Abstract
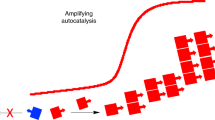
The single chirality of biological molecules is a signature of life. Yet, rationalizing how single chirality emerged remains a challenging goal1. Research has commonly focused on initial symmetry breaking and subsequent enantioenrichment of monomer building blocks—sugars and amino acids—that compose the genetic polymers RNA and DNA as well as peptides. If these building blocks are only partially enantioenriched, however, stalling of chain growth may occur, whimsically termed in the case of nucleic acids as “the problem of original syn”2. Here, in studying a new prebiotically plausible route to proteinogenic peptides3,4,5, we discovered that the reaction favours heterochiral ligation (that is, the ligation of l monomers with d monomers). Although this finding seems problematic for the prebiotic emergence of homochiral l-peptides, we demonstrate, paradoxically, that this heterochiral preference provides a mechanism for enantioenrichment in homochiral chains. Symmetry breaking, chiral amplification and chirality transfer processes occur for all reactants and products in multicomponent competitive reactions even when only one of the molecules in the complex mixture exhibits an imbalance in enantiomer concentrations (non-racemic). Solubility considerations rationalize further chemical purification and enhanced chiral amplification. Experimental data and kinetic modelling support this prebiotically plausible mechanism for the emergence of homochiral biological polymers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All source data are available in the Supplementary Information, including a video of the 3D plot in Fig. 1c. CoPaSi modelling files are available from the authors upon request. Crystallographic data for structures have been deposited at the Cambridge Crystallographic Data Centre, with numbers 2237664 (ld-dl) and 2238268 (ll-dd).
References
Blackmond, D. G. The origin of biological homochirality. Cold Spring Harb. Perspect. Biol. 11, a032540 (2019).
Brazil, R. The origin of homochirality. Chemistry World (26 October 2015).
Canavelli, P., Islam, S. & Powner, M. W. Peptide ligation by chemoselective aminonitrile coupling in water. Nature 571, 546–549 (2019).
Foden, C. S. et al. Prebiotic synthesis of cysteine peptides that catalyze peptide ligation in neutral water. Science 370, 865–869 (2020).
Singh, J. et al. Prebiotic catalytic peptide ligation yields proteinogenic peptides by intramolecular amide catalysed hydrolysis facilitating regioselective lysine ligation in neutral water. J. Am. Chem. Soc. 144, 10151–10155 (2022).
Flack, H. D. Louis Pasteur’s discovery of molecular chirality and spontaneous resolution in 1848, together with a review of his crystallographic and chemical work. Acta Cryst. A A65, 371–389 (2009).
Blackmond, D. G. Asymmetric autocatalysis and its implications for the origin of homochirality. Proc. Natl Acad. Sci. USA 101, 5732–5736 (2004).
Viedma, C., Ortiz, J. E., de Torres, T., Izumi, T. & Blackmond, D. G. Evolution of solid-phase homochirality for a proteinogenic amino acid. J. Am. Chem. Soc. 130, 15274–15275 (2008).
Klussmann, M. et al. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature 441, 621–623 (2006).
Hein, J. E., Tse, E. & Blackmond, D. G. A route to enantiopure RNA from nearly racemic precursors. Nat. Chem. 3, 704–706 (2011).
Hein, J. E. & Blackmond, D. G. On the origin of single chirality of amino acids and sugars in biogenesis. Acc. Chem. Res. 45, 2045–2054 (2012).
Powner, M. W., Gerland, B. & Sutherland, J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009).
Yu, J., Jones, A. X., Legnani, L. & Blackmond, D. G. Prebiotic access to enantioenriched glyceraldehyde mediated by peptides. Chem. Sci. 12, 6350–6354 (2021).
Legnani, L., Darù, A., Jones, A. X. & Blackmond, D. G. Mechanistic insight into the origin of stereoselectivity in the ribose-mediated Strecker synthesis of alanine. J. Am. Chem. Soc. 143, 7852–7858 (2021).
Leman, L., Orgel, L. & Ghadiri, M. R. Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306, 283–286 (2004).
Campbell, T. D. et al. Prebiotic condensation through wet–dry cycling regulated by deliquescence. Nat. Commun. 10, 4508 (2019).
Gillams, R. J. & Jia, T. Z. Mineral surface-templated self-assembling systems: case studies from nanoscience and surface science towards origins of life research. Life 8, 10 (2018).
Doran, D., Abul-Haija, Y. M. & Cronin, L. Emergence of function and selection from recursively programmed polymerisation reactions in mineral environments. Angew. Chem. Int. Ed. 58, 11253–11256 (2019).
Saghatelian, A., Yokobayashi, Y., Soltani, K. & Ghadiri, M. R. A chiroselective peptide replicator. Nature 409, 797–801 (2001).
Schmidt, J. G., Nielsen, P. E. & Orgel, L. E. Enantiomeric cross-inhibition in the synthesis of oligonucleotides on a nonchiral template. J. Am. Chem. Soc. 119, 1494–1495 (1997).
Bolli, M., Micura, R. & Eschenmoser, A. Pyranosyl-RNA: chiroselective self-assembly of base sequences by ligative oligomerization of tetranucleotide-29,39-cyclophosphates (with a commentary concerning the origin of biomolecular homochirality). Chem. Biol. 4, 309–320 (1997).
Munegami, T. & Shimoyama, A. Development of homochiral peptides in the chemical evolutionary process: separation of homochiral and heterochiral peptides. Chirality 15, S108–S115 (2003).
Sczepanski, J. T. & Joyce, G. F. A cross-chiral polymerase ribozyme. Nature 515, 440–442 (2014).
Tjhung, K., Sczepanski, J. T., Murtfeldt, E. R. & Joyce, G. F. RNA-catalyzed cross-chiral polymerization of RNA. J. Am. Chem. Soc. 142, 15331–15339 (2020).
Bare, G. A. K. & Joyce, G. F. Cross-chiral, RNA-catalyzed exponential amplification of RNA. J. Am. Chem. Soc. 143, 19160–19166 (2021).
Hoops, S. COPASI – A COmplex PAthway SImulator. Bioinformatics 22, 3067–3074 (2006).
Frank, F. C. On spontaneous asymmetric synthesis. Biochim. Biophys. Acta 11, 459–463 (1953).
Ozturk, S. F., Liu, Z., Sutherland, J. D. & Sasselov, D. D. Origin of biological homochirality by crystallization of an RNA precursor on a magnetic surface. Sci. Adv. 9, eadg8274 (2023).
Schimmel, P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu. Rev. Biochem. 56, 125–158 (1987).
Acknowledgements
D.G.B. acknowledges funding from the Simons Foundation under the Simons Collaboration on the Origins of Life (SCOL 287625) and the John C. Martin Endowed Chair in Chemistry. We acknowledge Y. Zhou for help in producing the 3D plot in Fig. 1c; L. Pasternack of the Scripps NMR facility for help with quantitative 13C-NMR experiments; the Scripps Automated Synthesis Facility, including for development of chiral assays; and M. Gembicky and J. Bailey for completing the crystallographic work at the X-ray Crystallography Facility at the University of California, San Diego.
Author information
Authors and Affiliations
Contributions
D.G.B. conceived the project, verified kinetic modelling, constructed the figures and wrote the first draft of the manuscript. M.D. carried out all experimental work (including developing 13C-NMR methodology for chiral analyses of complex product mixtures), developed the chiral amplification model and carried out kinetic modelling. J.Y. aided with critical chiral analyses. All authors contributed to discussion, interpretation of results and writing of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file is composed of five sections of experimental details, including Supplementary Schemes 1–3, Figs. 1–303 and Tables 1–109, and References.
Supplementary Video 1
Rotation of the 3D homochiral product e.e. prediction figure. Simulations of homochiral product 3 final e.e. for reaction of equimolar 1 and 2 with various initial e.e. of 1 and 2 at different diasterselectivities.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, M., Yu, J. & Blackmond, D.G. Symmetry breaking and chiral amplification in prebiotic ligation reactions. Nature 626, 1019–1024 (2024). https://doi.org/10.1038/s41586-024-07059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07059-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.