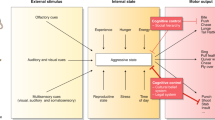
Abstract
Our understanding of the neurobiology of primate behaviour largely derives from artificial tasks in highly controlled laboratory settings, overlooking most natural behaviours that primate brains evolved to produce1,2,3. How primates navigate the multidimensional social relationships that structure daily life4 and shape survival and reproductive success5 remains largely unclear at the single-neuron level. Here we combine ethological analysis, computer vision and wireless recording technologies to identify neural signatures of natural behaviour in unrestrained, socially interacting pairs of rhesus macaques. Single-neuron and population activity in the prefrontal and temporal cortex robustly encoded 24 species-typical behaviours, as well as social context. Male–female partners demonstrated near-perfect reciprocity in grooming, a key behavioural mechanism supporting friendships and alliances6, and neural activity maintained a running account of these social investments. Confronted with an aggressive intruder, behavioural and neural population responses reflected empathy and were buffered by the presence of a partner. Our findings reveal a highly distributed neurophysiological ledger of social dynamics, a potential computational foundation supporting communal life in primate societies, including our own.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data, including supplementary videos 1–9, are available at the Open Science Framework (https://osf.io/e2xsu/).
Code availability
Code is available at GitHub (https://github.com/camilletestard/Datalogger).
References
Krakauer, J. W., Ghazanfar, A. A., Gomez-Marin, A., MacIver, M. A. & Poeppel, D. Neuroscience needs behavior: correcting a reductionist bias. Neuron 93, 480–490 (2017).
Miller, C. T. et al. Natural behavior is the language of the brain. Curr. Biol. 32, R482–R493 (2022).
Testard, C., Tremblay, S. & Platt, M. From the field to the lab and back: neuroethology of primate social behavior. Curr. Opin. Neurobiol. 68, 76–83 (2021).
Maestripieri, D. & Hoffman, C. L. in Bones, Genetics, and Behavior of Rhesus Macaques: Macaca Mulatta of Cayo Santiago and Beyond (ed. Wang, Q.) 247–262 (Springer, 2012).
Snyder-Mackler, N. et al. Social determinants of health and survival in humans and other animals. Science 368, eaax9553 (2020).
Schino, G. Grooming and agonistic support: a meta-analysis of primate reciprocal altruism. Behav. Ecol. 18, 115–120 (2007).
Silk, J. B. Social components of fitness in primate groups. Science 317, 1347–1351 (2007).
Testard, C. et al. Rhesus macaques build new social connections after a natural disaster. Curr. Biol. 31, 2299–2309 (2021).
Holt-Lunstad, J., Robles, T. F. & Sbarra, D. A. Advancing social connection as a public health priority in the United States. Am. Psychol. 72, 517–530 (2017).
Sterling, P. & Platt, M. L. Why deaths of despair are increasing in the US and not other industrial nations—insights from neuroscience and anthropology. JAMA Psychiatry 79, 368–374 (2022).
Chang, S. W. C., Gariépy, J.-F. & Platt, M. L. Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci. 16, 243–250 (2013).
Bonini, L., Maranesi, M., Livi, A., Fogassi, L. & Rizzolatti, G. Ventral premotor neurons encoding representations of action during self and others’ inaction. Curr. Biol. 24, 1611–1614 (2014).
Haroush, K. & Williams, Z. M. Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell 160, 1233–1245 (2015).
Barat, E., Wirth, S. & Duhamel, J.-R. Face cells in orbitofrontal cortex represent social categories. Proc. Natl Acad. Sci. USA 115, E11158–E11167 (2018).
Ong, W. S., Madlon-Kay, S. & Platt, M. L. Neuronal correlates of strategic cooperation in monkeys. Nat. Neurosci. 24, 116–128 (2020).
Dal Monte, O. et al. Widespread implementations of interactive social gaze neurons in the primate prefrontal-amygdala networks. Neuron 110, 2183–2197 (2022).
Cooper, E. B. et al. The rhesus macaque as a success story of the Anthropocene. eLife 11, e78169 (2022).
Aparicio, P. L., Issa, E. B. & DiCarlo, J. J. Neurophysiological organization of the middle face patch in macaque inferior temporal Cortex. J. Neurosci. 36, 12729–12745 (2016).
Bizley, J. K. & Cohen, Y. E. The what, where and how of auditory-object perception. Nat. Rev. Neurosci. 14, 693–707 (2013).
Sliwa, J. & Freiwald, W. A. A dedicated network for social interaction processing in the primate brain. Science 356, 745–749 (2017).
Boussaoud, D., Desimone, R. & Ungerleider, L. G. Visual topography of area TEO in the macaque. J. Comp. Neurol. 306, 554–575 (1991).
Kravitz, D. J., Saleem, K. S., Baker, C. I., Ungerleider, L. G. & Mishkin, M. The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn. Sci. 17, 26–49 (2013).
Passingham, R. E. & Wise, S. P. The Neurobiology of the Prefrontal cortex: Anatomy, Evolution, and the Origin of Insight (Oxford Univ. Press, 2014).
Holt-Lunstad, J., Smith, T. B. & Layton, J. B. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316 (2010).
Silk, J., Cheney, D. & Seyfarth, R. A practical guide to the study of social relationships. Evol. Anthropol. 22, 213–225 (2013).
Rust, N. C. & DiCarlo, J. J. Balanced increases in selectivity and tolerance produce constant sparseness along the ventral visual stream. J. Neurosci. 32, 10170–10182 (2012).
Rigotti, M. et al. The importance of mixed selectivity in complex cognitive tasks. Nature 497, 585–590 (2013).
Tremblay, S., Testard, C., DiTullio, R. W., Inchauspé, J. & Petrides, M. Neural cognitive signals during spontaneous movements in the macaque. Nat. Neurosci. 26, 295–305 (2022).
Laughlin, S. B. Energy as a constraint on the coding and processing of sensory information. Curr. Opin. Neurobiol. 11, 475–480 (2001).
Levy, W. B. & Baxter, R. A. Energy efficient neural codes. Neural Comput. 8, 531–543 (1996).
Quian Quiroga, R. & Panzeri, S. Extracting information from neuronal populations: information theory and decoding approaches. Nat. Rev. Neurosci. 10, 173–185 (2009).
McInnes, L., Healy, J. & Melville, J. UMAP: Uniform manifold approximation and projection for dimension reduction. Preprint at arxiv.org/abs/1802.03426 (2018).
Chang, C.-C. & Lin, C.-J. LIBSVM: a library for support vector machines. ACM Trans. Intell. Syst. Technol. 2, 1–27 (2011).
King, A. J., Clark, F. E. & Cowlishaw, G. The dining etiquette of desert baboons: the roles of social bonds, kinship, and dominance in co-feeding networks. Am. J. Primatol. 73, 768–774 (2011).
Musall, S., Kaufman, M. T., Juavinett, A. L., Gluf, S. & Churchland, A. K. Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci. 22, 1677–1686 (2019).
Cisek, P. & Pastor-Bernier, A. On the challenges and mechanisms of embodied decisions. Philos. Trans. R. Soc. Lond. B 369, 20130479 (2014).
Gintis, H. Strong reciprocity and human sociality. J. Theor. Biol. 206, 169–179 (2000).
Schweinfurth, M. K. & Call, J. Reciprocity: different behavioural strategies, cognitive mechanisms and psychological processes. Learn. Behav. 47, 284–301 (2019).
Gomes, C. M. & Boesch, C. Reciprocity and trades in wild West African chimpanzees. Behav. Ecol. Sociobiol. 65, 2183–2196 (2011).
Majolo, B., Schino, G. & Aureli, F. The relative prevalence of direct, indirect and generalized reciprocity in macaque grooming exchanges. Anim. Behav. 83, 763–771 (2012).
de Waal, F. B. & Luttrell, L. M. Mechanisms of social reciprocity in three primate species: symmetrical relationship characteristics or cognition? Ethol. Sociobiol. 9, 101–118 (1988).
Young, C., Majolo, B., Schülke, O. & Ostner, J. Male social bonds and rank predict supporter selection in cooperative aggression in wild Barbary macaques. Anim. Behav. 95, 23–32 (2014).
McEwen, B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007).
McEwen, B. S. Brain on stress: how the social environment gets under the skin. Proc. Natl Acad. Sci. USA 109, 17180–17185 (2012).
Decety, J., Bartal, I. B.-A., Uzefovsky, F. & Knafo-Noam, A. Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos. Trans. R. Soc. Lond. B 371, 20150077 (2016).
Hinde, R. A. & Rowell, T. E. Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta). Proc. Zool. Soc. Lond. 138, 1–21 (1962).
Schino, G., di Sorrentino, E. P. & Tiddi, B. Grooming and coalitions in Japanese macaques (Macaca fuscata): partner choice and the time frame reciprocation. J. Comp. Psychol. 121, 181–188 (2007).
Niell, C. M. & Stryker, M. P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Stringer, C. et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255 (2019).
Avitan, L. & Stringer, C. Not so spontaneous: multi-dimensional representations of behaviors and context in sensory areas. Neuron 110, 3064–3075 (2022).
Tremblay, S., Testard, C., Inchauspé, J. & Petrides, M. Non-necessary neural activity in the primate cortex. Preprint at bioRxiv https://doi.org/10.1101/2022.09.12.506984 (2022).
Blackman, R. K. et al. Shared neural activity but distinct neural dynamics for cognitive control in monkey prefrontal and parietal cortex. J. Neurosci. 43, 2767–2781 (2023).
Bala, P. C. et al. Automated markerless pose estimation in freely moving macaques with OpenMonkeyStudio. Nat. Commun. 11, 4560 (2020).
Freidin, E., Carballo, F. & Bentosela, M. Direct reciprocity in animals: the roles of bonding and affective processes. Int. J. Psychol. 52, 163–170 (2017).
Dunbar, R. I. M. The social brain hypothesis. Evol. Anthropol. 6, 178–190 (1998).
Richter-Levin, G. & Akirav, I. Emotional tagging of memory formationin the search for neural mechanisms. Brain Res. Brain Res. Rev. 43, 247–256 (2003).
Heiligenberg, W. Neural Nets in Electric Fish (MIT Press, 1991).
Brent, L. J. N., Chang, S. W. C., Gariépy, J.-F. & Platt, M. L. The neuroethology of friendship. Ann. N. Y. Acad. Sci. 1316, 1–17 (2014).
Pearson, J. M., Watson, K. K. & Platt, M. L. Decision making: the neuroethological turn. Neuron 82, 950–965 (2014).
Stringer, C. et al. Rastermap: a discovery method for neural population recordings. Preprint at bioRxiv https://doi.org/10.1101/2023.07.25.550571 (2023).
Altmann, J. Observational study of behavior: sampling methods. Behaviour 49, 227–267 (1974).
Cheney, D. L. & Seyfarth, R. M. How Monkeys See the World: Inside the Mind of Another Species (Univ. of Chicago Press, 2018).
Chen, K. et al. MMDetection: open MMLab detection toolbox and benchmark. Preprint at arxiv.org/abs/1906.07155 (2019).
MMPose Contributors. OpenMMLab pose estimation toolbox and benchmark. (2020); github.com/open-mmlab/mmpose.
Pan, S. J. & Yang, Q. A survey on transfer learning. IEEE Trans. Knowl. Data Eng. 22, 1345–1359 (2010).
Labuguen, R. et al. MacaquePose: a novel ‘in the wild’ macaque monkey pose dataset for markerless motion capture. Front. Behav. Neurosci. 14, 581154 (2021).
Ren, S., He, K., Girshick, R. & Sun, J. Faster R-CNN: Towards real-time object detection with region proposal networks. In Proc. Advances in Neural Information Processing Systems vol. 28 (eds Cortes, C. et al.) 1–9 (Curran Associates, 2015).
Sun, K., Xiao, B., Liu, D. & Wang, J. Deep high-resolution representation learning for human pose estimation. In Proc. 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) 5693–5703 (IEEE, 2019).
Yao, Y. et al. OpenMonkeyChallenge: dataset and benchmark challenges for pose estimation of non-human primates. Int. J. Comput. Vis. 131, 243–258 (2023).
Dwyer, B. et. al. Roboflow v.1.0, roboflow.com (2022).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. 12, 2825–2830 (2012).
Candan, Ç. & Inan, H. A unified framework for derivation and implementation of Savitzky–Golay filters. Signal Process. 104, 203–211 (2014).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 (2019).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Groppe, D. fdr_bh v.2.3.0.0 (https://www.mathworks.com/matlabcentral/fileexchange/27418-fdr_bh), MATLAB Central File Exchange (accessed 25 February 2024).
Keverne, E. B., Martensz, N. D. & Tuite, B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 14, 155–161 (1989).
Humphries, M. D. Strong and weak principles of neural dimension reduction. Neurons Behav. Data Anal. Theory 5, 1–28 (2021).
Meehan, C., Ebrahimian, J., Moore, W. & Meehan, S. Uniform manifold approximation and projection (UMAP), www.mathworks.com/matlabcentral/fileexchange/71902 (2022).
Aggarwal, C. C., Hinneburg, A. & Keim, D. A. On the surprising behavior of distance metrics in high dimensional space. In ICDT 2001: Database Theory — ICDT 2001 (Lecture Notes in Computer Science, vol 1973) (eds Bussche, J. & Vianu, V.) 420–434 (Springer, 2001).
Beyer, K., Goldstein, J., Ramakrishnan, R. & Shaft, U. When is “nearest neighbor” meaningful? In ICDT 1999: Database Theory — ICDT’99 (Lecture Notes in Computer Science, vol 1540) (eds. Beeri, C. & Buneman, P.) 217–235 (Springer, 1999).
Acknowledgements
We thank R. Seyfarth, A. Lowet, R. Lange, M. Jazeyari laboratories and, in particular, J. I. Sanguinetti-Scheck, for conceptual, theoretical and methodological advice; X. Jiang for help with pose tracking; J. Matelsky for artwork; and our subject monkeys Lovelace, Amos, Sallyride and Hooke, from whom all the data included in this paper are sourced. R01MH095894, R01MH108627, R37MH109728, R21AG073958, R01MH118203,866 R56MH122819 and R01NS123054 to M.L.P., Blavatnik Family Foundation Fellowship to CT, Canada Banting Fellowship and Human Frontier Science Program Fellowship to S.T.
Author information
Authors and Affiliations
Contributions
Study conceptualization: C.T., S.T. and M.L.P. Surgical implantation: S.T. and K.L.G. Data collection: C.T. and F.P. Behavioural labelling: C.T., A.A.-I. and S.T. Video motion tracking: F.P. Data analyses: C.T., S.T., R.W.D. and F.P. Figure editing: C.T. and S.T. Funding acquisition: C.T., F.P., S.T. and M.L.P. All of the authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.L.P. is a scientific advisory board member, consultant and/or co-founder of Blue Horizons International, NeuroFlow, Amplio, Cogwear Technologies, Burgeon Labs and Glassview, and receives research funding from AIIR Consulting, the SEB Group, Mars, Slalom, the Lefkort Family Research Foundation, Sisu Capital and Benjamin Franklin Technology Partners. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Michael Brecht and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Behaviour transition matrix, neurotechnology and neural data quality.
a, Absolute number of transitions from one behaviour to the next for one example session. Top: To reveal ethologically relevant behavioural patterns, transitions excluded “rest” epochs, behaviours performed by other monkeys in the colony (“Other monkeys vocalize” and “Rowdy Room”), as well as ephemeral behaviours (“vocalization”, “lip smack”, “anogenital investigation”, “scratch” and “yawning”). Bottom: Transition matrix with all behaviours included. b, Sum of transitions from one behaviour to the next across all sessions, pooled across monkeys. Top: ethologically relevant behaviours. Bottom: all behaviours in our ethogram. c, Images of wireless neural recording device. Note the minimal footprint of the cranial implant on the monkey’s head. d, Example medium-amplitude unit (green) with average peak at 50 uV. Bottom timeline plot (zoomed in) shows raw signal showing the high signal-to-noise ratio of spikes recording with the wireless logger. Red line indicates the threshold used, which is 3.7 SD from noise amplitude. Only spikes crossing that line compose both the yellow and green clusters above. Noise typically has a low amplitude of +/− 15 uV. Bottom right quadrant shows principal components vs time, used to confirm stability of the waveform shape over the entire session. e, Number of units recorded across vlPFC and TEO in our two subject monkeys in each session, chronologically (N = 12 sessions, 6 per monkey). All sessions were recording within 2 months of implantation.
Extended Data Fig. 2 Single unit responses during behaviour.
a, Top: behaviour during example session; Middle: Z-scored firing rate of all units recorded in the same example session as Fig. 2, ordered by activity pattern and units ordered using Rastermap60; Bottom: Average z-scored firing across all recorded units in the same session (N = 297). Shaded area is the standard deviation. b, Neuro-ethogram from Fig. 2d statistically thresholded at FDR-corrected P < 0.01 (two-sided t-tests). c, Number of behaviours a neuron is selective for if limiting to 100 observations per behaviour (sub-sampling 100 times through the data). d, Same as Fig. 2f but for well-isolated single units only for two sessions (one in each monkey). e, Same as Fig. 2b–d & Extended Data Fig. 2b but only considering fully-isolated single units (N = 67), as defined in Methods. f, Raw firing aligned to behaviour for two additional sessions to the one in Fig. 2b and Extended Data Fig. 2a, one from each monkey. Each row is an individual neural unit, ordered using the Rastermap toolbox60. Firing rates are Z-scored to maximum firing rate for each unit. Colour bar on top represents behaviours performed over time with the same colour code as in Fig. 2b. g, Z-scored raw firing aligned to behaviour for only fully-isolated units for another session than in e, from the other monkey.
Extended Data Fig. 3 Neural population states segregate by behaviour and context across sessions and are not reducible to visuo-motor contingencies.
a-b, Whole session UMAP visualization (same session as in Fig. 2a–d). c, Average distance between neural states across behaviours in UMAP space. Pooled across sessions and monkeys. d, Average distance between neural states across social contexts. (e-h) UMAP plots as in Fig. 2a but with different parameter values. Results remain qualitatively the same. i, Left panel: Table summarizing behaviour quantification models and their accuracies for monkey detection, monkey pose estimation, and datalogger pose estimation. Right panel: Confidence score by body landmark thresholding at c = 0.2. j, Comparison of elbow joint angle for two behaviours, self-groom and groom partner. Top: depiction of the stereotypic self-groom behaviour (left), groom partner (middle), and the shared motion pattern of shoulder-elbow-wrist across both behaviours (right). Bottom: plot of joint angle θ over time for self-grooming (green) and groom partner (yellow). Shaded portion indicates onset and offset of both behaviours. k, Histograms of field of view across social contexts: alone, paired female neighbour, paired male neighbour. Field of view varied substantially within a social context and overlapped across contexts, decorrelating social context coding from visual inputs. l, Schematic of ridge regression model to test the relative importance of behaviour vs. movements and field of view to explain neural variance (Fig. 3i).
Extended Data Fig. 4 Behavioural evidence for grooming reciprocity and underlying neural correlates.
a, Simulations assuming monkeys were grooming randomly. 1 = perfectly reciprocal, 0 = fully one-sided. Simulations were run 10,000 times to obtain likelihood of the observed reciprocity if monkeys were not intentionally trying to reciprocate number of bouts (top), engage in turn-taking (middle) or reciprocate grooming duration (bottom) (see Methods). b, Correlation in duration of consecutive alternating grooming bouts (e.g. I groom you, then you groom me). c, Interbout interval for alternating grooming bouts (Give-Receive-Give, blue) vs non-alternating (Give-Give or Receive-Receive, red). Alternating bouts showed a bi-modal distribution with short inter-bout intervals <20 s and longer ones. Based on this distribution we considered alternating bouts spaced by less than 20 s to be “Turn-taking”. d, Distribution of grooming bout duration for Amos (brown) vs. Hooke (yellow). Inset: Empirical distribution of grooming bout durations (blue) and approximated distribution (turquoise). Approximation uses an exponential distribution with empirical mean bout length as a parameter. e, Correlation between ridge regression model fit and how linear & monotonic the response variable is. Top: Net groom duration; Bottom: Net number of grooming bouts. f, Ridge regression model fit (cross-validated R2) for net grooming duration, net number of bouts and time combining separated by monkey (brain areas pooled). g, Ridge regression model fit (cross-validated R2) for net grooming duration, net number of bouts and time combining separated by brain areas (monkeys pooled). h, Cross-validation (5-fold) linear decoding accuracy of (from left to right): categorical net groom duration, categorical net number of bouts, chronological grooming bout number, chronological rest bout number. Red: shuffled control. i, Distribution of single neuron cross-validated ridge regression fit for net groom duration, net groom bouts and time. Distributions are not statistically distinguishable (one-way Anova, two-sided). Boxplots in panels f & g: central mark = median; box bottom and top edges = 25th and 75th percentiles, respectively; whiskers = min-max data points.
Extended Data Fig. 5 Neural signatures of social support and empathy in response to human threats.
a, Agitation score in response to human threats as in Fig. 5b separated by monkey. b, Probability of grooming given or received in the 5 min following a threat. c, Head direction during threats to self vs. partner, when paired. d, Correlation in average neural responses to threats towards the subject vs. his partner when the subject is looking in front (left), towards the threatening stimulus, or not (right). Each point represents one unit, all units across sessions with video data were included (10 sessions). Note that, because of missing head direction data during threat bouts, we averaged neural activity across all threat data points and lost temporal variation occurring within a bout. This explains why we obtained an even higher correlation than calculated in Fig. 5d. e, Average activity during threat to subject when alone vs. paired separated by brain area. f, Representational similarity scores as if Fig. 5d separated by brain area. g, Mean Euclidean distance from baseline (centre of mass of ‘rest’ states) in UMAP neural space during threats towards the subject in the alone (dark red) vs. paired conditions (yellow), separated by monkey (same as in Fig. 4e). Shaded area represents SEM.
Supplementary information
Supplementary Information 1
Cayo ethogram, basis for the experiment’s ethogram.
Supplementary Information 2
Comparison of Cayo and Lab ethogram.
Supplementary Information 3
Ethogram (description of behaviours, grooming contexts, visuomotor variables).
Supplementary Information 4
Intervention description and schedule.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Testard, C., Tremblay, S., Parodi, F. et al. Neural signatures of natural behaviour in socializing macaques. Nature 628, 381–390 (2024). https://doi.org/10.1038/s41586-024-07178-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07178-6
This article is cited by
-
Natural primate neurobiology
Nature Reviews Neuroscience (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.