Abstract
Mycobacterium tuberculosis (Mtb) is a bacterial pathogen that causes tuberculosis (TB), an infectious disease that is responsible for major health and economic costs worldwide1. Mtb encounters diverse environments during its life cycle and responds to these changes largely by reprogramming its transcriptional output2. However, the mechanisms of Mtb transcription and how they are regulated remain poorly understood. Here we use a sequencing method that simultaneously determines both termini of individual RNA molecules in bacterial cells3 to profile the Mtb transcriptome at high resolution. Unexpectedly, we find that most Mtb transcripts are incomplete, with their 5′ ends aligned at transcription start sites and 3′ ends located 200–500 nucleotides downstream. We show that these short RNAs are mainly associated with paused RNA polymerases (RNAPs) rather than being products of premature termination. We further show that the high propensity of Mtb RNAP to pause early in transcription relies on the binding of the σ-factor. Finally, we show that a translating ribosome promotes transcription elongation, revealing a potential role for transcription–translation coupling in controlling Mtb gene expression. In sum, our findings depict a mycobacterial transcriptome that prominently features incomplete transcripts resulting from RNAP pausing. We propose that the pausing phase constitutes an important transcriptional checkpoint in Mtb that allows the bacterium to adapt to environmental changes and could be exploited for TB therapeutics.
Similar content being viewed by others
Main
TB is the leading cause of death among infectious diseases4. The aetiological agent of TB, Mtb, has an exceptional ability to evade host defence mechanisms and drug treatment1. Mtb achieves this feat in part by enacting the appropriate transcriptional output in response to changing environments2. Thus, a detailed characterization of the Mtb transcriptome is key to understanding the pathogenesis and persistence of TB. However, many aspects of Mtb transcription remain poorly understood, and previous studies suggest that the operation of the Mtb transcription machinery differs substantially from that of its well-studied Escherichia coli (Eco) counterpart5,6.
Recently, we developed SEnd-seq (simultaneous 5′ and 3′ end sequencing) that enables full-length RNA profiling in a bacterial transcriptome3. This method provides a greater resolution of transcript boundaries than standard RNA-seq and has generated new insights into the mechanism of Eco transcription in our previous study3. In the current work, we applied SEnd-seq in combination with genetic manipulation and in vitro biochemistry to characterize the Mtb transcriptome and study transcriptional regulation in Mtb.
Profiling the Mtb transcriptome by SEnd-seq
Compared to Eco, Mtb has a much slower growth rate and is more difficult to lyse. We developed a SEnd-seq method customized for mycobacteria (Extended Data Fig. 1a–c and Methods), which captured the correlated 5′- and 3′-end sequences of individual Mtb transcripts (Fig. 1a,b and Extended Data Fig. 1d,e). Using a SEnd-seq protocol that specifically enriches for 5′-triphosphorylated primary RNAs, we identified 8,873 transcriptional start sites (TSSs) in Mtb as compared to 5,038 in the fast-growing model mycobacterium Mycobacterium smegmatis (Msm) and 4,358 in Eco (Fig. 1c, Extended Data Fig. 2a,b and Supplementary Table 1). SEnd-seq also identified 747 leaderless TSSs that lack a 5′ untranslated region (UTR), a known feature of the Mtb trancriptome7,8 (Extended Data Fig. 2c). Our dataset recapitulates most of the previously identified TSSs but also reveals many new sites (Extended Data Fig. 2d,e). Sequence analysis of our TSS dataset as well as the leaderless TSS subset showed the conserved −10 motif (TANNNT) recognized by the housekeeping σA-factor9 (Extended Data Fig. 2f,g), lending support to the validity of the identified sites. In addition, by using a SEnd-seq protocol for profiling total RNAs that contain both 5′-triphosphorylated primary RNAs and 5′-monophosphorylated processed RNAs, we found that Mtb uses a dearth of strong transcription termination sites (TTSs), defined here as sites where a sharp reduction in the total RNA coverage was observed (Fig. 1d, Extended Data Fig. 2h–k and Supplementary Table 2). The paucity of canonical intrinsic terminators in the Mtb genome is consistent with previous results10,11. SEnd-seq also revealed pervasive antisense RNAs (asRNAs) in the Mtb transcriptome (Fig. 1e, Extended Data Fig. 2a,l–p and Supplementary Table 3), also consistent with previous work8. Our data further show that the abundance of asRNAs within a given gene is inversely correlated with the corresponding sense transcript level (Extended Data Fig. 2n). Together, these results demonstrate the utility of SEnd-seq in profiling mycobacterial transcriptomes with high resolution and sensitivity.
a, A circos plot showing the transcriptomic profile of log-phase Mtb cells. Outer circle: gene annotation; middle circle: RNA coverage; inner circle: TSS intensity. Red and blue colours represent positive and negative strands, respectively. Mb, megabase. b, A SEnd-seq data track for an example Mtb genomic region showing primary and total RNA coverage (summed over signals from both strands), aligned SEnd-seq reads (red lines: positive-strand transcripts; blue lines: negative-strand transcripts) and TSSs (orange arrows: positive-strand TSSs; blue arrows: negative-strand TSSs). The primary RNA SEnd-seq data were used for TSS identification. The total RNA SEnd-seq data were used to evaluate the overall RNA level. c,d, Bar graphs showing the number of TSSs (c) and TTSs (d) detected by SEnd-seq for Eco (genome size: 4.6 Mb), Mtb (genome size: 4.4 Mb) and Msm (genome size: 7.0 Mb). The same criteria were used across species for TSS and TTS identification. e, Distribution of the number of asRNAs detected by SEnd-seq for each Mtb coding gene.
The Mtb transcriptome is dominated by short RNAs
A major advantage of SEnd-seq is the precise definition of 5′ and 3′ ends of individual RNA transcripts. By applying this approach, we observed a striking pattern in the RNA coverage in Mtb that differed markedly from what we had observed in Eco3. In Eco, the RNA coverage remains largely constant across open reading frames. By contrast, the Mtb RNA coverage drops significantly between 200 and 500 nucleotides (nt) downstream of TSSs (Figs. 1b and 2a,b). The RNA coverage drop-off tends to occur well before the encoded gene’s stop codon, yielding a large fraction of incomplete transcripts with heterogeneous 3′ ends. To systematically characterize this RNA drop-off pattern, we annotated 1,930 protein-coding transcription units (TUs) in Mtb, each containing one or several co-directional genes controlled by a major TSS (Extended Data Fig. 3a–d). For each TU, we calculated a numerical ‘progression factor’ (PF) defined as the ratio of RNA coverage between an upstream zone and a downstream zone using the total (that is, primary and processed) RNA SEnd-seq dataset. Smaller PF values indicate higher fractions of incomplete transcripts (Fig. 2c,d). Indeed, we found that the PF values are predominantly distributed below 1.0, although they do span a wide range of values (Fig. 2e and Supplementary Table 4). TUs with a relatively high or low PF are enriched in genes involved in distinct aspects of Mtb physiology (Extended Data Fig. 3e,f), the implications of which await further investigation. We did not observe a noticeable dependence of PF on the length of the 5′ UTR (Extended Data Fig. 3g). The RNA coverage drop-off was observed for both log-phase and stationary-phase Mtb cells (Extended Data Fig. 3h). Moreover, the coverage for Mtb asRNAs exhibited a steeper drop-off compared to that for coding TUs (Fig. 2f). Indeed, Mtb asRNAs generally feature a lower PF value than coding TUs (Fig. 2e). We also analysed previous Mtb transcriptomic data obtained by standard RNA-seq12—which reported RNA coverage from fragmented short reads—and found a similar drop-off pattern, supporting the findings from our SEnd-seq data (Extended Data Fig. 4a–f).
a, A total RNA SEnd-seq data track for an example Mtb TU showing predominantly incomplete RNAs with 5′ ends aligned at the TSS and heterogeneous 3′ ends (red lines: sense transcripts; blue lines: antisense transcripts). kb, kilobase. b, Summed SEnd-seq intensities aligned at TSSs for log-phase Mtb (red) and Eco (blue) cells. TSSs without another strong TSS within 700 nt downstream were selected for analysis (n = 1,431 for Mtb; n = 930 for Eco). The RNA intensities were normalized to the maximum value within a 200-nt window downstream of the corresponding TSS. Coloured lines represent median values and shaded regions represent standard deviations (s.d.). c,d, SEnd-seq signals for an example Mtb coding TU with a relatively high (c) or low (d) PF. The upstream and downstream zones for PF calculation are indicated. e, Distribution of the PF value for coding TUs (longer than 700 nt) and asRNAs expressed at high levels from log-phase Mtb cells (mean PF = 0.43 for coding TUs; mean PF = 0.09 for asRNAs). Only PF values lower than 1.2 are shown. f, Summed SEnd-seq intensities aligned at TSSs for coding TUs (n = 1,277) and asRNAs (n = 1,440) expressed at high levels. Coloured lines represent median values and shaded regions represent s.d.
Short Mtb transcripts are not due to RNA degradation
We carried out several experiments and analyses to rule out the possibility that the short Mtb transcripts resulted from RNA degradation. First, we found a similar coverage drop-off pattern in both primary RNA and total RNA SEnd-seq datasets (Fig. 1b and Extended Data Fig. 1d,e), ruling out the possibility that the short RNAs are generated by endonucleolytic cleavage, which would yield 5′-monophosphorylated RNAs excluded from the primary RNA sample but preserved in the total RNA sample. Second, we used an anhydrotetracycline (ATc)-inducible expression system to monitor heterologous transcription of the Eco lacZ gene in Mtb, which allowed us to track the lacZ mRNA length by quantitative PCR (qPCR) as a function of post-induction time. If the short RNAs were mainly due to degradation of full-length transcripts, we would expect to see a burst of RNA signal in the downstream zone at early time points after induction, followed by a decay in signal. However, we observed the accumulation of lacZ signal only within 500 nt of the TSS (Extended Data Fig. 4g–i), mirroring the steady-state length profile of endogenous Mtb TUs mapped by SEnd-seq. Last, we used CRISPR interference13 to individually knockdown RNase or RNase-related genes in Mtb14 (Extended Data Fig. 5a). After verifying the knockdown efficiency for each strain, we monitored their effects on the PF of select TUs and found that none of the gene knockdowns consistently caused an increase in the PF value (Extended Data Fig. 5b–d). Taken together, these results suggest that the short RNAs prevalently found in the Mtb transcriptome are generated during RNA synthesis rather than during post-transcriptional processing.
Short RNAs are mostly bound to paused RNAPs
Short transcripts could represent released RNAs due to premature transcription termination15 or, alternatively, represent nascent RNAs bound to paused transcription elongation complexes. To distinguish between these scenarios, we developed a native elongating transcript SEnd-seq (NET-SEnd-seq) method adapted from a NET-seq protocol developed for Eco16. This method uses a His-tagged Mtb RNAP β′-subunit to enrich for RNAP-bound nascent transcripts, which are then subjected to SEnd-seq analysis (Extended Data Fig. 6a–e). Using NET-SEnd-seq, we observed a drop-off pattern in the RNA coverage 200–500 nt downstream of TSSs for both coding TUs and asRNAs, very similar to the drop-off pattern observed in the total RNA SEnd-seq dataset (Fig. 3a,b and Extended Data Fig. 6f–l). This similarity suggests that a large fraction of the short transcripts were associated with RNAPs that paused downstream of the TSS. To further test this interpretation, we carried out chromatin immunoprecipitation followed by high-throughput sequencing (ChIP–seq) experiments for Mtb RNAP using an optimized protocol. Consistent with strong RNAP pausing predicted by NET-SEnd-seq, our ChIP–seq data show RNAP occupancy peaks within the RNA coverage drop-off region, both for individual TUs (Fig. 3a,c and Extended Data Fig. 7a–f) and in the averaged profile (Fig. 3d). In comparison, we carried out Eco RNAP ChIP–seq experiments and found that Eco RNAP occupancy peaks were located much closer to TSSs (Extended Data Fig. 7g–i), in agreement with previous results17. Last, we carried out ChIP–seq experiments for the Mtb σA-factor and again observed occupancy peaks within the RNA coverage drop-off region overlapping with the RNAP occupancy peaks (Fig. 3d and Extended Data Fig. 7j–m). Together, our NET-SEnd-seq and ChIP–seq results support a model in which the Mtb RNAP–σA holoenzyme has a strong propensity to pause 200–500 nt downstream of the TSS, producing incomplete transcripts. Notably, we did not find strong consensus motifs around the pausing sites (Extended Data Fig. 7n), suggesting that Mtb RNAP pausing in these regions is not sequence specific.
a, RNAP ChIP–seq, NET-SEnd-seq and total RNA SEnd-seq data tracks for an example Mtb genomic region. b, Summed intensities of the 5′-end (green) and 3′-end (red) positions of nascent RNAs from the NET-SEnd-seq dataset (n = 4,362). a.u., arbitrary units. c, RNAP ChIP–seq, σA-factor ChIP–seq and total RNA SEnd-seq data tracks for an example Mtb genomic region. d, Summed intensities of NET-SEnd-seq (red; n = 1,412), RNAP ChIP–seq (green; n = 996) and σA-factor ChIP–seq (blue; n = 635) for Mtb genomic regions aligned at TSSs. Strong TSSs without another strong TSS within 700 nt downstream were selected for NET-SEnd-seq data analysis. Transcribed regions that exhibited strong RNAP or σA-factor occupancy were selected for ChIP–seq data analysis. The intensities for each position were normalized to the maximum value of the corresponding data track within a 200-nt window downstream of the TSS. Coloured lines represent median values and shaded regions represent s.d.
Rho depletion does not globally lengthen Mtb RNAs
To further support our model that the short transcripts in Mtb are largely associated with paused RNAPs rather than being terminated products, we depleted the transcription termination factor Rho from Mtb cells by CRISPR interference (Extended Data Fig. 8a,b). Consistent with the known essentiality of Rho in Mtb, Rho depletion impaired cell growth and significantly changed the expression level of some essential genes (Extended Data Fig. 8c,d). However, Rho depletion did not significantly alter the PF values for coding TUs as shown by SEnd-seq (Extended Data Fig. 8e), suggesting that the incomplete transcripts were not primarily caused by Rho-mediated premature termination. Instead, we found that rho knockdown resulted in a genome-wide increase in the abundance of asRNAs but without a substantial change in their lengths (Extended Data Fig. 8f,g). Our results are consistent with those of a previous RNA-seq study that also reported pervasive transcription in a Rho-depleted Mtb strain18. Analysis of the data from both studies showed that the RNA coverage drop-off pattern downstream of TSSs remained largely unchanged following Rho depletion (Extended Data Fig. 8h,i).
Mtb RNAP–σA synthesizes short transcripts in vitro
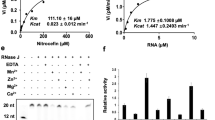
The prevalence of short RNAs in Mtb suggests that its transcription machinery has an unexpectedly high probability to enter long-lived pauses well before complete synthesis of full-length transcripts. To determine whether this behaviour is an intrinsic feature of the Mtb RNAP or is mediated by other factors, we carried out in vitro transcription experiments using purified Mtb RNAP–σA holoenzyme and plasmid DNA templates. We used qPCR to analyse the RNA abundances at various locations within the transcribed region as a function of time. In agreement with the in vivo SEnd-seq results, in vitro reactions produced mostly transcripts shorter than 500 nt in length (Fig. 4a and Extended Data Fig. 9a,b). By contrast, the Eco RNAP–σ70 holoenzyme was able to read through the transcribed region and synthesize full-length transcripts in vitro (Fig. 4b and Extended Data Fig. 9c,d). Next, we added Mtb NusA and NusG factors to the in vitro transcription reaction and found that neither factor substantially enhanced or impaired the synthesis of full-length transcripts by Mtb RNAP–σA (Extended Data Fig. 9e,f).
a, Length profile of the RNA products from in vitro transcription assays using the Mtb RNAP–σA holoenzyme and a plasmid DNA template harbouring an AP3 promoter, a transcribed region (coloured in yellow) and an intrinsic terminator (red hairpin). RNA abundances at different locations of the transcribed region were assessed by qPCR. b, Length profile of the RNA products using the Eco RNAP–σ70 holoenzyme and the same plasmid template as in a. Identical reaction conditions were used in a,b. c, Length profile of the RNA products using the Mtb RNAP core enzyme (without σA-factor) and a linear DNA template containing a preformed transcription bubble and the same transcribed region and terminator as in the plasmid template in a. d, Length profile of the RNA products using the Mtb RNAP–σA holoenzyme and the same template as in c. Data are from three independent measurements.
Given the overlapping Mtb RNAP and σA-factor occupancy observed in vivo from our ChIP–seq experiments, we sought to test whether σA-factor contributes to RNAP pausing in vitro. To this end, we constructed a template containing a preformed DNA bubble that allows RNAP to bypass the need for a σ-factor to initiate transcription. We found that the Mtb RNAP core enzyme alone was able to synthesize full-length transcripts on this template (Fig. 4c), but the addition of σA-factor restored the short-RNA profile (Fig. 4d). Together, these results indicate that the σ-factor has a crucial role in high propensity of Mtb RNAP to pause within the transcribed region.
Transcription elongation depends on translation in Mtb
Transcription–translation coupling is an important mechanism for gene regulation in bacteria19 and has been shown to promote transcription elongation in Eco20. We thus posited that active translation may be key to the synthesis of full-length transcripts in Mtb. The steeper drop-off in SEnd-seq coverage for asRNAs than for protein-coding RNAs (Fig. 2f) is consistent with this hypothesis. Moreover, we analysed published Mtb ribosome profiling data21 and found a positive correlation between the ribosome binding intensity within a coding TU and the corresponding PF value (Fig. 5a,b and Extended Data Fig. 10a). To further examine the impact of translation on transcription elongation in Mtb, we carried out SEnd-seq using Mtb cells treated with the translation inhibitor linezolid22. We found that linezolid treatment caused a reduction in RNA coverage in the downstream zone of coding TUs (Fig. 5c and Extended Data Fig. 10e) and significantly decreased the average PF (Fig. 5d), with the notable exception of whiB7, which encodes a transcription factor that coordinates a stress response to stalled ribosomes23,24 (Extended Data Fig. 10b,c). In comparison, rifampicin treatment resulted in a modest increase in the average PF (Fig. 5d). A reduced average PF was also observed for Mtb cells treated with the ribosome-targeting antibiotic clarithromycin25 (Extended Data Fig. 10d–f) but not for cells treated with streptomycin, which induces ribosome miscoding without preventing translation26 (Extended Data Fig. 10g–i). Linezolid’s negative effect on PF was still observed in Rho-depleted cells (Fig. 5d), reinforcing our conclusion that Rho is not chiefly responsible for the pervasive short RNAs found in Mtb. By contrast, linezolid had little impact on the RNA coverage for asRNAs, consistent with the fact that these transcripts are not efficiently translated (Extended Data Fig. 10j–l).
a,b, SEnd-seq (red) and ribosome profiling (Ribo-seq; blue) signals for a high-PF (a) and a low-PF (b) coding TU in the Mtb genome. c, SEnd-seq signals for an example TU from Mtb cells treated with linezolid (black) and control cells treated with DMSO (red). d, A violin plot showing the PF distribution for coding TUs from non-targeting or rho-knockdown Mtb cells treated with DMSO, rifampicin (RIF) or linezolid (LZD). TUs expressed at high levels in the DMSO-treated condition were selected for comparison (n = 1,380). Solid lines represent the median and dashed lines represent the quartiles. P values were determined using two-tailed Student’s t-test. e, Length profile of the RNA products from heterologous lacZ transcription in log-phase Mtb cells pre-treated with DMSO or linezolid and measured at 5 min or 15 min after ATc induction. RNA abundances at different positions (P1–P8) were quantified by qPCR and normalized to the DMSO-treated and uninduced condition. P, promoter; O, operator. f, Length profile of the RNA products from the wild-type (WT) lacZ template or a template containing two nonsense mutations 750 nt downstream of the TSS measured at 5 min or 30 min after ATc induction. RNA abundances were normalized to the uninduced condition for the wild-type template. Data are from three independent measurements. g, The working model for Mtb transcription in which the RNAP predominantly pauses 200–500 nt downstream of the TSS and resumes elongation when coupled to a translating ribosome. The binding of the σ-factor contributes to Mtb RNAP pausing, but whether RNAP retains σ-factor throughout the elongation phase remains unclear. Such prevalent transcriptional pausing may allow Mtb cells to integrate regulatory signals and adeptly respond to environmental changes.
We further used the inducible lacZ system to probe the response of transcription kinetics to translational perturbation. We treated Mtb cells with linezolid immediately before inducing lacZ transcription with ATc and found that the RNA abundance in the downstream zone of the lacZ gene body was substantially reduced compared to that from cells treated with dimethylsulfoxide (DMSO; Fig. 5e). In addition, we introduced nonsense mutations to the lacZ gene body and observed a reduction in the RNA abundance downstream of the ectopic stop codons (Fig. 5f). Together, these results provide evidence that active translation aids in RNAP elongation and full-length mRNA synthesis in Mtb.
Discussion
In this study, we used SEnd-seq to profile the Mtb transcriptome with high resolution. This led us to the unexpected discovery of pervasive short transcripts in Mtb. The prevalence of short RNAs has also been reported for the Eco transcriptome, but they were found to be predominantly decay intermediates27. By contrast, we showed here that the short RNAs in Mtb are primarily nascent transcripts associated with paused RNAPs (Fig. 5g). We further showed that the binding of the housekeeping σA-factor induces Mtb RNAP pausing. σ70-dependent RNAP pausing was also found to be widespread in Eco16. However, there are important distinctions between σ-dependent pausing in Eco and Mtb. First, pausing occurs much closer to TSSs in Eco (10–20 nt) than in Mtb (200–500 nt). It is generally thought that σ70-factor is released by Eco RNAP immediately after promoter escape28, although it can sometimes be retained for longer distances29,30. Our results showed that Mtb RNAP and σA-factor remain associated until at least 200 nt downstream of TSSs. Secondly, Eco σ70-factor is known to recognize conserved −10-like sequences to induce pausing31, whereas Mtb σA-factor does not seem to have such sequence specificity on the basis of our results. How the diverse σ-factors in Mtb differentially influence RNAP pausing will be an important subject for future research. For example, NET-SEnd-seq assays using His-tagged σ-factors would yield valuable insights into σ-factor occupancy in paused elongation complexes16. In addition, it will be interesting to examine whether co-transcribing RNAPs, which regulate the dynamics of Eco transcription via DNA supercoiling32, also affect the elongation and pausing behaviour of the Mtb transcription machinery.
The functional relevance of transcription–translation coupling varies among different bacterial species33,34,35, and its occurrence in mycobacteria has remained largely unexplored. Our results provide evidence that active translation plays a major role in rescuing Mtb RNAP from the paused state and promoting transcription elongation (Fig. 5g). This may confer a fitness advantage to ensure that cells do not waste energy on producing untranslated full-length transcripts20,36. Although transcription–translation coupling occurs in Eco and seems to also occur in Mtb, the molecular mechanisms differ. In Eco, Rho is required for transcriptional polarity by mediating premature termination when RNAP is uncoupled from the ribosome37. By contrast, Mtb Rho depletion does not relieve the negative effect on transcription elongation by translational inhibition. Whether other factors are involved in transcription–translation coupling in Mtb awaits further investigation. Notably, the universal transcription factor NusG was recently shown to be pro-pausing in Mtb in contrast to its anti-pausing role in Eco38. In the event of prolonged pausing, the untranslated elongation complexes may eventually undergo premature termination.
The pervasive RNAP pausing downstream of TSSs in Mtb is reminiscent of the promoter-proximal pausing of eukaryotic RNA polymerase II (Pol II), which is a well-established transcriptional checkpoint in metazoans39. Undoubtedly, the regulatory factors that induce and release paused polymerases differ across kingdoms. Pol II also tends to pause earlier (within 100 nt downstream of TSSs) than Mtb RNAP. Nevertheless, some of the physiological functions found for Pol II pausing, such as rapid and synchronous activation of gene expression, integration of multiple environmental cues, and coupling of co-transcriptional processes, may be applicable to mycobacterial RNAP pausing. Elucidating the regulatory function of the pervasive transcriptional pausing in Mtb, if any, requires further investigation.
RNAP is a prominent drug target for the antitubercular arsenal40. Our study highlights that transcriptional regulation in Mtb departs remarkably from the Eco paradigm. This insight may present unique opportunities for therapeutic intervention. For example, most current RNAP-targeting antibiotics inhibit transcription initiation. It is plausible that paused Mtb RNAP could be targeted for antibiotic development in ways distinct from the mechanism of action for existing antibiotics. Simultaneous targeting of both the ribosome and RNAP may yield synergistic effects on the Mtb transcriptome and proteome. Mtb strains resistant to the key front-line drug rifampicin harbour mutations in RNAP that come with a fitness cost and may affect the pausing behaviour41, which could make these cells more sensitive to such interventions. Finally, this work serves as a benchmark for dissecting transcriptional reprogramming of Mtb under stress conditions inside the host or following drug treatment.
Methods
Bacterial strains and growth conditions
The Mtb H37Rv strain (obtained from C. Sassetti) was grown at 37 °C in a minimal medium (Difco Middlebrook 7H9 broth (BD, 271310) supplemented with 0.5% (v/v) glycerol, 0.05% (v/v) tyloxapol (Sigma, T8761), 0.2 g l−1 casamino acids (BD, 223050), and 10% (v/v) OADC (oleic acid, albumin, dextrose and catalase; BD, 212351)). The double-auxotrophic Mtb mc26206 strain (H37Rv ΔpanCDΔleuCD)42 (obtained from W. Jacobs Jr) was grown in the minimal medium with an additional 50 mg l−1 l-leucine (Sigma, L8000) and 24 mg l−1 pantothenic acid (Sigma, P5155). The Msm mc2155 strain (obtained from S. Fortune) was grown in the Middlebrook 7H9 medium supplemented with 0.2% (v/v) glycerol, 0.05% (v/v) Tween-80 (VWR, M126), and 10% (v/v) albumin–dextrose–catalase. Liquid Mtb and Msm cultures were grown at 37 °C in Nalgene sterile square PETG medium bottles with constant agitation. The solid Mtb culture was grown on 7H11 agar (Sigma, M0428) supplemented as described above except for tyloxapol.
CRISPR interference
Plasmid pIRL58 (Addgene, 166886) bearing the Streptococcus thermophilus CRISPR–dCas9 system (dCas9Sth1)13 was used to modulate the RNA expression level of target genes in Mtb mc26206 cells. Oligonucleotides for single guide RNAs (sgRNAs; Integrated DNA Technologies) were cloned into pIRL58. After verification by Sanger sequencing, pIRL58 and pIRL19 (Addgene, 163634, which supplied the L5 integrase function on a separate suicide vector) were co-transformed into Mtb cells by electroporation using GenePulser (BioRad) at 2,500 V, 700 Ω, and 25 μF. Single colonies were picked from the solid culture plates with 20 μg ml−1 kanamycin (Goldbio, K-120) selection after 14–21 days of culture. Target gene knockdown was induced by adding 100 ng ml−1 ATc (Sigma, 37919). The sgRNA and primer sequences are listed in Supplementary Table 5.
SEnd-seq
RNA isolation
Bacterial cells were quenched by adding 1× vol of GTC buffer (600 g l−1 guanidium thiocyanate, 5 g l−1 N-laurylsarcosine, 7.1 g l−1 sodium citrate, and 0.7% (v/v) β-mercaptoethanol) to the culture medium immediately before collection and placed at room temperature for 15 min. Cell pellets were collected by centrifugation at 4,000g for 10 min at 4 °C, and then thoroughly resuspended in 100 μl TE buffer (10 mM Tris-HCl pH 8.0 and 1 mM EDTA). After the addition of 1 ml TRIzol reagent (Invitrogen, 15596) and 350 mg of glass beads (Sigma, G1145), the cells were immediately lysed in a screw-cap tube by bead beating with the Precellys Evolution homogenizer (Bertin Technologies, 02520-300-RD000) at 10,000 r.p.m. for 4× 45-s cycles with a 60-s interval and chilled with dry ice. After removal of the beads by spinning samples at 12,000 r.p.m. for 5 min at 4 °C, the liquid phase was transferred to a new tube. A 200 μl volume of chloroform was added, and the sample was gently inverted several times until reaching homogeneity. The sample was then incubated for 15 min at room temperature before spinning at 12,000g for 10 min at 4 °C. The upper phase (about 600 μl) was gently collected and mixed at a 1:1 ratio with 100% isopropanol. The sample was incubated for 2 h at −20 °C and then centrifuged at 14,000 r.p.m. for 15 min at 4 °C. The pellet was washed twice with 1 ml of 75% (v/v) ethanol, air dried for 5 min and dissolved in nuclease-free water. RNA integrity was assessed with 1% (m/v) agarose gel and the Agilent 2100 Bioanalyzer System (Agilent Technologies, 5067-4626). For antibiotic treatment conditions, Mtb mc26206 cells were exponentially grown to an optical density at 600 nm (OD600) of about 0.8 followed by treatment with a specific antibiotic (30 μg ml−1 linezolid (Sigma, PZ0014), 40 μg ml−1 clarithromycin (Sigma, C9742), 300 μg ml−1 streptomycin (Sigma, S9137), or 50 μg ml−1 rifampicin (Sigma, R3501)). At each time point following the treatment, 4 ml of cell culture medium was withdrawn and mixed quickly with 4 ml GTC buffer. The cells were then collected, and the RNA was isolated as described above.
Library preparation for total RNA SEnd-seq
A 5 μg quantity of total RNA was mixed with pooled spike-in RNAs used in our previous study3 at a mass ratio of 300:1 in a total volume of 12 μl. The RNA sample was incubated with a 5′-adaptor ligation mix (1 μl of 100 μM 5′ adaptor (Supplementary Table 5), 0.5 μl of 50 mM ATP, 2 μl DMSO, 5 μl of 50% PEG8000, 1 μl RNase Inhibitor (New England BioLabs, M0314), and 1 μl of High Concentration T4 RNA Ligase 1 (New England BioLabs, M0437)) at 23 °C for 5 h. The sample was then diluted to 40 μl with nuclease-free water and cleaned twice with 1.5× vol of Agencourt RNAClean XP beads (Beckman Coulter, A63987). Immediately following the 5′ adaptor ligation, the eluted RNA was ligated to the 3′ adaptor (Supplementary Table 5) using the same procedure. After incubation at 23 °C for 5 h, the reaction was diluted to 40 μl with water and cleaned twice with 1.5× vol of Agencourt RNAClean XP beads to remove excess adaptors. The sample was subsequently eluted with 0.1× TE buffer and subjected to ribosomal RNA removal with RiboMinus Transcriptome Isolation Kit (Thermo Fisher, K155004) following the manufacturer’s instructions. After recovery by ethanol precipitation, the RNA was reverse transcribed to cDNA with Eubacterium rectale maturase (recombinantly purified from Eco, obtained from A. M. Pyle)43 and 5′-phosphorylated and biotinylated reverse transcription primer (Supplementary Table 5). After purification, the cDNA was circularized with TS2126 RNA ligase44 (obtained from K. Ryan). Double-stranded DNA was synthesized by DNA PolI (New England BioLabs, M0209S). After enzyme inactivation and DNA purification with 1.5× vol of AMPure beads (Beckman Coulter, A63882), the DNA was subsequently fragmented by dsDNA Fragmentase (New England BioLabs, M0348S) at 37 °C for 15 min. The reaction was stopped by adding 5 μl of 0.5 M EDTA and incubated at 65 °C for 15 min in the presence of 50 mM dithiothreitol (DTT). Next, the DNA was diluted to 40 μl with TE buffer and purified with 1.5× vol of AMPure beads. The eluted DNA was used for sequencing library preparation with NEBNext Ultra II DNA Library Prep Kit (New England BioLabs, E7645). Biotinylated DNA fragments were enriched by 5 μl of Dynabeads M-280 Streptavidin (Thermo Fisher, 11205D) and further amplified for 12 cycles by PCR.
Library preparation for primary RNA SEnd-seq
A 5 μg quantity of total RNA was used for primary transcript enrichment with our previously published method3. In brief, the 5′-triphosphorylated RNA species was specifically capped with 3′-desthiobiotin-GTP (New England BioLabs, N0761) by the Vaccinia Capping System (New England BioLabs, M2080S). The RNA was subjected to 3′ adaptor ligation using the same procedure as described above and subsequently enriched with Hydrophilic Streptavidin Magnetic Beads (New England BioLabs, S1421). After washing thoroughly, the RNA was eluted and reverse transcribed to cDNA as described above. The remaining steps were the same as those for library preparation for total RNA SEnd-seq, except that the DNA library was amplified for 15 cycles.
Illumina sequencing
Following PCR amplification, each amplicon was cleaned by 1× vol of AMPure XP beads twice and quantified with a Qubit 2.0 fluorometer (Invitrogen). The amplicon size and purity were further evaluated on an Agilent 2200 Tape Station (Agilent Technologies, 5067-5576). Equal amounts of amplicon were then multiplexed and sequenced with 2 × 150 cycles on an Illumina NextSeq500 or NovaSeq6000 platform (Rockefeller University Genomics Resource Center).
SEnd-seq data analysis
Data processing
After quality filtering and Illumina sequencing adaptor trimming with FASTX-Toolkit (v0.0.13), the raw paired-end reads were merged to single-end reads by using FLASh software (v1.2.11). The correlated 5′-end and 3′-end sequences were extracted by the custom script (fasta_to_paired.sh) using the SeqKit (v2.4.0) and Cutadapt (v4.1) packages. The inferred full-length reads were generated by Bedtools (v2.31.0) and Samtools (v1.17) after mapping to the reference genome (NC_000913.3 for Eco, NC_008596.1 for Msm and NC_018143.2 for Mtb) with Bowtie 2 (v2.5.1). The full-length reads with an insert length greater than 10,000 nt were discarded. The mapping results were visualized using the IGV genome viewer (v2.4.10). Data analysis and visualization scripts used Python packages including Matplotlib (v3.7.1), Numpy (v1.24.3), Scipy (v1.10.1), bioinfokit (v0.3), and pyCircos (v0.3.0).
RNA coverage
Each full-length read was first mapped to the genome in a specific direction. Directional RNA coverage was quantified by summing the number of aligned reads at each mapped nucleotide position. When comparing RNA coverage between samples, data were normalized by the total non-ribosomal RNA amount in each sample. For the samples treated with translation inhibitors, the abundance of spike-in RNAs was used for normalization. Coding TUs and asRNAs with high levels of expression were defined as those with an average RNA coverage of at least 10 for the first 100 nt downstream of the TSS. The circos plot was generated using the Python package pyCircos (github.com/ponnhide/pyCircos, version 0.2.0). The RNA coverage plots were generated using Matplotlib package45 and custom Python scripts.
TSS identification
TSSs were identified from the primary RNA SEnd-seq data using a custom Python script. Only positions with more than 10 reads starting at that position, and with an increase of at least 50% in read coverage compared to its upstream neighbouring position (for example, 50 reads at position −1 and 150 reads at position 0), were retained. Candidate TSS positions within 5 nt in the same orientation were grouped together, and the position with the largest amount of read increase was used as the representative TSS position. Motif analysis around the TSS regions (−40 nt to +5 nt) was carried out by MEME (v5.5.2)46.
TTS identification
Potential TTSs were identified from the total RNA SEnd-seq data at genomic positions with more than 10 reads ending at that position (outside rRNA genes) and with a reduction of more than 40% in read coverage compared to its upstream neighbouring position (for example, 100 reads at position −1 and 50 reads at position 0).
TU annotation
TUs were used in this work to analyse the transcription of coding genes. The genome was first segmented into preliminary TUs that contained annotated genes of the same direction. A preliminary unit was further segmented into multiple units if it contained any internal TSS with a strong activity (>2-fold increase in RNA coverage between downstream and upstream of the site for log-phase cell sample). As such, each TU contains a major TSS (TU start site) and possibly additional minor TSSs (<2-fold increase in RNA coverage). The end site of a TU was set to 10 nt before the start of a following co-directional TU, or the middle position between opposite genes that belong to two convergent TUs. TUs shorter than 700 nt and TUs annotated with only rRNA or tRNA genes were excluded from further analysis.
Antisense transcript annotation
asRNAs were called if there existed a strong antisense TSS within a given coding TU or if an opposite-direction TSS was found within the non-annotated 400-nt region downstream of a coding TU. The end site of an asRNA was set to the position where the RNA coverage dropped below 25% of the peak value.
PF analysis
Each coding TU was assigned with an upstream zone (from 0 to 200 nt downstream of the TSS) and a downstream zone (from 500 nt downstream of the TSS to the end of the TU). If there was another qualified TSS located within the downstream zone, the region downstream of that TSS was excluded from analysis. The ratio between the average RNA intensity of the downstream zone and that of the upstream zone was calculated as the PF for the corresponding TU. For asRNAs, the upstream and downstream zones were defined as 0–200 nt and 500–700 nt downstream of the TSS, respectively. The lower and upper bounds of PF values were set to be 0.0 and 2.0, respectively.
Gene ontology analysis
The Database for Annotation, Visualization, and Integrated Discovery (DAVID; v2023q2; https://david.ncifcrf.gov/)47 was used to carry out gene ontology analysis for Mtb genes with different PF values. The complete list of genes within each set was uploaded to DAVID under the headings of Cellular Compartment, Biological Process, and Molecular Function. Enriched categories with a P value < 0.05 were presented.
NET-SEnd-seq
Cell collection, lysis, and elongation complex pulldown protocols were adapted from a published study16 with modifications. Briefly, an ATc-inducible pIRL58 backbone plasmid bearing Mtb rpoC–6×His was transformed into Mtb mc2 6206 cells and the genome-integrated expression strain was picked as described above. For each pulldown sample, 55 ml of cell culture was prepared. When the cell culture reached the mid-log phase (OD600 = 0.5), 100 ng ml−1 ATc or an equivalent volume of solvent methanol was added to the medium, and the cells were cultured for another 12 h. After removing 4 ml of cell culture for total RNA extraction, the remaining cell culture was mixed with an equal volume of frozen 2× crush buffer (20 mM Tris-HCl pH 7.8, 10 mM EDTA, 100 mM NaCl, 1 M urea, 25 mM NaN3, 2 mM β-mercaptoethanol, 10% ethanol, 0.4% NP40, and 1 mM phenylmethylsulfonyl fluoride). The cells were subsequently precipitated by centrifugation at 4,000g for 10 min at 4 °C, immediately frozen in liquid nitrogen, and stored at −80 °C for at least 1 day. After thawing on ice, the cells were washed twice with 25 ml of cold PBS pH 7.4 and once with 5 ml of cold lysis buffer (20 mM KOH-HEPES pH 7.9, 50 mM KCl, 0.5 mM DTT, 5 mM CaCl2, 10% glycerol, 0.3 mM MgCl2, and 2.5 mM imidazole). The cells were then resuspended in 2 ml of lysis buffer, transferred to two 2-ml lysing matrix B tubes (MP Biomedicals, 116911050), and immediately lysed by bead beating with the Precellys Evolution homogenizer at 10,000 r.p.m. for 4× 45-s cycles with 60-s interval and chilled with dry ice. After centrifugation at 13,000g for 5 min, the supernatant was collected into a new 15-ml RNase-free tube. Each lysing matrix B tube was subjected to an additional round of bead beating with 1 ml of fresh lysis buffer and the supernatants were combined. Next, the collected sample was treated with 1 μl TURBO DNase (Life Technologies, AM2238) and incubated at room temperature for 10 min. After centrifugation at 4,000g for 10 min at 4 °C, the supernatant was transferred to a new 15-ml tube and incubated with 40 μl pre-washed Ni-NTA beads (Qiagen, 30230) for 1 h at 4 °C with continuous shaking at 100 r.p.m. After immobilization, the beads were washed four times with 5 ml of wash buffer (20 mM Tris-HCl pH 7.8, 1 M betaine, 5% glycerol, 2 mM β-mercaptoethanol, and 2.5 mM imidazole) and five times with 5 ml of pre-elution buffer (20 mM Tris-HCl pH 7.8, 40 mM KCl, 5% glycerol, 2 mM β-mercaptoethanol, and 2.5 mM imidazole). The immobilized complex was subsequently eluted with 300 μl of the pre-elution buffer containing 0.3 M imidazole. The nucleic acids in the eluates were extracted once with 200 μl phenol/chloroform/isoamyl alcohol (25:24:1, v/v/v) and once with 200 µl chloroform. The top aqueous phase was collected and precipitated by 3× volumes of ethanol, 0.1× vol of 3 M sodium acetate pH 5.2, and 2 μl glycogen (Thermo Fisher, AM9510). After precipitation at −20 °C overnight and maximum-speed centrifugation for 20 min, the pellet was washed twice with 300 µl of 75% ethanol. The pellet was then dissolved in 50 µl nuclease-free water and treated with 0.5 U Turbo DNase at 37 °C for 15 min. The residual RNA was extracted by phenol/chloroform/isoamyl alcohol, precipitated by ethanol and recovered in 11.5 µl of nuclease-free water. A 1 µl volume of spike-in RNA was added to each RNA sample, and the RNAs were ligated to a 3′ adaptor. The remaining steps were the same as those described above for total RNA SEnd-seq. The DNA library was amplified for 16 cycles by PCR.
ChIP–seq
A 50 ml volume of mid-log phase Mtb cells (OD600 = 0.8–1.0) were treated with 1% formaldehyde while the culture was agitated at room temperature for 30 min. Crosslinking was quenched by adding glycine to a final concentration of 250 mM for another 30 min while stirring at room temperature. The cells were pelleted by centrifugation at 4,000g for 10 min at 4 °C and washed three times with 20 ml of cold PBS and 0.1× protease inhibitor (Sigma, P8465). The cell pellet was stored at −80 °C for at least one day. After thawing on ice, the cells were washed once with 5 ml of IP lysis buffer (20 mM KOH-HEPES pH 7.9, 50 mM KCl, 0.5 mM DTT, 5 mM CaCl2, and 10% glycerol) and resuspended in 2 ml of IP lysis buffer. The cells were then transferred to two 2-ml lysing matrix B tubes (MP Biomedicals, 116911050) and immediately lysed by bead beating with the Precellys Evolution homogenizer at 10,000 r.p.m. for 4× 45-s cycles with a 60-s interval and chilled with dry ice. After centrifugation at 13,000g for 5 min, the supernatant was collected into a new 15-ml RNase-free tube. Each lysing matrix B tube was subjected to an additional round of bead beating after adding 1 ml of fresh IP lysis buffer. After centrifugation at 4,000g for 10 min at 4 °C and sampling for input control, 4 ml of supernatant was transferred to a new 15-ml tube and incubated with 0.75 µl of micrococcal nuclease (New England BioLabs, M0247S) at 37 °C for 15 min with continuous shaking. The reaction was stopped by adding EDTA at a final concentration of 25 mM, and the supernatant was transferred to a new 15-ml tube after centrifugation at 4,000g for 10 min at 4 °C. A 3 µl volume of anti-Eco σ70-factor antibody (BioLegend, 663208; 1:1,333 dilution) or 5 µl of anti-Eco RNAP β-subunit antibody (BioLegend, 663903; 1:800 dilution) was used to immunoprecipitate Mtb σA-factor and Mtb RNAP, respectively. After overnight incubation, 40 µl of pre-washed protein A/G agarose beads (Thermo Fisher, 26159) were added and incubated for 2 h at 4 °C and for another 30 min at room temperature. The beads were then washed ten times with 5 ml IPP150 buffer (10 mM Tris-HCl pH 8.0, 150 mM NaCl, and 0.1% NP40) and once with 5 ml TE buffer. Next, the DNA was eluted with 150 µl of elution buffer (50 mM Tris-HCl pH 8.0, 10 mM EDTA, and 1% SDS) followed by 100 µl TE buffer with 1% SDS. After thoroughly removing the beads by centrifugation at 2,000g for 5 min at 4 °C, the combined supernatants were incubated with 1 mg ml−1 Pronase (Sigma, 537088) at 42 °C for 2 h and then at 65 °C for 9 h. The sample was cleaned twice with 200 µl of phenol/chloroform/isoamyl alcohol (25:24:1, v/v/v) and recovered by ethanol precipitation. Finally, the sequencing libraries for immunoprecipitated DNA and input control were prepared using the NEBNext Ultra II DNA Library Prep Kit. After sequencing and quality filtering, the reads were mapped to the Mtb genome using Bowtie 2. The ChIP–seq signals were extracted and plotted using custom Python scripts.
Analysis of deposited RNA-seq data
The RNA-seq datasets SRR5689224 and SRR5689225 (BioProject PRJNA390669)12 from log-phase Mtb cells cultured in dextrose-containing medium were used to compare the RNA coverage between SEnd-seq and RNA-seq. The RNA-seq datasets SRR5061507, SRR5061514, SRR5061706 and SRR5061510 (BioProject PRJNA354066)18 from Mtb cells with Rho depletion were used to compare to the rho-knockdown SEnd-seq datasets. The deposited datasets were downloaded from the National Center for Biotechnology Information. After read extraction and quality filtering, the reads were mapped to the Mtb genome using Bowtie 2 (v2.5.1). The RNA intensities were extracted and plotted using custom Python scripts.
Analysis of deposited Ribo-seq data
Mtb Ribo-seq data were downloaded from the EMBL-EBI database (E-MTAB-8835)21. After read extraction and quality filtering, the reads were mapped to the Mtb genome using Bowtie 2. The directional ribosome binding signals were extracted and plotted using a custom Python script.
Immunoblot
Mtb cells were lysed with TRIzol reagent as described above, and protein samples were extracted following a TRIzol-based protein extraction protocol provided by the manufacturer. Immunoblotting was carried out as described previously48. Antibodies against His-tag (Santa Cruz, sc-8036; 1:1,000 dilution), Mtb Rho (obtained from D. Schnappinger; 1:200 dilution), and Eco RpoB (BioLegend, 663903; 1:1,000 dilution) were used.
qPCR
A 1–10 μg amount of total RNA was treated with 0.5 μl of TURBO DNase (Life Technologies, AM2238) at 37 °C for 30 min to remove the genomic DNA. The sample was diluted to 100 μl with RNase-free water and then cleaned three times with 100 μl of H2O-saturated phenol/chloroform/isoamyl alcohol (25:24:1, v/v/v). After ethanol precipitation, 1 μg of RNA was reverse transcribed to cDNA with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher, 4368814) following the manufacturer’s instructions. qPCR was conducted using synthesized primers and the SYBR green master mix (Thermo Fisher, 4309155) on a QuantStudio5 Real-Time PCR System (Thermo Fisher). The relative RNA abundance was presented as the signal ratio between the target transcript and the reference 16S rRNA from the same sample using the formula: 2Ct(16S) − Ct(target), in which Ct denotes the cycle threshold.
Inducible lacZ transcription in Mtb
Plasmid pIRL58 was modified by removing the sgRNA expression cassette and replacing the dCas9Sth1 gene body with the Eco lacZ coding region, allowing the synthesis of lacZ RNA under the control of ATc-inducible promoter Ptet. The modified plasmid was co-transformed into Mtb mc26206 cells with pIRL19 as described above. Cells from a single colony of Mtb Ptet-lacZ after selection were exponentially grown to an OD600 of about 0.8 followed by the addition of 100 ng ml−1 ATc to induce lacZ transcription. After induction, 4 ml of cell culture was withdrawn at indicated time points and mixed with 4 ml GTC buffer in a new tube as sample t (St). One extra sample taken immediately before ATc addition was referred to as S0. After RNA isolation and TURBO DNase treatment as described above, 1 µg of total RNA was used to synthesize the cDNA for qPCR. The relative lacZ mRNA abundance at each time point is defined as 2Ct(S0) − Ct(St), in which Ct denotes the cycle threshold.
In vitro transcription
DNA fragments were amplified by PCR from Mtb genomic DNA with primer sets listed in Supplementary Table 5. An AP3 promoter sequence was inserted into one end of the fragment and an intrinsic terminator (derived from TsynB in pIRL58) was placed at the other end. The DNA fragment was then incorporated into the pUC19 plasmid. The plasmid templates were prepared from Eco DH5α cells and subsequently treated with 2 µl RNase A (Thermo Fisher, EN0531) for 30 min and 2 µl Proteinase K (New England BioLabs, P8107S) for 1 h. The plasmid templates were cleaned three times with phenol/chloroform/isoamyl alcohol (25:24:1, v/v/v) and recovered by ethanol precipitation.
To prepare templates with a preformed bubble, the DNA fragment containing the intrinsic terminator was amplified from the plasmid DNA described above by PCR. The product was cleaned with QIAQuick PCR purification kit (Qiagen, 28104) and phenol/chloroform/isoamyl alcohol (25:24:1, v/v/v). The bubble template was constructed by ligating a DNA adaptor (NEBNext adaptor for Illumina) to each end of the DNA fragment using NEBNext Ultra II DNA Library Prep Kit. After XbaI digestion (cut site immediately after the terminator), the DNA template was purified using AMPure XP beads.
Purified Mtb RNAP, σA-factor, NusA, and NusG were prepared as described previously38,49,50. The in vitro transcription mixture contained 2 μl of 10× transcription buffer (200 mM Tris-acetate pH 7.9, 0.5 M potassium acetate, 100 mM magnesium acetate, 10 mM DTT, and 50 µg ml−1 BSA), 1 μl RNase inhibitor, 0.5 pmol of DNA template, and 2 pmol of Mtb RNAP holoenzyme (or core RNAP alone) in a 20 μl volume. The mixture was incubated at 37 °C for 15 min before the addition of rNTPs (100 μM each). At indicated time points, the reaction was quenched by adding EDTA at a final concentration of 20 mM and 2 μl of Proteinase K and incubating for 30 min. The reaction was then diluted to 100 μl with RNase-free H2O and cleaned three times with phenol/chloroform/isoamyl alcohol (25:24:1, v/v/v). After ethanol precipitation and resuspension with 30 μl RNase-free H2O, 0.5 μl DNase I (New England BioLabs, M0303S), 3.5 μl of DNase buffer, and 1 μl RNase inhibitor were added. After incubation at 37 °C for 30 min, the RNA product was cleaned three times with phenol/chloroform/isoamyl alcohol (25:24:1, v/v/v) and recovered by ethanol precipitation. Half of the RNA was converted to cDNA with the High-Capacity cDNA Reverse Transcription Kit and evaluated by qPCR as described above. RNA abundances were normalized to a diluted plasmid DNA sample with a concentration of 0.033 ng ml−1.
Statistics
Statistical analyses were conducted with Excel (version 16.178.3) or GraphPad Prism (version 10.1.0). GraphPad Prism (version 10.1.0) or the Python Matplotlib package (version 3.7.1) was used for plotting.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
SEnd-seq, NET-SEnd-seq and ChIP–seq datasets from this study have been deposited in the Gene Expression Omnibus with the accession number GSE211992 (BioProject PRJNA873109). Source data are provided with this paper.
Code availability
The custom scripts in this study are available at https://github.com/LiuLab-codes/Mtb_transcriptome_profiling.
References
Pai, M. et al. Tuberculosis. Nat. Rev. Dis. Primers 2, 16076 (2016).
Flentie, K., Garner, A. L. & Stallings, C. L. Mycobacterium tuberculosis transcription machinery: ready to respond to host attacks. J. Bacteriol. 198, 1360–1373 (2016).
Ju, X., Li, D. & Liu, S. Full-length RNA profiling reveals pervasive bidirectional transcription terminators in bacteria. Nat. Microbiol. 4, 1907–1918 (2019).
Global Tuberculosis Report 2023 (World Health Organization, 2023).
Boyaci, H., Saecker, R. M. & Campbell, E. A. Transcription initiation in mycobacteria: a biophysical perspective. Transcription 11, 53–65 (2020).
Uplekar, S., Rougemont, J., Cole, S. T. & Sala, C. High-resolution transcriptome and genome-wide dynamics of RNA polymerase and NusA in Mycobacterium tuberculosis. Nucleic Acids Res. 41, 961–977 (2013).
Shell, S. S. et al. Leaderless transcripts and small proteins are common features of the mycobacterial translational landscape. PLoS Genet. 11, e1005641 (2015).
Cortes, T. et al. Genome-wide mapping of transcriptional start sites defines an extensive leaderless transcriptome in Mycobacterium tuberculosis. Cell Rep. 5, 1121–1131 (2013).
Sachdeva, P., Misra, R., Tyagi, A. K. & Singh, Y. The sigma factors of Mycobacterium tuberculosis: regulation of the regulators. FEBS J. 277, 605–626 (2010).
Arnvig, K. B. et al. Sequence-based analysis uncovers an abundance of non-coding RNA in the total transcriptome of Mycobacterium tuberculosis. PLoS Pathog. 7, e1002342 (2011).
Czyz, A., Mooney, R. A., Iaconi, A. & Landick, R. Mycobacterial RNA polymerase requires a U-tract at intrinsic terminators and is aided by NusG at suboptimal terminators. mBio 5, e00931 (2014).
Aguilar-Ayala, D. A. et al. The transcriptome of Mycobacterium tuberculosis in a lipid-rich dormancy model through RNAseq analysis. Sci. Rep. 7, 17665 (2017).
Rock, J. M. et al. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat. Microbiol. 2, 16274 (2017).
Bechhofer, D. H. & Deutscher, M. P. Bacterial ribonucleases and their roles in RNA metabolism. Crit. Rev. Biochem. Mol. Biol. 54, 242–300 (2019).
D’Halluin, A. et al. Premature termination of transcription is shaped by Rho and translated uORFS in Mycobacterium tuberculosis. iScience 26, 106465 (2023).
Sun, Z., Yakhnin, A. V., FitzGerald, P. C., McIntosh, C. E. & Kashlev, M. Nascent RNA sequencing identifies a widespread sigma70-dependent pausing regulated by Gre factors in bacteria. Nat. Commun. 12, 906 (2021).
Mooney, R. A. et al. Regulator trafficking on bacterial transcription units in vivo. Mol. Cell 33, 97–108 (2009).
Botella, L., Vaubourgeix, J., Livny, J. & Schnappinger, D. Depleting Mycobacterium tuberculosis of the transcription termination factor Rho causes pervasive transcription and rapid death. Nat. Commun. 8, 14731 (2017).
Blaha, G. M. & Wade, J. T. Transcription-translation coupling in bacteria. Annu. Rev. Genet. 56, 187–205 (2022).
Proshkin, S., Rahmouni, A. R., Mironov, A. & Nudler, E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science 328, 504–508 (2010).
Sawyer, E. B., Phelan, J. E., Clark, T. G. & Cortes, T. A snapshot of translation in Mycobacterium tuberculosis during exponential growth and nutrient starvation revealed by ribosome profiling. Cell Rep. 34, 108695 (2021).
Marks, J. et al. Context-specific inhibition of translation by ribosomal antibiotics targeting the peptidyl transferase center. Proc. Natl Acad. Sci. USA 113, 12150–12155 (2016).
Poulton, N. C. et al. Beyond antibiotic resistance: the whiB7 transcription factor coordinates an adaptive response to alanine starvation in mycobacteria. Cell Chem. Biol. https://doi.org/10.1016/j.chembiol.2023.12.020 (2024).
Morris, R. P. et al. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 102, 12200–12205 (2005).
Peters, D. H. & Clissold, S. P. Clarithromycin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic potential. Drugs 44, 117–164 (1992).
Demirci, H. et al. A structural basis for streptomycin-induced misreading of the genetic code. Nat. Commun. 4, 1355 (2013).
Herzel, L., Stanley, J. A., Yao, C. C. & Li, G. W. Ubiquitous mRNA decay fragments in E. coli redefine the functional transcriptome. Nucleic Acids Res. 50, 5029–5046 (2022).
Mooney, R. A., Darst, S. A. & Landick, R. Sigma and RNA polymerase: an on-again, off-again relationship? Mol. Cell 20, 335–345 (2005).
Harden, T. T. et al. Bacterial RNA polymerase can retain σ70 throughout transcription. Proc. Natl Acad. Sci. USA 113, 602–607 (2016).
Bar-Nahum, G. & Nudler, E. Isolation and characterization of σ70-retaining transcription elongation complexes from Escherichia coli. Cell 106, 443–451 (2001).
Perdue, S. A. & Roberts, J. W. σ70-dependent transcription pausing in Escherichia coli. J. Mol. Biol. 412, 782–792 (2011).
Kim, S., Beltran, B., Irnov, I. & Jacobs-Wagner, C. Long-distance cooperative and antagonistic RNA polymerase dynamics via DNA supercoiling. Cell 179, 106–119.e16 (2019).
Zhu, M., Mori, M., Hwa, T. & Dai, X. Disruption of transcription-translation coordination in Escherichia coli leads to premature transcriptional termination. Nat. Microbiol. 4, 2347–2356 (2019).
Johnson, G. E., Lalanne, J. B., Peters, M. L. & Li, G. W. Functionally uncoupled transcription–translation in Bacillus subtilis. Nature 585, 124–128 (2020).
O’Reilly, F. J. et al. In-cell architecture of an actively transcribing-translating expressome. Science 369, 554–557 (2020).
McGary, K. & Nudler, E. RNA polymerase and the ribosome: the close relationship. Curr. Opin. Microbiol. 16, 112–117 (2013).
Richardson, J. P. Preventing the synthesis of unused transcripts by Rho factor. Cell 64, 1047–1049 (1991).
Delbeau, M. et al. Structural and functional basis of the universal transcription factor NusG pro-pausing activity in Mycobacterium tuberculosis. Mol. Cell 83, 1474–1488 (2023).
Adelman, K. & Lis, J. T. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat. Rev. Genet. 13, 720–731 (2012).
Chakraborty, S. & Rhee, K. Y. Tuberculosis drug development: history and evolution of the mechanism-based paradigm. Cold Spring Harb. Perspect. Med. 5, a021147 (2015).
Stefan, M. A., Ugur, F. S. & Garcia, G. A. Source of the fitness defect in rifamycin-resistant Mycobacterium tuberculosis RNA polymerase and the mechanism of compensation by mutations in the beta’ subunit. Antimicrob. Agents Chemother. 62, e00164-18 (2018).
Jain, P. et al. Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. mBio 5, e01245-14 (2014).
Zhao, C., Liu, F. & Pyle, A. M. An ultraprocessive, accurate reverse transcriptase encoded by a metazoan group II intron. RNA 24, 183–195 (2018).
Lama, L. & Ryan, K. Adenylylation of small RNA sequencing adapters using the TS2126 RNA ligase I. RNA 22, 155–161 (2016).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Bailey, T. L., Johnson, J., Grant, C. E. & Noble, W. S. The MEME suite. Nucleic Acids Res. 43, W39–W49 (2015).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221 (2022).
Ju, X. et al. Neuraminidase of influenza A virus binds lysosome-associated membrane proteins directly and induces lysosome rupture. J. Virol. 89, 10347–10358 (2015).
Hubin, E. A., Lilic, M., Darst, S. A. & Campbell, E. A. Structural insights into the mycobacteria transcription initiation complex from analysis of X-ray crystal structures. Nat. Commun. 8, 16072 (2017).
Hubin, E. A. et al. Structural, functional, and genetic analyses of the actinobacterial transcription factor RbpA. Proc. Natl Acad. Sci. USA 112, 7171–7176 (2015).
Yeh, H. Y. et al. The core-independent promoter-specific interaction of primary sigma factor. Nucleic Acids Res. 39, 913–925 (2011).
Acknowledgements
We thank R. Landick, A. M. Pyle and D. Schnappinger for sharing reagents; M. DeJesus, R. Gong, G. Chua and Rockefeller University Genomics Resource Center for technical assistance; and S. Darst for a critical reading of the manuscript. This work was supported by a National Institutes of Health (NIH) grant (5R01GM114450 to E.A.C.), NIH New Innovator Awards (1DP2AI144850-01 to J.M.R.; 1DP2HG010510-01 to S. Liu), the Stavros Niarchos Foundation Institute for Global Infectious Disease Research (to E.A.C., J.M.R. and S. Liu), the Rita Allen Foundation (to J.M.R.), the Robertson Foundation (to S. Liu) and the Alfred P. Sloan Foundation (to S. Liu).
Author information
Authors and Affiliations
Contributions
X.J., J.M.R. and S. Liu conceived the project. X.J. carried out the experiments and data analysis. S. Li and J.M.R. provided resources for mycobacterial sample collection. R.F., L.W., M.L., M.D. and E.A.C. assisted with in vitro transcription experiments. X.J., J.M.R. and S. Liu wrote the manuscript with inputs from S. Li, R.F. and E.A.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Workflow, quality control, and example data tracks of Mtb SEnd-seq.
a, Workflow of Mtb SEnd-seq for primary RNA, total RNA, and nascent RNA associated with elongation complexes. See Methods for details. b, Gel analysis for the total RNA isolated from Mtb cells showing the abundant ribosomal RNA species. This experiment was repeated more than three times with similar results. c, SEnd-seq data tracks for three spike-in RNAs with different lengths, which were pooled with total cellular RNA before the preparation of sequencing libraries. The uniform coverage of the spike-in RNAs indicates that the library preparation steps had minimal impact on the RNA integrity. d-e, Primary RNA and total RNA SEnd-seq data tracks for two example genomic regions from log-phase Mtb cells (red lines: positive-strand transcripts; blue lines: negative-strand transcripts; orange arrows: positive-strand TSSs; blue arrows: negative-strand TSSs).
Extended Data Fig. 2 Characterization of Mtb TSSs, TTSs, and asRNAs detected by SEnd-seq.
a, (Top) Schematic showing different categories of TSS based on its location and orientation. (Bottom) Number of gTSSs, iTSSs, and asTSSs in the Mtb genome identified by SEnd-seq. b, Distribution of TSS intensities for the gTSSs (n = 2,584), iTSSs (n = 2,681), and asTSSs (n = 3,608) described in a. The bars indicate mean values with interquartile range. P values were determined using two-tailed Student’s t-test. c, Primary RNA SEnd-seq data track showing an example leaderless TSS in Mtb. d-e, Venn diagram showing the overlap of all TSSs (d) or leaderless TSSs (e) identified by SEnd-seq in this study with those reported by two previous studies7,8. f-g, Motif analysis for the +1 site, −10 element, and −35 element for all Mtb TSSs (f) and leaderless TSSs (g) identified by SEnd-seq. h, SEnd-seq data track showing an example TTS in Mtb. i, Distribution of the termination efficiencies for the TTSs in the Mtb genome identified by SEnd-seq. The lower bound to qualify for a TTS was set to 40%. j, Secondary RNA structure upstream of the example TTS shown in h. (Inset) Motif analysis for the 3′ flanking sequences of the RNA hairpins upstream of all identified Mtb TTSs (n = 121) showing a lack of conserved motif. k, Distribution of the RNA coverage upstream of each TTS identified by SEnd-seq. Only sites with an RNA coverage less than 3,000 are shown here. Note that we cannot detect potential TTSs for very lowly expressed genes due to the read threshold used in our TTS identification criteria (see Methods). l-m, Primary RNA and total RNA SEnd-seq data tracks for two example Mtb genomic regions showing an abundance of antisense RNAs (blue lines). n, Scatter plot showing the anticorrelation between the percentage of asRNAs in each TU (n = 1,930) and the summed coverage of the corresponding coding RNAs. Spearman correlation coefficient is shown. o, SEnd-seq signals for an example Mtb asRNA demonstrating the definition of asRNA length used in this study. p, Distribution of the length of asRNAs identified by SEnd-seq in log-phase Mtb cells.
Extended Data Fig. 3 Characterization of Mtb coding transcription units (TUs) detected by SEnd-seq.
a-b, Primary and total RNA SEnd-seq data tracks for two example Mtb genomic regions showing a TU consisting of several co-directional genes (a) and two convergent TUs (b). c, Distribution of the length of Mtb coding TUs. TUs shorter than 6,000 nt are shown here. d, Distribution of the number of annotated Mtb genes within each coding TU. e-f, Gene Ontology analysis for Mtb genes in TUs with a progression factor (PF) > 0.5 (e) or with a PF < 0.3 (f). g, Scatter plot showing the lack of strong correlation between the distance from the major TSS of a coding TU to its start codon (0 indicates a leaderless TSS) and the PF of the same TU. Pearson correlation coefficient is shown. h, Summed SEnd-seq intensities aligned at TSSs for log-phase (red; n = 1,390) and stationary-phase (blue; n = 302) Mtb cells. Colored lines represent median values and shaded regions represent s.d.
Extended Data Fig. 4 Supporting evidence for the pervasive short transcripts in Mtb cells.
a-b, Standard RNA-seq (ref. 12) and SEnd-seq (this study) data tracks for two example TUs in log-phase Mtb cells. As expected based on methodological differences, the boundary features are less pronounced in the RNA-seq dataset than in the SEnd-seq dataset. Nonetheless, the overall expression levels are comparable between the two methods. c, Summed intensities of RNA-seq and SEnd-seq reads for Mtb genomic regions aligned at TSSs. Strong TSSs that did not show another strong TSS within a 700-nt downstream window were selected for analysis (n = 1,431). Colored lines represent median values and shaded regions represent s.d. d, Standard RNA-seq12 and SEnd-seq data tracks for an example asRNA. e, Summed intensities of RNA-seq and SEnd-seq reads aligned at TSSs for highly expressed asRNAs in both datasets (n = 216). Colored lines represent median values and shaded regions represent s.d. f, Scatter plot showing the correlation between the percentage of asRNAs in selected TU obtained by RNA-seq and the corresponding value obtained by SEnd-seq. TUs that exhibited high expression of either sense or antisense transcripts in both datasets were selected (n = 1,826). Pearson correlation coefficient is shown. g, Experimental scheme for measuring the kinetics of heterologous lacZ transcription in Mtb cells induced by ATc. h, qPCR results for the RNA abundances at various locations (P1 to P8) across the lacZ gene body measured at different time points after ATc induction. i, Length profile of mRNA products from ATc-induced lacZ transcription in Mtb measured at different time points after ATc induction. RNA abundances were normalized to the 0-min values for each probe position.
Extended Data Fig. 5 Evaluation of the effects of RNases on the PF of Mtb TUs.
a, Schematic of the experimental strategy to knockdown individual Mtb RNase genes using CRISPRi and measure the PF of target TUs using qPCR. PF was calculated relative to a control strain expressing scrambled sgRNA. b, PF values for five selected Mtb coding TUs in control cells. c, Validation of the knockdown efficiency for each Mtb RNase gene. d, Relative PF values for the five target TUs upon the individual knockdown of nine RNase or RNase-related genes, namely rne, gpsI, rhlE, rnj, rphA, orn, rnhB, rnc, and rnpA. Data are mean ± s.d. from three independent measurements. P values were determined using two-tailed Student’s t-test.
Extended Data Fig. 6 Developing NET-SEnd-seq for nascent RNA profiling in Mtb.
a, Induced expression of His-tagged RpoC in Mtb cells verified by western blotting using anti-His antibody. His-tagged σA was used as a positive control. Asterisk indicates a non-specific band. Molecular weight markers are shown on the left. This experiment was repeated twice with similar results. b, NET-SEnd-seq data track for an example Mtb TU with or without ATc-induced RpoC-His expression. c, Percentage of mRNAs in total cellular RNAs plus spike-in RNAs before and after ATc induction. The same amount of spike-in RNAs was added to each sample before library preparation. The significantly increased mRNA population upon RpoC-His expression indicates the specific enrichment of RNAP-bound nascent RNAs in the NET-SEnd-seq dataset. Data are mean ± s.d. from three independent measurements. P value was determined using two-tailed Student’s t-test. d, NET-SEnd-seq and total RNA SEnd-seq data tracks for the rnpB gene. e, Bar graphs comparing the percentage of rnpB mRNAs in total non-ribosomal RNAs for NET-SEnd-seq and total RNA SEnd-seq datasets. As expected for a highly abundant mRNA species, rnpB transcripts account for a significant fraction of total non-ribosomal RNAs. Its lower percentage in the NET-SEnd-seq dataset indicates the specific enrichment of nascent RNAs. Data are mean ± s.d. from four independent measurements. P value was determined using two-tailed Student’s t-test. f-g, SEnd-seq data tracks for an example low-PF TU (f) and an example high-PF TU (g) comparing the RNAs profiled by NET-SEnd-seq vs. total RNA SEnd-seq. h-i, Summed SEnd-seq intensities for TUs with a PF < 0.3 (n = 464) (h) or with a PF ≥ 0.3 (n = 1,234) (i) aligned at TSSs for nascent RNA (blue) and total RNA (red) isolated from log-phase Mtb cells. TUs that exhibited high expression within a 200-nt region downstream of the TSS in both datasets were selected for analysis. Colored lines represent median values and shaded regions represent s.d. j, NET-SEnd-seq and total RNA SEnd-seq data tracks for an example Mtb asRNA. k, Summed SEnd-seq intensities for asRNAs aligned at TSSs for nascent RNA (blue) and total RNA (red). asRNAs that exhibited high expression in both datasets were selected for analysis (n = 1,852). Colored lines represent median values and shaded regions represent s.d. l, Distribution of the percentage of asRNAs in each TU for primary RNA, total RNA, and nascent RNA isolated from log-phase Mtb cells. P values were determined using two-tailed Student’s t-test.
Extended Data Fig. 7 Additional Mtb RNAP and σA ChIP-seq results.
a-c, Mtb RNAP ChIP-seq, NET-SEnd-seq, and total RNA SEnd-seq data tracks for three example Mtb genomic regions. Input DNA signals are shown on the top. d-e, Mtb RNAP ChIP-seq and total RNA SEnd-seq data tracks for two example Mtb genomic regions from cells treated with DMSO or rifampicin. f, Summed RNAP ChIP-seq intensities for transcribed regions aligned at TSSs from DMSO-treated or rifampicin-treated Mtb cells. TSSs without another strong TSS within 700 nt downstream and associated with strong RNAP occupancy in the rifampicin-treated condition were selected for analysis (n = 65). Colored lines represent median values and shaded regions represent s.d. g-h, Eco RNAP ChIP-seq and total RNA SEnd-seq data tracks for two example Eco genomic regions. i, Summed RNAP ChIP-seq intensities for TUs from log-phase Mtb and Eco cells. TSSs without another strong TSS within 700 nt downstream were selected for analysis (n = 1,432 for Mtb; n = 932 for Eco). Colored lines represent median values and shaded regions represent s.d. j-m, Mtb RNAP ChIP-seq and σA ChIP-seq data tracks for four example Mtb genomic regions and the corresponding total RNA SEnd-seq data tracks. Input DNA signals are shown on the top. Additional σA peaks that did not have a corresponding RNAP peak were sometimes observed, presumably due to RNAP-independent σA-–DNA interaction51. n, Motif analysis for DNA sequences surrounding the Mtb RNAP pause sites defined by the RNA 3′ ends in the NET-SEnd-seq data. Each panel uses a different read threshold. Positions with a higher read indicate stronger pause sites.
Extended Data Fig. 8 Effects of rho knockdown on the Mtb transcriptome.
a, SEnd-seq data track around the Mtb rho gene (Rv1297) from cells with or without ATc-induced sgRNA expression. The sgRNA targeting site is indicated by the purple line. b, Depletion of Rho proteins upon sgRNA expression is verified by western blotting. The RpoB signal serves as a loading control. M indicates the molecular weight marker lane. c, Mtb growth curves with or without ATc-induced rho knockdown. d, Volcano plot showing changes in the expression level of essential Mtb genes (n = 696) upon rho knockdown measured by SEnd-seq. P values were determined using two-tailed Student’s t-test. e, Violin plot showing the distribution of PFs for Mtb coding TUs with or without rho knockdown. TUs that exhibited high expression within a 200-nt window downstream of the TSS in both datasets were selected for analysis (n = 1,679). The solid lines represent the median and the dashed lines represent the quartiles. P value was determined using two-tailed Student’s t-test. f, SEnd-seq data track for an example Mtb genomic region showing an increase in asRNA abundance upon rho knockdown. g, Summed SEnd-seq intensities for Mtb asRNAs aligned at TSSs with or without rho knockdown. Highly expressed asRNAs in the control condition were selected for analysis (n = 1,565). The intensities for each position were normalized to the peak value in the control group. h, Data track for an example Mtb TU showing the RNA-seq results before and after Rho depletion from a previous study18 and the corresponding total RNA SEnd-seq results from the current study. i, Summed RNA-seq and SEnd-seq intensities for Mtb TUs aligned at TSSs before and after Rho depletion. TUs with a highly expressed upstream zone and a PF < 0.3 in the control condition for wildtype cells were selected for analysis (n = 431). Colored lines represent median values and shaded regions represent s.d.
Extended Data Fig. 9 Additional in vitro transcription results.
a-b, Length profile of RNA products measured by qPCR using Mtb RNAP–σA holoenzyme and two plasmid DNA templates with different transcribed regions (template 2 in a and template 3 in b) than that used in Fig. 4a (template 1). c-d, Length profile of RNA products using Eco RNAP–σ70 holoenzyme and plasmid DNA templates 2 (c) and 3 (d). e, Length profile of RNA products using plasmid DNA template 1 and Mtb RNAP–σA holoenzyme alone (control) or in the presence of Mtb NusA or NusG measured at the 3-min time point. f, Same as e except that the RNA abundances were measured at the 10-min time point. Data are from three independent measurements.
Extended Data Fig. 10 Effects of translation inhibitors on transcription elongation in Mtb.
a, Scatter plot showing the correlation between the ribosome binding intensity for Mtb coding TUs (measured by ribo-seq and normalized by the TU length) and the corresponding RNA coverage in the TU’s downstream zone (measured by SEnd-seq and normalized by the length of the downstream zone from 500 nt downstream of TSS to the end of TU). TUs that exhibited high expression within a 200-nt window downstream of the TSS in the SEnd-seq dataset were selected for analysis (n = 1,855). Spearman correlation coefficient is shown. b, Total RNA SEnd-seq data track for the whiB7 gene from Mtb cells treated with DMSO or linezolid. c, Bar graphs comparing the PF for whiB7 transcription from Mtb cells treated with DMSO or linezolid. Data are mean ± s.d. from three independent measurements. P value was determined using two-tailed Student’s t-test. d, SEnd-seq signals for an example TU from Mtb cells treated with clarithromycin (black) and control cells treated with DMSO (red). e, Summed SEnd-seq intensities aligned at TSSs for DMSO-treated (red), linezolid-treated (blue), and clarithromycin-treated (black) Mtb cells. The RNA intensities for each position were normalized to the peak value in the corresponding condition. Highly expressed TUs in the DMSO-treated condition with a PF > 0.3 were selected for analysis (n = 1,144). Colored lines represent median values and shaded regions represent s.d. f, Distribution of PFs for TUs from DMSO-treated or clarithromycin-treated Mtb cells. TUs that exhibited high expression within a 200-nt window downstream of the TSS in both datasets were selected for analysis (n = 1,543). The solid lines represent the median and the dashed lines represent the quartiles. P value was determined using two-tailed Student’s t-test. g, SEnd-seq signals for an example TU from Mtb cells treated with streptomycin (black) and control cells treated with DMSO (red). h, Summed SEnd-seq intensities aligned at TSSs for DMSO-treated (red), linezolid-treated (blue), and streptomycin-treated (black) Mtb cells. The RNA intensities for each position were normalized to the peak value in the corresponding condition. Highly expressed TUs in the DMSO-treated condition with a PF > 0.3 were selected for analysis (n = 1,144). Colored lines represent median values and shaded regions represent s.d. i, Distribution of PFs for TUs from DMSO-treated or streptomycin-treated Mtb cells. TUs that exhibited high expression within a 200-nt window downstream of the TSS in both datasets were selected for analysis (n = 1,535). The solid lines represent the median and the dashed lines represent the quartiles. P value was determined using two-tailed Student’s t-test. j, Summed SEnd-seq intensities for antisense RNAs from DMSO-treated (red) and linezolid-treated (blue) Mtb cells aligned at TSSs. Highly expressed asRNAs in the DMSO-treated condition were selected for analysis (n = 1,370). Colored lines represent median values and shaded regions represent s.d. k, SEnd-seq and ribo-seq signals for an example Mtb asRNA. l, Distribution of ribosome binding intensities for highly expressed Mtb coding TUs (n = 1,685) and asRNAs (n = 2,162) (normalized by their lengths). Only units with a normalized ribosome binding intensity of less than 100 are shown here.
Supplementary information
Supplementary Information
Supplementary Fig. 1 (original gels) and full descriptions for Supplementary Tables 1–4.
Supplementary Table 1
TSS information for Mtb and Msm detected by SEnd-seq
Supplementary Table 2
TTS information for Mtb and Msm detected by SEnd-seq
Supplementary Table 3
Antisense transcript information for Mtb detected by SEnd-seq
Supplementary Table 4
Transcription unit (TU) information annotated for the Mtb transcriptome
Supplementary Table 5
Oligonucleotides and plasmids used in this study
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ju, X., Li, S., Froom, R. et al. Incomplete transcripts dominate the Mycobacterium tuberculosis transcriptome. Nature 627, 424–430 (2024). https://doi.org/10.1038/s41586-024-07105-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07105-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.