Abstract
A hallmark of cancer is the avoidance of immune destruction. This process has been primarily investigated in locally advanced or metastatic cancer1,2,3; however, much less is known about how pre-malignant or early invasive tumours evade immune detection. Here, to understand this process in early colorectal cancers (CRCs), we investigated how naive colon cancer organoids that were engineered in vitro to harbour Apc-null, KrasG12D and Trp53-null (AKP) mutations adapted to the in vivo native colonic environment. Comprehensive transcriptomic and chromatin analyses revealed that the endoderm-specifying transcription factor SOX17 became strongly upregulated in vivo. Notably, whereas SOX17 loss did not affect AKP organoid propagation in vitro, its loss markedly reduced the ability of AKP tumours to persist in vivo. The small fraction of SOX17-null tumours that grew displayed notable interferon-γ (IFNγ)-producing effector-like CD8+ T cell infiltrates in contrast to the immune-suppressive microenvironment in wild-type counterparts. Mechanistically, in both endogenous Apc-null pre-malignant adenomas and transplanted organoid-derived AKP CRCs, SOX17 suppresses the ability of tumour cells to sense and respond to IFNγ, preventing anti-tumour T cell responses. Finally, SOX17 engages a fetal intestinal programme that drives differentiation away from LGR5+ tumour cells to produce immune-evasive LGR5− tumour cells with lower expression of major histocompatibility complex class I (MHC-I). We propose that SOX17 is a transcription factor that is engaged during the early steps of colon cancer to orchestrate an immune-evasive programme that permits CRC initiation and progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Datasets generated in this study are available at the Gene Expression Omnibus (GEO) repository (GSE222713, token: ifylaumkxlsvvqh): including RNA-seq data (GSE222709, GSE222710 and GSE245668), ATAC-seq data (GSE222712) and single-cell RNA-seq data (GSE222711). Any additional information required to reanalyse the data reported in this paper is available upon request.
References
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752.e4720 (2021).
Lin, J. R. et al. Multiplexed 3D atlas of state transitions and immune interaction in colorectal cancer. Cell 186, 363–381.e319 (2023).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543 (2018).
Shankaran, V. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
Koebel, C. M. et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907 (2007).
Beyaz, S. et al. Dietary suppression of MHC class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. Cell Stem Cell 28, 1922–1935 e1925 (2021).
Roper, J. et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 35, 569–576 (2017).
Roper, J. et al. Colonoscopy-based colorectal cancer modeling in mice with CRISPR–Cas9 genome editing and organoid transplantation. Nat. Protoc. 13, 217–234 (2018).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Goto, N. et al. Lymphatics and fibroblasts support intestinal stem cells in homeostasis and injury. Cell Stem Cell 29, 1246–1261 e1246 (2022).
Spence, J. R. et al. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev. Cell 17, 62–74 (2009).
Shivdasani, R. A. Molecular regulation of vertebrate early endoderm development. Dev. Biol. 249, 191–203 (2002).
Kanai-Azuma, M. et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129, 2367–2379 (2002).
Schepers, A. G. et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337, 730–735 (2012).
The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Westcott, P. M. K. et al. Low neoantigen expression and poor T-cell priming underlie early immune escape in colorectal cancer. Nat. Cancer 2, 1071–1085 (2021).
Heide, T. et al. The co-evolution of the genome and epigenome in colorectal cancer. Nature 611, 733–743 (2022).
Fordham, R. P. et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13, 734–744 (2013).
Mustata, R. C. et al. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 5, 421–432 (2013).
Nusse, Y. M. et al. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature 559, 109–113 (2018).
Llosa, N. J. et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 5, 43–51 (2015).
Beltra, J. C. et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841.e828 (2020).
Miller, B. C. et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 326–336 (2019).
Khan, O. et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 571, 211–218 (2019).
Di Pilato, M. et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment. Cell 184, 4512–4530.e4522 (2021).
Ikeda, H., Old, L. J. & Schreiber, R. D. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 13, 95–109 (2002).
Pan, D. et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 359, 770–775 (2018).
Miao, D. et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat. Genet. 50, 1271–1281 (2018).
Reschke, R. & Gajewski, T. F. CXCL9 and CXCL10 bring the heat to tumors. Sci. Immunol. 7, eabq6509 (2022).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Shimokawa, M. et al. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 545, 187–192 (2017).
de Sousa e Melo, F. et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Fumagalli, A. et al. Plasticity of Lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell 26, 569–578.e567 (2020).
He, S., Kim, I., Lim, M. S. & Morrison, S. J. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes Dev. 25, 1613–1627 (2011).
Kim, I., Saunders, T. L. & Morrison, S. J. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130, 470–483 (2007).
Agudo, J. et al. Quiescent tissue stem cells evade immune surveillance. Immunity 48, 271–285 e275 (2018).
Drukker, M. et al. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 24, 221–229 (2006).
Li, L. et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells 22, 448–456 (2004).
Manguso, R. T. et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 547, 413–418 (2017).
Patel, S. J. et al. Identification of essential genes for cancer immunotherapy. Nature 548, 537–542 (2017).
Gao, J. et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167, 397–404.e399 (2016).
Zaretsky, J. M. et al. Mutations Associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Baldominos, P. et al. Quiescent cancer cells resist T cell attack by forming an immunosuppressive niche. Cell 185, 1694–1708.e1619 (2022).
Zhang, W. et al. Epigenetic inactivation of the canonical Wnt antagonist SRY-box containing gene 17 in colorectal cancer. Cancer Res. 68, 2764–2772 (2008).
Wang, L. et al. SOX17 antagonizes the WNT signaling pathway and is epigenetically inactivated in clear-cell renal cell carcinoma. OncoTargets Ther. 14, 3383–3394 (2021).
Wang, M. et al. Loss-of-function mutations of SOX17 lead to YAP/TEAD activation-dependent malignant transformation in endometrial cancer. Oncogene 42, 322–334 (2023).
Delgiorno, K. E. et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology 146, 233–244.e235 (2014).
Tan, D. S., Holzner, M., Weng, M., Srivastava, Y. & Jauch, R. SOX17 in cellular reprogramming and cancer. Semin. Cancer Biol. 67, 65–73 (2020).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
el Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
Kuraguchi, M. et al. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2, e146 (2006).
Johnson, L. et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116 (2001).
Marino, S., Vooijs, M., van Der Gulden, H., Jonkers, J. & Berns, A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 14, 994–1004 (2000).
Hogquist, K. A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994).
Chu, V. T. et al. Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes. BMC Biotechnol. 16, 4 (2016).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Hao, Z. & Rajewsky, K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 194, 1151–1164 (2001).
Dow, L. E. et al. Apc restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 161, 1539–1552 (2015).
Boutin, A. T. et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 31, 370–382 (2017).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Miyoshi, H. & Stappenbeck, T. S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 8, 2471–2482 (2013).
Schwank, G. & Clevers, H. CRISPR/Cas9-mediated genome editing of mouse small intestinal organoids. Methods Mol. Biol. 1422, 3–11 (2016).
Matano, M. et al. Modeling colorectal cancer using CRISPR–Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Koo, B. K. et al. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods 9, 81–83 (2011).
Pelossof, R. et al. Prediction of potent shRNAs with a sequential classification algorithm. Nat. Biotechnol. 35, 350–353 (2017).
Dow, L. E. et al. A pipeline for the generation of shRNA transgenic mice. Nat. Protoc. 7, 374–393 (2012).
Fellmann, C. et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 5, 1704–1713 (2013).
Mana, M. D. et al. High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep. 35, 109212 (2021).
Cheng, C. W. et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell 178, 1115–1131.e1115 (2019).
Beyaz, S. et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58 (2016).
Sheridan, B. S. & Lefrancois, L. Isolation of mouse lymphocytes from small intestine tissues. Curr. Protoc. Immunol. 99, 3.19.1–3.19.11 (2012).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.21–21.29.29 (2015).
Skene, P. J., Henikoff, J. G. & Henikoff, S. Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat. Protoc. 13, 1006–1019 (2018).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinform. 12, 323 (2011).
Bullard, J. H., Purdom, E., Hansen, K. D. & Dudoit, S. Evaluation of statistical methods for normalization and differential expression in mRNA-seq experiments. BMC Bioinform. 11, 94 (2010).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Zhu, A., Ibrahim, J. G. & Love, M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092 (2019).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Han, T. et al. Lineage reversion drives WNT independence in intestinal cancer. Cancer Discov. 10, 1590–1609 (2020).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Feng, J., Liu, T., Qin, B., Zhang, Y. & Liu, X. S. Identifying ChIP-seq enrichment using MACS. Nat. Protoc. 7, 1728–1740 (2012).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Wang, Q. et al. Exploring epigenomic datasets by ChIPseeker. Curr. Protoc. 2, e585 (2022).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Meers, M. P., Tenenbaum, D. & Henikoff, S. Peak calling by sparse enrichment analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin 12, 42 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Acknowledgements
The authors thank the Swanson Biotechnology Center at the Koch Institute, which encompasses the Flow Cytometry, Histology, Microscopy and Genomics and Bioinformatics Core facilities (NCI P30-CA14051); the Department of Comparative Medicine for mouse husbandry support; S. Holder and members of the Hope Babette Tang (1983) Histology Facility for substantial histology support; members of the Yilmaz laboratory for discussions; K. Kelley for laboratory management; L. Galoyan for administrative assistance; K. W. Wucherpfennig for reading of the manuscript; L. M. LaFave for help and advice with the ATAC-seq experiments; C. P. Concepcion for help and advice with the CUT&RUN experiments; and A. Bhutkar for help and advice for the RNA-seq and ATAC-seq analyses. N.G. is supported by Postdoctoral Fellowship for Research Abroad from Japan Society for the Promotion of Science and 1K99AG076987-01A1. S.G. is supported by the research grant of Astellas Foundation and Postdoctoral Fellowship for Research Abroad from Japan Society for the Promotion of Science. Ö.H.Y. is supported by R01CA254314, R01CA257523, R01DK126545, R01DK133919, and U54CA224068; a Pew-Stewart Trust scholar award; AFAR and Glenn Foundation for Medical Research Breakthroughs in Gerontology (BIG) Award; Kenneth Rainin Foundation Innovator Award; and a Crohn’s & Colitis Foundation Senior Research Award. Ö.H.Y. receives support from the MIT Stem Cell Initiative via Fondation MIT. J.A. is supported by R01DK132544, the Smith Family Awards Program for Excellence in Biomedical Research, the Ludwig Center at Harvard, the Ira Schneider Foundation, the Claudia Adams Barr Program in Innovative Basic Cancer Research Program, and the Innovation research Funds at DFCI. J.A. is a member of the Parker Institute for Cancer Immunotherapy and a New York Stem Cell Foundation–Robertson Investigator, and her research is funded, in part, by these two institutes. Ö.H.Y. and J.A. together are supported by the Footbridge and Traditional Bridge Koch Institute–Dana-Farber Cancer Institute awards.
Author information
Authors and Affiliations
Contributions
N.G., P.M.K.W., T.J., J.A. and Ö.H.Y. conceived the study. N.G., P.M.K.W., S.G., J.A. and Ö.H.Y. designed the experiments. N.G. performed most of the experiments. P.M.K.W. assisted with co-culture experiments of T cells and organoids and provided reagents. S.G. performed the autochthonous mouse adenoma model experiments. P.M.K.W. assisted with the RNA-seq and ATAC-seq analyses. S.I. and S.G. assisted organoid orthotopic transplantation experiments. J.B. generated mouse naive AKP organoids. G.E. established healthy human colon organoids from patients. M.S.T. and V.D. provided human histopathological samples with diagnostic information and assisted in the interpretation of histology. N.G., J.A, and Ö.H.Y wrote the paper. All the authors assisted in the interpretation of the experiments and the editing of the paper.
Corresponding authors
Ethics declarations
Competing interests
Ö.H.Y. holds equity and is a scientific advisory board member in Ava Lifesciences and AI Proteins. Ö.H.Y. receives research support from Microbial Machines. Ö.H.Y. is a consultant for Nestle. T.J. is a member of the Board of Directors of Amgen and Thermo Fisher Scientific and a co-founder of Dragonfly Therapeutics and T2 Biosystems; serves on the Scientific Advisory Board of Dragonfly Therapeutics, SQZ Biotech, and Skyhawk Therapeutics; and is president of Break Through Cancer. The laboratory of T.J. currently receives funding from Johnson & Johnson and The Lustgarten Foundation, but these funds did not support the research described in this manuscript. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ophir Klein, Sean Morrison and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 In vivo transplantation of naïve AKP organoids induces SOX17 expression.
a, Schematic of generation of mouse naïve AKP organoids by shApc and primary tumour-derived AKP organoids from immunocompetent mice by colonoscopy-based orthotopic transplantation. b, c, PCA plot (b) and volcano plot (c) of RNA-seq in naïve and primary tumour-derived AKP organoids (shApc model) from immunocompetent mice. n = 3-6 mice per group. d, Schematic of RNA-seq in bulk resected primary tumours as well as in flow cytometry-sorted tdTomato+ tumour cells that were freshly isolated from primary tumours. e, f, PCA plot (e) and volcano plot (f) of RNA-seq in naïve AKP organoids and bulk resected primary tumours. n = 3-6 mice per group. g, h, PCA plot (g) and volcano plot (h) of RNA-seq in naïve AKP organoids and flow cytometry-sorted tdTomato+ tumour cells that were freshly isolated from primary tumours. n = 3-4 mice per group.
Extended Data Fig. 2 Autochthonous tumours and transplanted tumours in immunodeficient mice show elevated SOX17 expression.
a, Autochthonous tumour models by colonoscopy-guided 4-OH-Tamoxifen injection into the colonic mucosa of Villin-CreERT2; Apc f/f; Kras LSL-G12D; P53 f/f; Rosa LSL-tdTomato (AKPVT) mice. b-d, PCA plot (b), volcano plot (c), and GSEA (d) of RNA-seq in normal colon and autochthonous AKPVT tumours. n = 4-7 mice per group. e, Schematic of generation of mouse naïve AKP organoids and mouse primary tumour-derived AKP organoids from immunodeficient mice (Rag2−/− mice) by colonoscopy-based orthotopic transplantation. f, g, PCA plot (f) and volcano plot (g) of RNA-seq in naïve and primary tumour-derived AKP organoids from immunodeficient mice. n = 3 mice per group.
Extended Data Fig. 3 Generation of human naive AKP organoids.
a, Schematic of generation of human naive AKP organoids by CRISPR-Cas9 editing. b, Representative image of human APC−/− organoids and Sanger sequencing result confirming the biallelic frameshift mutations in APC. c, Representative image of human APC−/−; P53−/− organoids and Sanger sequencing result confirming the biallelic frameshift mutations in TP53. d, e, Strategy to knock in a KRASG12D point mutation by homology directed repair (d), and PCR results confirming the correct homology directed repair (e). f, Representative image of human APC−/−; KRASG12D; P53−/− organoids and the Sanger sequencing result confirming the KRASG12D point mutation. g, Immunoblots for SOX17 in human naive AKP organoids and in vivo tumour-derived human AKP organoids.
Extended Data Fig. 4 SOX17 loss does not affect tumour growth/proliferation in immunodeficient mice.
a, IHC for SOX17 in sgScramble, sgSox17-1, and sgSox17-2 in vivo tumour-derived AKP organoids. b, Time-course images of sgScramble, sgSox17-1, and sgSox17-2 AKP organoids. 2,000 single cells in 10 µl Matrigel were plated for each well. Representative of 10 wells. Representative of three independent experiments. See also Fig. 2c for organoid growth curve. c, d, IHC for BrdU (c) and the percentage of BrdU+ cells (d) in sgScramble, sgSox17-1, and sgSox17-2 AKP organoids that were incubated for 4 hrs with 10 µM BrdU in the culture media. n = 30 organoids per group. e, Representative images and H&E staining of orthotopically transplanted sgScramble, sgSox17-1, and sgSox17-2 AKP tumours in immunocompetent mice. f, g, IHC for Ki67 (f) and quantification (g) in orthotopically transplanted sgScramble, sgSox17-1, and sgSox17-2 AKP tumours in NCG mice. n = 20 fields from 5 mice per group. h, i, IHC for Ki67 (h) and quantification (i) in orthotopically transplanted sgScramble, sgSox17-1, and sgSox17-2 AKP tumours in Rag2−/− mice. n = 20 fields from 5 mice per group. One-way ANOVA (d, g, i). Data are mean ± SD. N.S. not significant. Scale bar, 20 μm (a, c, f, h), 1000 μm (b), 500 μm (e).
Extended Data Fig. 5 Sox17 knockdown in established AKP tumours inhibits tumour growth.
a, Constructs for Sox17 knockdown and control. b, qRT-PCR for Sox17 mRNA expression 48 hr after doxycycline administration. n = 3 organoid culture wells per group. c, Schematic of Sox17 knockdown in established AKP tumours using doxycycline-inducible system. d, Tumour formation was confirmed by colonoscopy 1 week after the transplantation. e, f, Representative images (e) and tumour size (f) 2 weeks after doxycycline administration. n = 15 mice per group. Pooled from three independent experiments. g, h, IHC for CD4 and CD8 (g) and quantification (h) in control (shRenilla) and Sox17 knockdown (shSox17) tumours. n = 20 fields from 5 mice per group. Unpaired two-tailed t-tests (b, f, h). Data are mean ± SD. *p < 0.05. Scale bar, 20 μm (g).
Extended Data Fig. 6 Time-course analysis of scRNA-seq of immune cells in control and SOX17-null tumours.
a, b, UMAP plots of CD45+ cells in control and SOX17KO AKP tumours at 1-, 2- and 4-weeks post-transplantation. Classified by sample groups (a) and cell clusters (b). Control at 1 wk; n = 5,296 cells, control at 2 wks; n = 4,585 cells, control at 4 wks; n = 6,230 cells, SOX17KO at 1 wk; n = 7,047 cells, SOX17KO at 2 wks; n = 2,894 cells, SOX17KO at 4 wks; n = 6,207 cells, pooled from 5 mice in each group. c, Expressions of key genes used for identification of major populations in the scRNA-seq analysis of control and SOX17KO tumours.
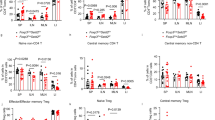
Extended Data Fig. 7 SOX17 alters CD4+ T cell subsets in AKP CRCs.
a, b, Time-course analysis of CD45+ cells in control and SOX17KO AKP tumours at 1-, 2- and 4-weeks post-transplantation by scRNA-seq. UMAP plots (a). Bar graphs showing % of each subcluster (b). c-e, UMAP plots (c) of re-clustering of CD4+ T cell clusters (clusters 5 and 9 in Extended Data Fig. 7b). Bar graphs showing % of each subcluster (d). Dot plots of gene expressions in each subcluster (e).
Extended Data Fig. 8 Effector Th1 CD4+ T cells contribute to the elimination of SOX17-null tumours.
a-c, IHC for SOX17 (a) and CD4 (b) and quantification (c) in sgScramble, sgSox17-1, and sgSox17-2 AKP tumours at 1-, 2- and 4-weeks post-transplantation. n = 20 fields from 5 mice per group. d, Violin plot for Ifng expression in scRNA-seq data from Extended Data Fig. 6a. e-g, Schematic (e), representative images (f), and tumour engraftment rate (g) of orthotopically transplanted SOX17KO (sgSox17-1 and sgSOX17-2) AKP tumours in isotype control- and anti-CD4 antibody-treated mice. n = 10 mice per group. Pooled from two independent experiments. h, SOX17-null tumour size in anti-CD4 antibody and anti-CD8 antibody-treated mice. n = 9-10 mice per group. Pooled from two independent experiments. i, j, IHC for CD8 (i) and quantification (j) in isotype control- and anti-CD4 antibody-treated mice. n = 20 fields from 5 mice. One-way ANOVA (c). Fisher’s exact test (g). Unpaired two-tailed t-tests (h, j). Data are mean ± SD. *p < 0.05. Scale bar, 25 μm (a), 20 μm (b, i).
Extended Data Fig. 9 SOX17 expression augments the resistance of colon cancer cells to CD8+ T cell-mediated killing.
a-b, Sanger sequencing chromatograms and TIDE analyses for sgH2k1 clones (a) and sgB2m clones (b). c-e, Schematic (c), representative images (d), and tumour engraftment rate (e) of orthotopic transplantation of SOX17KO (sgSox17-1); sgScramble, SOX17KO; H2K1 KO (sgH2k1 clone 3), and SOX17KO; B2M KO (sgB2m clone 23) AKP organoids into the colons of immunocompetent mice. n = 7-13 mice per group. f, Schematic of co-culture experiments of SOX17OE SIINFEKL-expressing colon cancer organoids with activated OT-1 T cells. g-i, Representative images (g), and quantification of organoids (h) and CD8+ T cell numbers (i) in the co-culture experiments. n = 3 per group. j, Ifnar1 and Ifnar2 expression by RNA-seq data of control and SOX17KO AKP organoids. n = 3 per group. k, l, Flow cytometry analysis of protein surface levels of IFNAR1 (k) and quantification (l). n = 3 per group. Chi-square test (e). Unpaired two-tailed t-tests (h, I, j). One-way ANOVA (l). Data are mean ± SD. *p < 0.05. N.S. not significant. Scale bar, 500 μm (h).
Extended Data Fig. 10 Generation of SOX17 overexpression organoids and IFNGR1 knockout organoids.
a, Constructs for constitutive SOX17 overexpression (SOX17OE). b, Immunoblots confirmed SOX17 overexpression in naïve AKP organoids transduced with a constitute SOX17OE cassette. c, Constructs for dox-inducible SOX17OE. d, Immunoblots confirmed SOX17 overexpression in naïve AKP organoids transduced with a dox-inducible SOX17OE cassette 72 h after doxycycline administration. e, qRT-PCR for Ifngr1 mRNA expression in constitutive and dox-inducible SOX17OE AKP organoids compared with controls. n = 3-6 each group. f, Sanger sequencing chromatograms for IFNGR1KO clones. g, h, Flow cytometry (g) and quantification (h) of IFNGR1 expression. n = 3 per group. i, j, Flow cytometry (i) and quantification (j) of MHC-I expressions 24 hr after IFNγ (10 ng/ml) administration in the organoids. n = 3 per group. One-way ANOVA (h). Unpaired two-tailed t-test (j). Data are mean ± SD. N.S. not significant.
Extended Data Fig. 11 In vivo APC loss induces SOX17 expression.
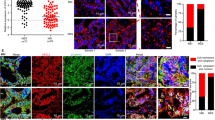
a-c, Schematic (a), representative images (b), and tumour engraftment rate (c) of orthotopic transplantation of SOX17KO (sgSox17-1) and SOX17KO; CXCL10KO (sgCxcl10 clones 6 and 9) AKP organoids into immunocompetent recipient mice. n = 20 mice per group. Sanger sequencing results of CXCL10KO organoids are in Supplementary Fig. 4. d, IHC for SOX17 in Villin-CreERT2; Apc f/f mouse normal colon and adenoma. e, Schematic of generation of naïve and primary tumour-derived Apc−/− organoids. f, qRT-PCR for Sox17 mRNA expression in naïve and primary tumour-derived Apc−/− organoids. n = 3 mice per group. g, H&E staining and IHC for β-Catenin and SOX17 in Lgr5-CreERT2; Apc f/f and Lgr5-CreERT2; Apc f/f; Sox17 f/f mouse intestines after tamoxifen injections. Representative of n = 6 mice per group. h, Flow cytometry of MHC-I (H-2Kb) expression in Lgr5-GFP− and Lgr5-GFP+ cells from Lgr5-CreERT2; Apc−/− adenoma. n = 4 mice. See Fig. 5i for quantification. Chi-square test (c). Unpaired two-tailed t-test (f). Data are mean ± SD. *p < 0.05. Scale bar, 20 μm (d, g).
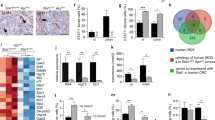
Extended Data Fig. 12 SOX17 expression in human adenomas and CRCs anti-correlates with CD8+ T cell infiltration.
a, b, Representative images (a) and classification (b) of IHC for SOX17 and CD8 in adenomas from patients with (n = 8) or without (n = 11) familial adenomatous polyposis (FAP). c-e, Representative images (c) and classification (d) of IHC for SOX17 and CD8 in CRC samples at different pathologic tumour stages (pT1: n = 9, pT2: n = 10, pT3: n = 10, pT4: n = 10). Plot (e) showing the % of CD8high tumours and CD8low tumours in SOX17high/med tumours versus SOX17low/neg tumours. n = 39 cases. Tumours were classified into SOX17high (≥70%), SOX17med (<70%, ≥30%), SOX17low (<30%, >0%), and SOX17neg (0%) based on the percentage of SOX17 nuclear staining-positive cells. Tumours with ≥ 50 CD8+ cells per x20 field were classified as CD8high tumours, <50 CD8+ cells per x20 field as CD8low tumours. Scale bar, 20 μm (a, c). Fisher’s exact test (e). *p < 0.05.
Extended Data Fig. 13 Frequency of Lgr5-GFP+ cells decreases over time in adenomas.
a, Schematic of time-course analysis of the tumours in Lgr5-CreERT2; Apc f/f mice after tamoxifen injections. b, c, IF for Lgr5-GFP and β-catenin in Lgr5-CreERT2; Apc f/f mouse intestines (b) and quantification for the percentage of Lgr5-GFP+ cells in β-catenin nuclear accumulation positive tumour cells (c). n = 30 fields from 5 mice per group. d, Schematic of anti-CD8 antibody treatment in Lgr5-CreERT2; Apc f/f mice with tamoxifen injections. e, f, IF for Lgr5-GFP (e) and quantification of Lgr5-GFP+ areas (f) in anti-CD8 or isotype antibody-treated Lgr5-CreERT2; Apc f/f mouse intestines. n = 10 fields from 5 mice per group. g, Lgr5 mRNA expression in RNA-seq of control and SOX17KO AKP organoids. n = 3 per group. h, ISH for Lgr5 in control and SOX17OE AKP tumours. Representative of n = 9-11 mice per group. i, qRT-PCR for Lgr5 mRNA expression in control and SOX17OE naïve AKP organoids. n = 3 per group. One-way ANOVA (c). Unpaired two-tailed t-test (f, g, i). Data are mean ± SEM (c) and mean ± SD (f, g, i). *p < 0.05. Scale bar, 50 μm (b, e), 20 μm (h).
Extended Data Fig. 14 LGR5+ and LGR5- tumour cells are highly plastic and give rise to one another during tumour growth.
a, Schematic of generation of Lgr5-GFP; AKP organoids and orthotopic transplantation. b, Flow cytometry for Lgr5-GFP+ cells from Lgr5-GFP; AKP tumours and wild-type colon epithelia (negative control). Representative of n = 6 mice. c, qRT-PCR for Lgr5 mRNA expression in sorted Lgr5-GFP− and Lgr5-GFP+ cells from Lgr5-GFP; AKP tumours. n = 3 mice per group. d, e, Schematic (d) and tumour engraftment rate (e) of orthotopic transplantation of sorted Lgr5-GFP− and Lgr5-GFP+ cells into the colons of immunocompetent mice. In each mouse, 4,000 cells were orthotopically transplanted. n = 10-13 mice per group. Representative of two independent experiments. f, IF for Lgr5-GFP and SOX17 in Lgr5-GFP− cell-derived tumours and Lgr5-GFP+ cell-derived tumours. Representative of n = 6 mice per each group. Unpaired two-tailed t-test (c). Fisher’s exact test (e). Data are mean ± SD. N.S. not significant. *p < 0.05. Scale bar, 50 μm (f).
Supplementary information
Supplementary Information
Supplementary Table 1 and Supplementary Figs 1–5.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goto, N., Westcott, P.M.K., Goto, S. et al. SOX17 enables immune evasion of early colorectal adenomas and cancers. Nature 627, 636–645 (2024). https://doi.org/10.1038/s41586-024-07135-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07135-3
This article is cited by
-
Programming immune escape
Nature Reviews Cancer (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.