmer, P. G., Cunnold, H. & Ballantyne, G. Insights from measuring pollen deposition: quantifying the pre-eminence of bees as flower visitors and effective pollinators. Arthropod Plant Interact. 11, 411–425 (2017).
Jordan, A., Patch, H. M., Grozinger, C. M. & Khanna, V. Economic dependence and vulnerability of United States agricultural sector on insect-mediated pollination service. Environ. Sci. Technol. 55, 2243–2253 (2021).
Potts, S. G. et al. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 49, 15–22 (2010).
Soroye, P., Newbold, T. & Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 367, 685–688 (2020).
Cameron, S. A. et al. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. 108, 662–667 (2011).
Zattara, E. E. & Aizen, M. A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4, 114–123 (2021).
Burkle, L. A., Marlin, J. C. & Knight, T. M. Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339, 1611–1615 (2013).
Michener, C. D. The Bees of the World (Johns Hopkins Univ. Press, 2007).
Hofmann, M. M., Fleischmann, A. & Renner, S. S. Changes in the bee fauna of a German botanical garden between 1997 and 2017, attributable to climate warming, not other parameters. Oecologia 187, 701–706 (2018).
Martínez-López, O. et al. Reduction in the potential distribution of bumble bees (Apidae: Bombus) in Mesoamerica under different climate change scenarios: conservation implications. Glob. Change Biol. 27, 1772–1787 (2021).
Jackson, H. M. et al. Climate change winners and losers among North American bumblebees. Biol. Lett. 18, 20210551 (2022).
Martinet, B. et al. Global effects of extreme temperatures on wild bumblebees. Conserv. Biol. 35, 1507–1518 (2021).
Pardee, G. L. et al. Life-history traits predict responses of wild bees to climate variation. Proc. R. Soc. B 289, 20212697 (2022).
Kammerer, M., Goslee, S. C., Douglas, M. R., Tooker, J. F. & Grozinger, C. M. Wild bees as winners and losers: relative impacts of landscape composition, quality, and climate. Glob. Change Biol. 27, 1250–1265 (2021).
Giannini, T. C. et al. Projected climate change threatens pollinators and crop production in Brazil. PLoS ONE 12, e0182274 (2017).
Decourtye, A., Alaux, C., Le Conte, Y. & Henry, M. Toward the protection of bees and pollination under global change: present and future perspectives in a challenging applied science. Curr. Opin. Insect Sci. 35, 123–131 (2019).
Scaven, V. L. & Rafferty, N. E. Physiological effects of climate warming on flowering plants and insect pollinators and potential consequences for their interactions. Curr. Zool. 59, 418–426 (2013).
Hamblin, A. L., Youngsteadt, E., López-Uribe, M. M. & Frank, S. D. Physiological thermal limits predict differential responses of bees to urban heat-island effects. Biol. Lett. 13, 20170125 (2017).
Oyen, K. J. & Dillon, M. E. Critical thermal limits of bumblebees (Bombus impatiens) are marked by stereotypical behaviors and are unchanged by acclimation, age or feeding status. J. Exp. Biol. 221, jeb165589 (2018).
Renner, S. S. & Zohner, C. M. Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu. Rev. Ecol. Evol. Syst. 49, 165–182 (2018).
Burdine, J. D. & McCluney, K. E. Differential sensitivity of bees to urbanization-driven changes in body temperature and water content. Sci. Rep. 9, 1643 (2019).
Céréghino, R. et al. Desiccation resistance traits predict freshwater invertebrate survival and community response to drought scenarios in a Neotropical ecosystem. Ecol. Indic. 119, 106839 (2020).
Harrison, J. F., Woods, H. A. & Roberts, S. P. Ecological and Environmental Physiology of Insects (Oxford Univ. Press, 2012).
Sheridan, J. A. & Bickford, D. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406 (2011).
Schoener, T. W. Field experiments on interspecific competition. Am. Nat. 122, 240–285 (1983).
Greenleaf, S. S., Williams, N. M., Winfree, R. & Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 153, 589–596 (2007).
Van Wyk, J. I., Amponsah, E. R., Ng, W. H. & Adler, L. S. Big bees spread disease: body size mediates transmission of a bumble bee pathogen. Ecology 102, e03429 (2021).
Gérard, M. et al. Shift in size of bumblebee queens over the last century. Glob. Change Biol. 26, 1185–1195 (2020).
Gérard, M. et al. Patterns of size variation in bees at a continental scale: does Bergmann’s rule apply? Oikos 127, 1095–1103 (2018).
Osorio‐Canadas, S. et al. Body size phenology in a regional bee fauna: a temporal extension of Bergmann’s rule. Ecol. Lett. 19, 1395–1402 (2016).
Osorio-Canadas, S., Flores-Hernández, N., Sánchez-Ortiz, T. & Valiente-Banuet, A. Changes in bee functional traits at community and intraspecific levels along an elevational gradient in a Mexical-type scrubland. Oecologia 200, 145–158 (2022).
Oyen, K. J., Giri, S. & Dillon, M. E. Altitudinal variation in bumble bee (Bombus) critical thermal limits. J. Therm. Biol 59, 52–57 (2016).
Földesi, R., Howlett, B. G., Grass, I. & Batáry, P. Larger pollinators deposit more pollen on stigmas across multiple plant species—a meta-analysis. J. Appl. Ecol. 58, 699–707 (2021).
Lawson, C. R., Vindenes, Y., Bailey, L. & van de Pol, M. Environmental variation and population responses to global change. Ecol. Lett. 18, 724–736 (2015).
Dillon, M. E. et al. Life in the frequency domain: the biological impacts of changes in climate variability at multiple time scales. Integr. Comp. Biol. 56, 14–30 (2016).
Minckley, R. L. & Radke, W. R. Extreme species density of bees (Apiformes, Hymenoptera) in the warm deserts of North America. J. Hymenopt. Res. 82, 317–345 (2021).
Zinnert, J. C. et al. State changes: insights from the U.S. Long Term Ecological Research Network. Ecosphere 12, e03433 (2021).
Danforth, B. N. Emergence dynamics and bet hedging in a desert bee, Perdita portalis. Proc. R. Soc. Lond. B Biol. Sci. 266, 1985–1994 (1999).
Danforth, B. N., Minckley, R. L. & Neff, J. L. The Solitary Bees: Biology, Evolution, Conservation (Princeton Univ. Press, 2019).
Minckley, R. L., Roulston, T. H. & Williams, N. M. Resource assurance predicts specialist and generalist bee activity in drought. Proc. R. Soc. B 280, 20122703 (2013).
Clusella-Trullas, S., Garcia, R. A., Terblanche, J. S. & Hoffmann, A. A. How useful are thermal vulnerability indices? Trends Ecol. Evol. 36, 1000–1010 (2021).
Prugh, L. R. et al. Ecological winners and losers of extreme drought in California. Nat. Clim. Change 8, 819–824 (2018).
Wang, T., Hamann, A., Spittlehouse, D. & Carroll, C. Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLoS ONE 11, e0156720 (2016).
Dew, R. M., Silva, D. P. & Rehan, S. M. Range expansion of an already widespread bee under climate change. Glob. Ecol. Conserv. 17, e00584 (2019).
Biesmeijer, J. C. et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354 (2006).
Hung, K.-L. J., Sandoval, S. S., Ascher, J. S. & Holway, D. A. Joint impacts of drought and habitat fragmentation on native bee assemblages in a California biodiversity hotspot. Insects 12, 135 (2021).
Kelemen, E. P. & Rehan, S. M. Opposing pressures of climate and land‐use change on a native bee. Glob. Change Biol. 27, 1017–1026 (2020).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Pincebourde, S. & Woods, H. A. There is plenty of room at the bottom: microclimates drive insect vulnerability to climate change. Curr. Opin. Insect Sci. 41, 63–70 (2020).
Ogilvie, J. E. et al. Interannual bumble bee abundance is driven by indirect climate effects on floral resource phenology. Ecol. Lett. 20, 1507–1515 (2017).
Cárdenas, P. A. et al. Declines in rodent abundance and diversity track regional climate variability in North American drylands. Glob. Change Biol. 27, 4005–4023 (2021).
Siepielski, A. M. et al. No evidence that warmer temperatures are associated with selection for smaller body sizes. Proc. R. Soc. B 286, 20191332 (2019).
Olden, J. D., Comte, L. & Giam, X. The Homogocene: a research prospectus for the study of biotic homogenisation. NeoBiota 37, 23–36 (2018).
Aldea-Sánchez, P., Ramírez-Cáceres, G. E., Rezende, E. L. & Bozinovic, F. Heat tolerance, energetics, and thermal treatments of honeybees parasitized with Varroa. Front. Ecol. Evol. 9, 463 (2021).
Peters, D. P. C. & Yao, J. Long-term experimental loss of foundation species: consequences for dynamics at ecotones across heterogeneous landscapes. Ecosphere 3, 27 (2012).
Collins, S. L. & Xia, Y. Long-term dynamics and hotspots of change in a desert grassland plant community. Am. Nat. 185, E30–E43 (2015).
Caracciolo, D., Istanbulluoglu, E., Noto, L. V. & Collins, S. L. Mechanisms of shrub encroachment into Northern Chihuahuan Desert grasslands and impacts of climate change investigated using a cellular automata model. Adv. Water Resour. 91, 46–62 (2016).
Wilson, J. S., Griswold, T. & Messinger, O. J. Sampling bee communities (Hymenoptera: Apiformes) in a desert landscape: are pan traps sufficient? J. Kans. Entomol. Soc. 81, 288–300 (2008).
Portman, Z. M., Bruninga-Socolar, B. & Cariveau, D. P. The state of bee monitoring in the United States: a call to refocus away from bowl traps and towards more effective methods. Ann. Entomol. Soc. Am. 113, 337–342 (2020).
Prendergast, K. S., Menz, M. H. M., Dixon, K. W. & Bateman, P. W. The relative performance of sampling methods for native bees: an empirical test and review of the literature. Ecosphere 11, e03076 (2020).
R Core Team. R: A Language and Environment for Statistical Computing, version 4.2.2. http://www.R-project.org/ (R Foundation for Statistical Computing, 2022).
Petrie, M. D., Collins, S. L., Gutzler, D. S. & Moore, D. M. Regional trends and local variability in monsoon precipitation in the northern Chihuahuan Desert, USA. J. Arid. Environ. 103, 63–70 (2014).
Beguería, S., Vicente-Serrano, S. M., Reig, F. & Latorre, B. Standardized precipitation evapotranspiration index (SPEI) revisited: parameter fitting, evapotranspiration models, tools, datasets and drought monitoring. Int. J. Climatol. 34, 3001–3023 (2014).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team. nlme: Linear and nonlinear mixed effects models. R package version 3.1-161 (2022).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002).
Gałecki, A. T. & Burzykowski, T. Linear Mixed-Effects Models Using R: A Step-By-Step Approach (Springer, 2013).
Stralberg, D. et al. Projecting boreal bird responses to climate change: the signal exceeds the noise. Ecol. Appl. 25, 52–69 (2015).
Jin, C., Wang, B. & Liu, J. Future changes and controlling factors of the eight regional monsoons projected by CMIP6 models. J. Clim. 33, 9307–9326 (2020).
Almazroui, M. et al. Projected changes in temperature and precipitation over the United States, Central America, and the Caribbean in CMIP6 GCMs. Earth Syst. Environ. https://doi.org/10.1007/s41748-021-00199-5 (2021).
Oksanen, J. et al. vegan: Community ecology package. R package version 2.6-4 (2022).
Gonzalez, V. H., Oyen, K., Ávila, O. & Ospina, R. Thermal limits of Africanized honey bees are influenced by temperature ramping rate but not by other experimental conditions. J. Therm. Biol 110, 103369 (2022).
Little, A. G. & Seebacher, F. Physiological performance curves: when are they useful? Front. Physiol. 12, 805102 (2021).
Diamond, S. E. et al. A physiological trait-based approach to predicting the responses of species to experimental climate warming. Ecology 93, 2305–2312 (2012).
Hoffmann, A. A. & Harshman, L. G. Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity 83, 637–643 (1999).
Li, D., Dinnage, R., Nell, L. A., Helmus, M. R. & Ives, A. R. phyr: an R package for phylogenetic species-distribution modelling in ecological communities. Methods Ecol. Evol. 11, 1455–1463 (2020).
Hedtke, S. M., Patiny, S. & Danforth, B. N. The bee tree of life: a supermatrix approach to apoid phylogeny and biogeography. BMC Evol. Biol. 13, 138 (2013).
Paradis, E. & Schliep, K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2018).
Pearse, W. D. et al. pez: phylogenetics for the environmental sciences. Bioinformatics 31, 2888–2890 (2015).
Duursma, R. A. Plantecophys - an R package for analysing and modelling leaf gas exchange data. PLoS ONE 10, e0143346 (2015).
Keck, F., Rimet, F., Bouchez, A. & Franc, A. phylosignal: an R package to measure, test, and explore the phylogenetic signal. Ecol. Evol. 6, 2774–2780 (2016).
Kendall, L. K. et al. Pollinator size and its consequences: robust estimates of body size in pollinating insects. Ecol. Evol. 9, 1702–1714 (2019).
Cane, J. H. Estimation of bee size using intertegular span (Apoidea). J. Kans. Entomol. Soc. 60, 145–147 (1987).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Breheny, P. & Burchett, W. Visualization of regression models using visreg. R J. 9, 56–71 (2017).
Kazenel, M. melaniekazenel/BeesClimateChangeSevilleta: Code for “Heat and desiccation tolerances predict bee abundance under climate change”. Zenodo https://doi.org/10.5281/zenodo.8412360 (2023).
Esri. World Imagery. https://www.arcgis.com/home/item.html?id=10df2279f9684e4a9f6a7f08febac2a9 (2022).
Clarke, K. R. & Gorley, R. N. PRIMER v7: User Manual/Tutorial (PRIMER-E, 2015).
Acknowledgements
Funding was provided by the NSF Long-Term Ecological Research programme (DEB-1655499), the Southwestern Association of Naturalists, the University of New Mexico (UNM) Department of Biology, the UNM Graduate and Professional Student Association, and an NSF REU Site Award to S. Collins (DBI-1950237). The authors thank M. Aizen, F. Bozinovic, M. Dillon, R. Irwin, V. Martinson, H. Wearing and N. Williams for providing feedback that improved the manuscript; B. Wolf for equipment and advice on physiological measurements; M. Litvak, T. Duman, K. Hall and L. Baur for help with climate and plant community analyses; and D. Lightfoot, J. Bettinelli, O. M. Carril, J. McLaughlin, B. Turnley, A. Garcia and R. Martinez for their contributions to laboratory and field data collection.
Author information
Authors and Affiliations
Contributions
M.R.K. created the conceptual framework, collected bee physiology and morphology data, analysed the data, and wrote the manuscript. K.W.W. designed the long-term bee monitoring study and completed specimen collection and identification. T.G. provided taxonomic expertise. J.A.R. and K.D.W. contributed to conceptualization, statistical analyses and writing. All authors helped to revise the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Bryan Danforth, Baptiste Martinet, Nicole Miller-Struttman and Justin Sheffield for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Temporal trends in historic and predicted future aridity (inverse SPEI).
(a, b) Trends for the monsoon season in Socorro, NM, USA. In (a), points show the 6-month integrated aridity index, calculated from temperature and precipitation data recorded since 1900, with positive values indicating hotter and drier conditions relative to the mean. In (b), points show the coefficient of variation (CV) in the aridity index for non-overlapping 5-yr time windows (adapted from Rudgers et al., Ecology, 2018). (c) Predicted future monsoon season aridity trends for the Sevilleta National Wildlife Refuge (NM, USA) under low, moderate, and high CO2 emissions scenarios (RCP 2.6, 4.5, and 8.5, respectively), using projected future climate data from six General Circulation Models (ACCESS 1.0, CanESM2, CCSM 4.0, CNRM-CM5, CSIRO-Mk3.6.0, and INM-CM4). RCP 2.6 data were only available for the CanESM2 GCM. Positive and negative values indicate hotter/drier and cooler/wetter conditions relative to the historic mean (2002–2019), respectively. In all panels, error bands represent 95% confidence intervals.
Extended Data Fig. 2 Research sites and equipment.
Left: Map of sampling sites at the Sevilleta National Wildlife Refuge, NM, USA (beige polygon in upper map). Bees were sampled in three focal ecosystem types: Chihuahuan Desert shrubland (green points), Chihuahuan Desert grassland (black points), and plains grassland (blue points). To sample bees, we installed one passive funnel trap at each end of five 200 m transects/site; traps are indicated by colored points in the lower panel. Maps were generated via ArcGIS v. 10.1 (ESRI 2012, Redlands, CA) using the World Imagery basemap93 (sources: Esri, Maxar, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AeroGRID, IGN, and the GIS User Community; accessed 23 February 2022 via https://www.arcgis.com/home/item.html?id=10df2279f9684e4a9f6a7f08febac2a9). Upper right: Differences between sites in climate conditions (table) and plant community composition (figures). Table values are results from paired, two-sided t-tests comparing temperature and precipitation metrics from the month of greatest difference between the Plains and Chihuahuan Desert meteorological stations. Figures are NMDS plots94 illustrating differences among ecosystems in plant cover for all species, and with the dominant species removed. Lower right: (a) Images of the environmental chamber used to assess thermal and desiccation tolerances of bees. The chamber consisted of an insulated ice chest (IceKool, Queensland, Australia). In the chamber, air temperature was controlled using a 162-W Peltier device (model AC-162, TE Technology, Traverse City, MI) and a custom-built controller that incorporated a TE Technology digital display (MP-2986) and control card (TC-36-25-RS486). (b) Traps used for bee collection. Each consisted of a 946 mL paint can filled with ~275 mL of propylene glycol and topped with a plastic automotive funnel (funnel height = 10 cm, top diameter = 14 cm, bottom diameter = 2.5 cm). The funnels’ interiors were painted with either blue or yellow fluorescent paint (Krylon, Cleveland, OH or Ace Hardware, Oak Brook, IL). Each trap was placed on a 45 cm high platform that was surrounded by a 60 cm high chicken wire cage to prevent wildlife and wind disturbance.
Extended Data Fig. 3 Relationship between air temperature and aridity, and alternate aridity index calculations.
(a) Aridity index (inverse SPEI) as a function of maximum air temperature for the period leading up to the monsoon season (April–September), for the historic period (2002–2020) in the plains and Chihuahuan Desert ecosystems, and for 2021–2100 under three predicted future climate scenarios (RCP 2.6, 4.5, and 8.5) for the midpoint between ecosystems, using data from six General Circulation Models (ACCESS 1.0, CanESM2, CCSM 4.0, CNRM-CM5, CSIRO-Mk3.6.0, and INM-CM4). The red bar with an asterisk on the x-axis indicates the critical thermal maximum (CTMax) of the least thermally tolerant bee taxon in the dataset. The error band represents the 95% confidence interval. (b) Year-to-year variation in the aridity index calculated using two different PET estimation methods (Thornthwaite and Penman) for the spring and monsoon seasons in the plains ecosystem and Chihuahuan Desert ecosystems.
Extended Data Fig. 4 Bee phylogeny and predicted change in abundance over time.
Phylogeny of the 339 bee species collected at the Sevilleta National Wildlife Refuge (NM, USA) from 2002–2019, with direction of predicted future change in abundance from 2002–2100 based on averaged projections from six General Circulation Models of global climate (white = insufficient data).
Extended Data Fig. 5 Projected trends in community-weighted mean body mass and total abundance under low and high climate change scenarios.
(a) Change in community-weighted mean (CWM) bee body mass with monsoon season aridity (inverse SPEI) and over time in the combined historic and predicted future datasets, for low (RCP 2.6) and high (RCP 8.5) climate change scenarios. Points represent means and error bars indicate s.e.m. for the linear or quadratic effect of aridity or year on CWM body mass using results from each of six General Circulation Models (GCMs; listed on y-axis). RCP 2.6 data were only available for the CanESM2 GCM. Positive and negative aridity values indicate hotter/drier and cooler/wetter conditions relative to the historic mean (2002–2019), respectively. Statistical results are from mixed effects models (see Methods). (b) Change over time in total bee abundance across study sites, using long-term historic data and predicted future data for low and high climate change scenarios (RCP 2.6 and 8.5). Each point represents the sum for each ecosystem × year combination of all species-level mean predicted abundance values that were calculated by averaging across predictions from the six GCMs. Points are colored by monsoon aridity averaged across the six GCMs. Positive and negative aridity values indicate hotter/drier and cooler/wetter conditions relative to the historic mean (2002–2019), respectively. Error bands represent 95% confidence intervals. Statistics are from linear regression analysis.
Extended Data Fig. 6 Body mass of 16 bee species as a function of time.
Points represent means and error bars indicate s.e.m. (mean n = 15 bee individuals/species/year; see Methods for sample sizes per species). Statistical results are from linear regressions. Mean body mass did not change over time within any species.
Extended Data Fig. 7 Aridity predicts floral availability.
Relationship between spring or monsoon season aridity and proportion of forb and shrub individuals in flower from long-term plant phenology data (2002–2019), in three focal ecosystem types. Positive and negative aridity values indicate hotter/drier and cooler/wetter conditions relative to the mean, respectively. Error bands represent 95% confidence intervals. Statistical results are from mixed effects models.
Extended Data Fig. 8 Monthly climate trends.
Mean air temperature and total monthly precipitation trends for the plains ecosystem and Chihuahuan Desert ecosystems (grassland and shrubland) at the Sevilleta National Wildlife Refuge, for each month averaged across the years 2002–2019 (top) and for each month within each year (bottom).
Extended Data Fig. 9 Relationships between aridity and other climate variables.
Monthly aridity index (inverse SPEI) as a function of four other climate variables (temperature, precipitation, relative humidity, and vapor pressure deficit) for each month of the year (1–12). Data are from two meteorological stations at the Sevilleta National Wildlife Refuge (Chihuahuan Desert and Plains). Error bands represent 95% confidence intervals.
Supplementary information
Supplementary Information
This file contains: Supplementary Tables 1–8, showing results from statistical analyses, with corresponding captions; a description of methods and results related to calculating potential evapotranspiration (PET) using two different estimation methods; and Supplementary Fig. 1, showing climate sensitivity function graphs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kazenel, M.R., Wright, K.W., Griswold, T. et al. Heat and desiccation tolerances predict bee abundance under climate change. Nature 628, 342–348 (2024). https://doi.org/10.1038/s41586-024-07241-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07241-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.