Abstract
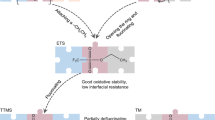
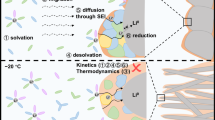
Li-ion batteries (LIBs) for electric vehicles and aviation demand high energy density, fast charging and a wide operating temperature range, which are virtually impossible because they require electrolytes to simultaneously have high ionic conductivity, low solvation energy and low melting point and form an anion-derived inorganic interphase1,2,3,4,5. Here we report guidelines for designing such electrolytes by using small-sized solvents with low solvation energy. The tiny solvent in the secondary solvation sheath pulls out the Li+ in the primary solvation sheath to form a fast ion-conduction ligand channel to enhance Li+ transport, while the small-sized solvent with low solvation energy also allows the anion to enter the first Li+ solvation shell to form an inorganic-rich interphase. The electrolyte-design concept is demonstrated by using fluoroacetonitrile (FAN) solvent. The electrolyte of 1.3 M lithium bis(fluorosulfonyl)imide (LiFSI) in FAN exhibits ultrahigh ionic conductivity of 40.3 mS cm−1 at 25 °C and 11.9 mS cm−1 even at −70 °C, thus enabling 4.5-V graphite||LiNi0.8Mn0.1Co0.1O2 pouch cells (1.2 Ah, 2.85 mAh cm−2) to achieve high reversibility (0.62 Ah) when the cells are charged and discharged even at −65 °C. The electrolyte with small-sized solvents enables LIBs to simultaneously achieve high energy density, fast charging and a wide operating temperature range, which is unattainable for the current electrolyte design but is highly desired for extreme LIBs. This mechanism is generalizable and can be expanded to other metal-ion battery electrolytes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tarascon, J.-M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Rodrigues, M.-T. F. et al. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2, 17108 (2017).
Liu, Y., Zhu, Y. & Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 4, 540–550 (2019).
Fan, X. & Wang, C. High-voltage liquid electrolytes for Li batteries: progress and perspectives. Chem. Soc. Rev. 50, 10486–10566 (2021).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4417 (2004).
Yao, N., Chen, X., Fu, Z.-H. & Zhang, Q. Applying classical, ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries. Chem. Rev. 122, 10970–11021 (2022).
Gupta, A. & Manthiram, A. Designing advanced lithium-based batteries for low-temperature conditions. Adv. Energy Mater. 10, 2001972 (2020).
Zhang, S., Xu, K. & Jow, T. The low temperature performance of Li-ion batteries. J. Power Sources 115, 137–140 (2003).
Yu, Z. et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 5, 526–533 (2020).
Huang, Y. et al. Eco-friendly electrolytes via a robust bond design for high-energy Li metal batteries. Energy Environ. Sci. 15, 4349–4361 (2022).
Fan, X. et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 4, 882–890 (2019).
Yao, Y. et al. Regulating interfacial chemistry in lithium-ion batteries by a weakly solvating electrolyte. Angew. Chem. Int. Ed. 60, 4090–4097 (2021).
Jiang, L. et al. Inhibiting solvent co-intercalation in a graphite anode by a localized high-concentration electrolyte in fast-charging batteries. Angew. Chem. Int. Ed. 60, 3402–3406 (2021).
Self, J., Fong, K. D. & Persson, K. A. Transport in superconcentrated LiPF6 and LiBF4/propylene carbonate electrolytes. ACS Energy Lett. 4, 2843–2849 (2019).
Siegel, D. J., Nazar, L., Chiang, Y.-M., Fang, C. & Balsara, N. P. Establishing a unified framework for ion solvation and transport in liquid and solid electrolytes. Trends Chem. 3, 807–818 (2021).
Aihara, Y., Sugimoto, K., Price, W. S. & Hayamizu, K. Ionic conduction and self-diffusion near infinitesimal concentration in lithium salt-organic solvent electrolytes. J. Chem. Phys. 113, 1981–1991 (2000).
Borodin, O. & Smith, G. D. Li+ transport mechanism in oligo(ethylene oxide)s compared to carbonates. J. Solut. Chem. 36, 803–813 (2007).
Yamada, Y., Wang, J., Ko, S., Watanabe, E. & Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Borodin, O. et al. Liquid structure with nano-heterogeneity promotes cationic transport in concentrated electrolytes. ACS Nano 11, 10462–10471 (2017).
Okoshi, M., Chou, C. P. & Nakai, H. Theoretical analysis of carrier ion diffusion in superconcentrated electrolyte solutions for sodium-ion batteries. J. Phys. Chem. B 122, 2600–2609 (2018).
Borodin, O., Self, J., Persson, K. A., Wang, C. & Xu, K. Uncharted waters: super-concentrated electrolytes. Joule 4, 69–100 (2020).
Sun, C. et al. 50C fast-charge Li-ion batteries using a graphite anode. Adv. Mater. 34, 2206020 (2022).
Xu, J. et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 614, 694–700 (2023).
Ren, X. et al. Role of inner solvation sheath within salt–solvent complexes in tailoring electrode/electrolyte interphases for lithium metal batteries. Proc. Natl Acad. Sci. 117, 28603–28613 (2020).
Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Ue, M. & Mori, S. Mobility and ionic association of lithium salts in a propylene carbonate-ethyl methyl carbonate mixed solvent. J. Electrochem. Soc. 142, 2577–2581 (1995).
Ren, Y. et al. Oxide electrolytes for lithium batteries. J. Am. Ceram. Soc. 98, 3603–3623 (2015).
Yang, Y. et al. Liquefied gas electrolytes for wide-temperature lithium metal batteries. Energy Environ. Sci. 13, 2209–2219 (2020).
Dong, X., Guo, Z., Guo, Z., Wang, Y. & Xia, Y. Organic batteries operated at −70°C. Joule 2, 902–913 (2018).
Seo, D. M. et al. Electrolyte solvation and ionic association III. Acetonitrile-lithium salt mixtures–transport properties. J. Electrochem. Soc. 160, A1061–A1070 (2013).
Kreuer, K.-D., Rabenau, A. & Weppner, W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew. Chem. Int. Ed. Engl. 21, 208–209 (1982).
Xing, L. et al. Deciphering the ethylene carbonate–propylene carbonate mystery in Li-ion batteries. Acc. Chem. Res. 51, 282–289 (2018).
Yao, Y.-X. et al. Unlocking charge transfer limitations for extreme fast charging of Li-ion batteries. Angew. Chem. Int. Ed. 62, e202214828 (2023).
Yang, X. et al. Enabling stable high‐voltage LiCoO2 operation by using synergetic interfacial modification strategy. Adv. Funct. Mater. 30, 2004664 (2020).
Zhi, H., Xing, L., Zheng, X., Xu, K. & Li, W. Understanding how nitriles stabilize electrolyte/electrode interface at high voltage. J. Phys. Chem. Lett. 8, 6048–6052 (2017).
Gao, Y. et al. Low-temperature and high-rate-charging lithium metal batteries enabled by an electrochemically active monolayer-regulated interface. Nat. Energy 5, 534–542 (2020).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Hayashi, A., Hama, S., Morimoto, H., Tatsumisago, M. & Minami, T. Preparation of Li2S–P2S5 amorphous solid electrolytes by mechanical milling. J. Am. Ceram. Soc. 84, 477–479 (2001).
Fang, C., Mistry, A., Srinivasan, V., Balsara, N. P. & Wang, R. Elucidating the molecular origins of the transference number in battery electrolytes using computer simulations. JACS Au 3, 306–315 (2023).
Fong, K. D., Self, J., McCloskey, B. D. & Persson, K. A. Onsager transport coefficients and transference numbers in polyelectrolyte solutions and polymerized ionic liquids. Macromolecules 53, 9503–9512 (2020).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (22161142017, 22072134, U21A2081, 20727001, 91121020, 21327802, 21973102 and 22003071), the Natural Science Foundation of Zhejiang Province (LR23B030002 and LZ21B030002), and the Fundamental Research Funds for the Central Universities (2021FZZX001-09). M.M.R. and E.H. are supported by the Assistant Secretary for Energy Efficiency and Renewable Energy (EERE), the Vehicle Technologies Office (VTO) of the U.S. Department of Energy (DOE) through the Advanced Battery Materials Research (BMR) Program under contract no. DE-SC0012704. This research used beamline 23-ID-2 of the National Synchrotron Light Source II, a U.S. DOE Office of Science user facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract number DE-SC0012704.
Author information
Authors and Affiliations
Contributions
D.L., R.L. and X.F. conceived the idea and designed the experiments. D.L., L.L., S.Y., Y.H., C.S., S.Z., H.Z. and J.Z. conducted the electrochemical experiments and characterizations, with the assistance of X.X., L.F., L.C. and X.F. M.M.R. performed XAS under the guidance of E.H. R.L. provided the theoretical calculations. P.Y. and J.W. performed the two-dimensional infrared spectroscopy test. D.L., T.D., J.W., E.H., C.W. and X.F. prepared the manuscript, with input from all the co-authors. All authors endorsed the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Chong Yan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–10, Supplementary Figs. 1–45, Supplementary Tables 1–6 and Supplementary References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, D., Li, R., Rahman, M.M. et al. Ligand-channel-enabled ultrafast Li-ion conduction. Nature 627, 101–107 (2024). https://doi.org/10.1038/s41586-024-07045-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07045-4
This article is cited by
-
Tiny sheaths of solvent boost battery performance
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.