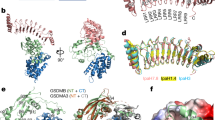
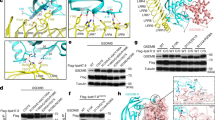
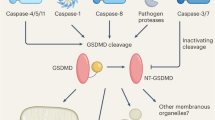
Abstract
In response to pathogen infection, gasdermin (GSDM) proteins form membrane pores that induce a host cell death process called pyroptosis1,2,3. Studies of human and mouse GSDM pores have revealed the functions and architectures of assemblies comprising 24 to 33 protomers4,5,6,7,8,9, but the mechanism and evolutionary origin of membrane targeting and GSDM pore formation remain unknown. Here we determine a structure of a bacterial GSDM (bGSDM) pore and define a conserved mechanism of pore assembly. Engineering a panel of bGSDMs for site-specific proteolytic activation, we demonstrate that diverse bGSDMs form distinct pore sizes that range from smaller mammalian-like assemblies to exceptionally large pores containing more than 50 protomers. We determine a cryo-electron microscopy structure of a Vitiosangium bGSDM in an active ‘slinky’-like oligomeric conformation and analyse bGSDM pores in a native lipid environment to create an atomic-level model of a full 52-mer bGSDM pore. Combining our structural analysis with molecular dynamics simulations and cellular assays, our results support a stepwise model of GSDM pore assembly and suggest that a covalently bound palmitoyl can leave a hydrophobic sheath and insert into the membrane before formation of the membrane-spanning β-strand regions. These results reveal the diversity of GSDM pores found in nature and explain the function of an ancient post-translational modification in enabling programmed host cell death.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Coordinates and density maps of the active-state Vitiosangium bGSDM have been deposited with the PDB and the Electron Microscopy Data Bank (EMDB) under the accession codes 8SL0 and EMD-40570, respectively. Complete 52-mer circular and elliptical models are deposited at the PDB-Dev under the accession codes PDBDEV_00000369, PDBDEV_00000370, and PDBDEV_00000371. Low-resolution density maps of the Vitiosangium bGSDM pore and slinky have been deposited at the EMDB under the accession codes EMDB-43508, EMD-43509, EMD-43510, EMD-43511 and EMD-43513. Simulation parameter files, raw trajectories and code used for analysis are deposited in two Zenodo repositories (https://doi.org/10.5281/zenodo.782840353 and https://doi.org/10.5281/zenodo.827214354). All other data are available in the manuscript or the supplementary materials, including source data for Figs. 1, 3 and 4 and Extended Data Figs. 1–4 and 8–10. Source data are provided with this paper.
Code availability
Custom scripts used to make the Vitiosangium bGSDM 52-mer pore model are available in a Zenodo repository (https://doi.org/10.5281/zenodo.782840353).
References
Broz, P., Pelegrín, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Lieberman, J., Wu, H. & Kagan, J. C. Gasdermin D activity in inflammation and host defense. Sci. Immunol. 4, eaav1447 (2019).
Shi, J., Gao, W. & Shao, F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci 42, 245–254 (2017).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Ruan, J., Xia, S., Liu, X., Lieberman, J. & Wu, H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
Wang, C. et al. Structural basis for GSDMB pore formation and its targeting by IpaH7.8. Nature 616, 590–597 (2023).
Zhong, X. et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature 616, 598–605 (2023).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1–17 (2019).
Hansen, J. M. et al. Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell 184, 3178–3191.e18 (2021).
Jiang, S., Zhou, Z., Sun, Y., Zhang, T. & Sun, L. Coral gasdermin triggers pyroptosis. Sci. Immunol. 5, eabd2591 (2020).
Daskalov, A., Mitchell, P. S., Sandstrom, A., Vance, R. E. & Glass, N. L. Molecular characterization of a fungal gasdermin-like protein. Proc. Natl Acad. Sci. USA 117, 18600–18607 (2020).
Clavé, C. et al. Fungal gasdermin-like proteins are controlled by proteolytic cleavage. Proc. Natl Acad. Sci. USA 119, e2109418119 (2022).
Johnson, A. G. et al. Bacterial gasdermins reveal an ancient mechanism of cell death. Science 375, 221–225 (2022).
Pang, S. S. et al. The cryo-EM structure of the acid activatable pore-forming immune effector macrophage-expressed gene 1. Nat. Commun. 10, 4288 (2019).
Dudkina, N. V. et al. Structure of the poly-C9 component of the complement membrane attack complex. Nat. Commun. 7, 10588 (2016).
Tilley, S. J., Orlova, E. V., Gilbert, R. J. C., Andrew, P. W. & Saibil, H. R. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell 121, 247–256 (2005).
van Pee, K. et al. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of Pneumolysin. eLife 6, e23644 (2017).
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778 (2016).
Schaefer, S. L. & Hummer, G. Sublytic gasdermin-D pores captured in atomistic molecular simulations. eLife 11, e81432 (2022).
Gilbert, R. J. C. et al. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell 97, 647–655 (1999).
Holm, L. Dali server: structural unification of protein families. Nucleic Acids Res. 50, W210–W215 (2022).
van Kempen, M. et al. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol. 42, 243–246 (2023).
Mari, S. A. et al. Gasdermin-A3 pore formation propagates along variable pathways. Nat. Commun. 13, 2609 (2022).
Johnson, A. G. & Kranzusch, P. J. What bacterial cell death teaches us about life. PLoS Pathog. 18, e1010879 (2022).
Peraro, M. D. & Van Der Goot, F. G. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92 (2016).
Strahl, H. & Errington, J. Bacterial membranes: structure, domains, and function. Annu. Rev. Microbiol. 71, 519–538 (2017).
Du, G. et al. ROS-dependent palmitoylation is an obligate licensing modification for GSDMD pore formation. Preprint at bioRxiv https://doi.org/10.1101/2023.03.07.531538 (2023).
Balasubramanian, A. et al. Palmitoylation of gasdermin D directs its membrane translocation and pore formation in pyroptosis. Preprint at bioRxiv https://doi.org/10.1101/2023.02.21.529402 (2023).
Tan, B. K. et al. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc. Natl Acad. Sci. USA 109, 16504–16509 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Abraham, M. J. et al. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996). 27–8.
Gowers, R. et al. in Proc. 15th Python in Science Conference (eds Benthall, S. & Rostrup, S.) 98–105 (2016).
Michaud‐Agrawal, N., Denning, E. J., Woolf, T. B. & Beckstein, O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 32, 2319–2327 (2011).
Schaefer, S. L. & Hummer, G. Raw data for: Structure and assembly of a bacterial gasdermin pore. Zenodo https://doi.org/10.5281/zenodo.7828403 (2023).
Schaefer, S. L. & Hummer, G. Additional raw data for: Structure and assembly of a bacterial gasdermin pore. Zenodo https://doi.org/10.5281/zenodo.8272143 (2023).
Acknowledgements
The authors thank members of their laboratories for helpful discussions; S. Rawson, S. Sterling, R. Walsh, M. Yip and S. Shao for advice on cryo-EM; A. Lu for assistance with protein purification, and the Max Planck Computing and Data Facility for computational resources. Cryo-EM data were collected at the Harvard Cryo-EM Center for Structural Biology at Harvard Medical School and at PNCC supported by NIH grant U24GM129547. The work was funded by grants to P.J.K. from the Pew Biomedical Scholars programme, the Burroughs Wellcome Fund PATH programme, The Mathers Foundation, The Mark Foundation for Cancer Research, the Parker Institute for Cancer Immunotherapy, and the National Institutes of Health (1DP2GM146250-01). A.G.J. is supported through a Life Science Research Foundation postdoctoral fellowship of the Open Philanthropy Project. G.H. and S.L.S. are supported by the Max Planck Society and the Collaborative Research Center 1507 funded by the Deutsche Forschungsgemeinschaft (DFG project number 450648163).
Author information
Authors and Affiliations
Contributions
The study was designed and conceived by A.G.J. and P.J.K. All cell growth and biochemical assays were performed by A.G.J. Protein purification and detergent screens were performed by A.G.J. and N.K.M.-B. Samples for cryo-EM were prepared by A.G.J. and M.L.M. Electron microscopy data collection and processing was performed by A.G.J. and M.L.M. Model building and analysis was performed by A.G.J., S.L.S. and P.J.K. Molecular dynamics simulations were performed by S.L.S. and G.H. Figures were prepared by A.G.J., M.L.M. and S.L.S. The manuscript was written by A.G.J. and P.J.K. All authors contributed to editing the manuscript and support the conclusions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Kevin Corbett, James Whisstock and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Engineering diverse bGSDMs for controlled activation.
a, Crystal structure of an inactive bGSDM from a Vitiosangium species (PDB ID 7N51) and indication of the disordered loop that was targeted for cleavage site engineering. b, Colony forming units (CFU) per mL of E. coli derived from spot assays shown in panel (c). Error bars represent the SEM of n = 3 biologically independent samples. The data are representative of three independent experiments. c, TOP10 E. coli harboring plasmids encoding full-length GSDMs (full) or the N-terminal pore-forming domain alone (∆CTD) were grown on LB-agar plates in triplicate. LB-agar contained either 1% glucose or 0.2% arabinose to repress or induce expression, respectively. Cells were serially diluted and plated out from left (100) to right (10−7) with 5 µL per spot. Though the Ideonella ∆CTD construct does not drastically reduce the CFUs compared to the full construct, colonies grow more slowly and appear fainter in agreement with toxicity from pore formation. d, Parental (W3310) or the triple cardiolipin synthase knockout (BKT12) E. coli harboring plasmids for the ∆CTD GSDMs were grown and spotted out onto LB agar plates with 1% glucose or 0.2% arabinose in duplicate as in panel (c). e, Engineered bGSDMs were treated with or without paired site-specific proteases for 18 h at room temperature and analyzed by 15% SDS-PAGE and visualized by Coomassie staining. Cleaved bGSDM proteins are indicated with a yellow asterisk. f, Full time-course of liposome leakage assays related to Fig. 1b. Error bars represent the SEM of three technical replicates. The data are representative of three independent experiments. g, Negative-stain EM micrographs representing larger view fields micrographs shown in Fig. 1d or second example micrographs used to measure pore sizes for Fig. 1c. Scale bar = 50 nm. Micrographs are representative of >10 replicates with similar results.
Extended Data Fig. 2 Engineered bGSDMs form pores in liposomes with simple and complex lipid compositions.
a, Liposome leakage assay of engineered mammalian GSDMs and bacterial GSDMs with matched site-specific proteases. The left plot shows the results from an experiment performed with liposomes prepared from DOPC lipids (DOPC liposomes), while the right plot shows the result from an experiment using liposomes prepared from E. coli polar lipid extract (E. coli liposomes). The species and/or paralog of mammalian GSDMs or bGSDMs or mammalian GSDMs (and protease sites) are as follows: mGSDMA3 (HRV 3 C site), hGSDMD (HRV 3 C site), Unclassified Bacteroidetes (TEV site), Nostoc sp. Moss4 (WELQ site), Vitiosangium sp. GDMCC 1.1324 (thrombin), and Ideonella sp. 201-F6 (WELQ). In most cases, engineered bGSDMs exhibited robust pore formation in the presence of E. coli liposomes. The Nostoc bGSDM exhibited comparatively weak pore formation activity, possibly due to inefficient cleavage or exogenous amino acids remaining following WELQ protease cleavage compared to the ΔCTD variant tested in in vivo growth assays. The data are representative of three independent experiments each with two technical replicates. b, Full time-course of liposome leakage assays related to panel (a). c, Negative-stain EM micrographs of Bacteroidetes bGSDM and Vitiosangium bGSDM pores in E. coli liposomes (left) and plot comparing pore inner diameters of these bGSDMs in DOPC liposomes versus the same bGSDMs in E. coli liposomes (right). The number of pores measured (n) for each species in E. coli liposomes is Bacteroidetes (n = 171) and Vitiosangium (n = 100). The inner diameters values for pores in DOPC liposomes are the same as in Fig. 1c. The black bar represents the average inner diameter of measured pores. Scale bar = 50 nm. Micrographs are representative of >10 replicates with similar results.
Extended Data Fig. 3 Electron microscopy of Bacteroidetes bGSDM pores and Vitiosangium bGSDM pores and slinkies.
a, Representative cryo-EM micrograph and select 2D class averages of Bacteroidetes bGSDM pores from pore-liposome samples. Scale bars = 20 nm. Micrographs are representative of >4,000 replicates with similar results. b, Representative cryo-EM micrograph and select 2D class averages of DDM-extracted Bacteroidetes bGSDM pores. Numbers in the upper left-hand corner of 2D classes represent the number of bGSDM protomers observed in that class. Scale bars = 20 nm. Micrographs are representative of >2,500 replicates with similar results. c, HECAMEG detergent-extracted Vitiosangium bGSDM pores. Scale bar = 50 nm. Micrographs are representative of >10 replicates with similar results. d, Comparison of inner diameters measured from pore-liposome samples (Fig. 1 and Extended Data Fig. 2) and HECAMEG detergent-extracted Vitiosangium bGSDM pores. The number of pores measured (n) from each sample is as follows: pore-liposome (n = 56), extracted pore (n = 189). The black bar represents the average inner diameter of measured pores. e, DDMAB detergent-extracted Vitiosangium bGSDM slinkies. Scale bars = 50 nm. Micrographs are representative of >10 replicates with similar results.
Extended Data Fig. 4 Cryo-EM data processing of Vitiosangium bGSDM pores and slinkies.
a, Representative cryo-EM micrograph and 2D class averages of HECAMEG detergent-extracted bGSDM pores. Scale bars = 50 nm. Micrographs are representative of >8,000 replicates with similar results. b, Single-particle processing schematic of DDMAB detergent-extracted bGSDM slinkies. From top to bottom: representative cryo-EM micrograph and 2D class averages (as in Fig. 2b), particle classification and map refinement, and local resolution estimate of final map. c, Fourier shell correlation (FSC) curves versus resolution of bGSDM slinky map. Resolution was estimated at an FSC of 0.143. Scale bars = 50 nm. Micrographs are representative of >8,000 replicates with similar results. d, Representative cryo-EM micrograph and 2D class averages of HECAMEG detergent-extracted bGSDM pore-slinky mixture (as in Fig. 2a). Micrographs are representative of >4,000 replicates with similar results.
Extended Data Fig. 5 Cryo-EM model to map fitting of bGSDM slinky and pore.
a, 54-mer model of the Vitiosangium bGSDM in a slinky-like oligomerization. b, Examples regions of model to map fit quality for a single Vitiosangium bGSDM protomer. The map surface has been contoured to 16σ.
Extended Data Fig. 6 Functional conservation of the GSDM active state.
a, Predicted bGSDM structures are organized from left to right based on percent sequence identity to the Vitiosangium bGSDM: Runella bGSDM (18%), Bradyrhizobium bGSDM (19%), and Lysobacter bGSDM (28%). Phyre homology model threading utilized the active hGSDMD structure (PDB ID 6VFE, left model) or the active Vitiosangium bGSDM structure (this study, right model). Each bGSDM structure was also predicted using AlphaFold after deleting ~20 amino acids from the C-termini and yielded inactive-like structures (center). The modeled sequences are as follows: Runella bGSDM (1–247), Bradyrhizobium bGSDM (1–237), and Lysobacter bGSDM (1–240). b, Structure-based alignment of bacterial and mammalian gasdermins. The Vitiosangium bGSDM active structure was aligned to the active mGSDMA3 (PDB ID 6CB8) and hGSDMD (PDB ID 6VFE). Secondary structures are indicated below the sequences for each structure, in addition to secondary structures from the Vitiosangium bGSDM inactive state crystal structure. Residues of the Vitiosangium bGSDM that surround the palmitoyl in the inactive state are boxed in black, asterisks indicate residues that have been mutated to test their effect on bGSDM-mediated bacterial cell death, and green boxes indicate resides that align with the conserved glycines in MACPF/CDC proteins. c, Topology diagrams showing the transitions from inactive to active structures of the Vitiosangium bGSDM (left) and mGSDMA3 (right). Conserved α-helices and β-strands are outlined in black, the positions of residues universally conserved in charge, identity, or aromaticity are indicated in active state topology diagrams, and green arrows indicate the sites of conserved glycines in MACPF/CDC protein structures.
Extended Data Fig. 7 Evidence for a possible divergent evolution of gasdermins and cytolysins.
a, Structure-based alignment of the Vitiosangium bGSDM (aa 6–229) and the pneumolysin (PLY) pore-forming domain (aa 4–355). The Vitiosangium bGSDM active state structure (this study, PDB 8SL0) was aligned to the active state PLY structure (PDB ID 5LY6). Secondary structures are indicated below the sequences for each structure and numbered according to prior conventions19. Conserved glycine residues of the PLY structure that are present in other MACFP/CDC proteins are boxed in green. b, A query of the AlphaFold database with experimentally determined bGSMD structures yielded putative bGSDM-like proteins with cytolysin-like features. A representative structure from a Pseudomonas species is shown on the right, indicating the N-terminal bGSDM-like domain in salmon color and the C-terminal immunoglobulin-like β-sandwich domain in green color with similarity to the membrane binding domain of PLY and other cytolysins. The sequence alignment shows the highly conserved undecapeptide present in the β-sandwich domains of multiple cytolysins c, Structure-based alignment of the Vitiosangium bGSDM (aa 2–229) and the Pseudomonas bGSDM-like protein pore-forming domain (aa 1–245). The Vitiosangium bGSDM inactive state structure (PDB 7N51) was aligned to the putative inactive state structure of the bGSDM-like protein (AF-A0A2N3PBA1). Secondary structures are indicated below the sequences for each structure, using α-helix and β-sheet numbering for the bGSDM-like protein that reflect homology to the bGSDM.
Extended Data Fig. 8 Extraction and cryo-EM data processing of Vitiosangium bGSDM pores with sideviews.
a, Fractions from the detergent extraction of Vitiosangium bGSDM pores from E. coli liposomes analyzed by SDS-PAGE and Coomassie staining. The bGSDM sample extracted at 50 nm HECAMEG was subsequently used for cryo-EM analysis b, Representative cryo-EM micrograph of HECAMEG detergent-extracted bGSDM pores from (a). Scale bar = 50 nm. Micrograph is representative of >30,000 replicates with similar results. c, 2D class averages from processing micrograph indicated in (b) and single-particle processing schematic of HECAMEG detergent-extracted bGSDM closed-ring pores and slinky-like oligomers. Scale bars = 50 nm. Data was processed using homogeneous (homo.) or non-uniform (NU) refinements. d, Fourier shell correlation (FSC) curves versus resolution of bGSDM slinky map. Resolution was estimated at an FSC of 0.143. e, Docking of the 52-mer elliptical pore-model into the 6.5 Å resolution map from (c). The left model represents a geometric model based on the structure of the slinky-like oligomer, with an eccentricity of 0.86. The right model represents the maximum eccentricity pore undulation observed in during the MD simulations of the 52-mer pore, with an eccentricity of 0.67.
Extended Data Fig. 9 Cell death and liposome rupture by the Vitiosangium bGSDM is robust to single mutations.
a, Residue-residue contacts between neighboring subunits occurring with a frequency of >0.01 over the last 900 ns of an MD simulation of the 52-mer pore with C4-palmitoylation. b, Structural representations of the Vitiosangium bGSDM oligomer. Center, an electrostatic charge model of a 4-mer of the slinky-like oligomer. Left inset, sites targeted for mutation on the ‘positive patch’ and α1 thumb on the pore exterior. Right, dimer of the Vitiosangium bGSDM along the interface shaded to indicate the frequency of contact occurring over the course of the MD simulation described in panel (a). Right inset, residues targeted for mutagenesis at the subunit interface. c, Colony forming units (CFU) per mL of E. coli derived from spot assays shown in panel (d). Growth assays test single charge-swap mutations to residues at select interfaces in the active model. The experiment was performed with n = 2 biologically independent samples and the data are representative of two or three independent experiments. d, E. coli harboring plasmids encoding full-length GSDMs (full) or the N-terminal pore-forming domain alone (∆CTD) were grown on LB-agar plates in duplicate. LB-agar contained either 1% glucose or 0.2% arabinose to repress or induce expression, respectively. Cells were serially diluted and plated out from left (100) to right (10−7) with 5 µL per spot.
Extended Data Fig. 10 Liposome rupture and cell death by the Vitiosangium bGSDM is sensitive to mutation of the palmitoylated cysteine but not other single amino acid residues.
a, Relative fluorescent units (RFUs) at six hours from liposome rupture experiment testing single amino acid mutants of the Vitiosangium bGSDM with DOPC liposomes. b, Full time-course RFU data for the plot shown in panel (a). c, RFU at six hours for liposome rupture experiment testing single amino acid mutants of the Vitiosangium bGSDM with DOPC liposomes. The data are representative of three independent experiments each with n = 2 technical replicates. d, Full time-course RFU data for the plot shown in panel (c). e, Bacterial spot assays testing mutation of residues proximal to the N-terminal cysteine of the Vitiosangium bGSDM. TOP10 E. coli harboring plasmids encoding full-length GSDMs (full) or the N-terminal pore-forming domain alone (∆CTD) were grown on LB-agar plates in triplicate. LB-agar contained either 1% glucose or 0.2% arabinose to repress or induce expression, respectively. Cells were serially diluted and plated out from left (100) to right (10−7) with 5 µL per spot.
Extended Data Fig. 11 MD simulations of small membrane pores formed by active 1, 2, and 3-mer bGSDM assemblies.
Snapshots of palmitoylated (left) and non-palmitoylated (right) mono- and oligomers after 3.3 μs of simulation in a bacterial membrane in top view and side view. Protein shown in purple cartoon representation, membrane phosphates shown as tan spheres, palmitoylated C4 shown in cyan opaque licorice and transparent surface representation. In the side views, water inside the small pores is shown in blue transparent surface representation. Otherwise, solvent molecules and lipid fatty acid tails are omitted for clarity.
Supplementary information
Supplementary Table 1
Restraints used during energy minimization (EM) and stepwise equilibration (EQ) in kJ mol−1 nm−2.
Supplementary Table 2
Overview of bGSDM production simulations.
Supplementary Video 1
Atomistic molecular dynamics simulation of the fully assembled, palmitoylated 52-meric Vitiosangium bGSDM pore in a bacterial lipid membrane.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Johnson, A.G., Mayer, M.L., Schaefer, S.L. et al. Structure and assembly of a bacterial gasdermin pore. Nature 628, 657–663 (2024). https://doi.org/10.1038/s41586-024-07216-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07216-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.