Abstract
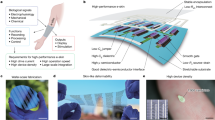
Electrode-based electrical stimulation underpins several clinical bioelectronic devices, including deep-brain stimulators1,2 and cardiac pacemakers3. However, leadless multisite stimulation is constrained by the technical difficulties and spatial-access limitations of electrode arrays. Optogenetics offers optically controlled random access with high spatiotemporal capabilities, but clinical translation poses challenges4,5,6. Here we show tunable spatiotemporal photostimulation of cardiac systems using a non-genetic platform based on semiconductor-enabled biomodulation interfaces. Through spatiotemporal profiling of photoelectrochemical currents, we assess the magnitude, precision, accuracy and resolution of photostimulation in four leadless silicon-based monolithic photoelectrochemical devices. We demonstrate the optoelectronic capabilities of the devices through optical overdrive pacing of cultured cardiomyocytes (CMs) targeting several regions and spatial extents, isolated rat hearts in a Langendorff apparatus, in vivo rat hearts in an ischaemia model and an in vivo mouse heart model with transthoracic optical pacing. We also perform the first, to our knowledge, optical override pacing and multisite pacing of a pig heart in vivo. Our systems are readily adaptable for minimally invasive clinical procedures using our custom endoscopic delivery device, with which we demonstrate closed-thoracic operations and endoscopic optical stimulation. Our results indicate the clinical potential of the leadless, lightweight and multisite photostimulation platform as a pacemaker in cardiac resynchronization therapy (CRT), in which lead-placement complications are common.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The research findings presented in this study are substantiated by the data included in the main body of the article, as well as its Supplementary information. The source data and raw data are made readily accessible at https://osf.io/kr67g/. mRNA accession numbers: Actc1: NM_019183(1); Gata4: NM_144730(1); Nrf1: NM_001100708(1); Fos: NM_022197(1); Gapdh: NM_017008(1); Gja1: NM_012567(1); cTnT: NM_012676.2. Source data are provided with this paper.
Code availability
Scripts used for data analysis in this study can be accessed from https://osf.io/kr67g/.
References
Kringelbach, M. L., Jenkinson, N., Owen, S. L. F. & Aziz, T. Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 8, 623–635 (2007).
Krauss, J. K. et al. Technology of deep brain stimulation: current status and future directions. Nat. Rev. Neurol. 17, 75–87 (2021).
Choi, Y. S. et al. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat. Biotechnol. 39, 1228–1238 (2021).
Gauvain, G. et al. Optogenetic therapy: high spatiotemporal resolution and pattern discrimination compatible with vision restoration in non-human primates. Commun. Biol. 4, 125 (2021).
Fernandez-Ruiz, A., Oliva, A. & Chang, H. High-resolution optogenetics in space and time. Trends Neurosci. 45, 854–864 (2022).
Entcheva, E. & Bub, G. All-optical control of cardiac excitation: combined high-resolution optogenetic actuation and optical mapping. J. Physiol. 594, 2503–2510 (2016).
Jiang, Y. et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 41, 652–662 (2023).
Maya-Vetencourt, J. F. et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 16, 681–689 (2017).
Ferlauto, L. et al. Design and validation of a foldable and photovoltaic wide-field epiretinal prosthesis. Nat. Commun. 9, 992 (2018).
Mickle, A. D. et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019).
Yang, Y. et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 24, 1035–1045 (2021).
Hu, H. et al. A wearable cardiac ultrasound imager. Nature 613, 667–675 (2023).
Hsueh, B. et al. Cardiogenic control of affective behavioural state. Nature 615, 292–299 (2023).
Nussinovitch, U. & Gepstein, L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol. 33, 750–754 (2015).
Huang, Y. et al. Bioresorbable thin-film silicon diodes for the optoelectronic excitation and inhibition of neural activities. Nat. Biomed. Eng. 7, 486–498 (2023).
Silverå Ejneby, M. et al. Chronic electrical stimulation of peripheral nerves via deep-red light transduced by an implanted organic photocapacitor. Nat. Biomed. Eng. 6, 741–753 (2022).
Prominski, A. et al. Porosity-based heterojunctions enable leadless optoelectronic modulation of tissues. Nat. Mater. 21, 647–655 (2022).
Jiang, Y. et al. Rational design of silicon structures for optically controlled multiscale biointerfaces. Nat. Biomed. Eng. 2, 508–521 (2018).
Chenais, N. A. L., Airaghi Leccardi, M. J. I. & Ghezzi, D. Photovoltaic retinal prosthesis restores high-resolution responses to single-pixel stimulation in blind retinas. Commun. Mater. 2, 28 (2021).
Mathieson, K. et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photonics 6, 391–397 (2012).
Gaillet, V. et al. Spatially selective activation of the visual cortex via intraneural stimulation of the optic nerve. Nat. Biomed. Eng. 4, 181–194 (2020).
Prévot, P. H. et al. Behavioural responses to a photovoltaic subretinal prosthesis implanted in non-human primates. Nat. Biomed. Eng. 4, 172–180 (2020).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents – EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Mao, X. & Chen, P. Inter-facet junction effects on particulate photoelectrodes. Nat. Mater. 21, 331–337 (2022).
Sambur, J. B. et al. Sub-particle reaction and photocurrent mapping to optimize catalyst-modified photoanodes. Nature 530, 77–80 (2016).
Lei, Y. et al. A fabrication process for flexible single-crystal perovskite devices. Nature 583, 790–795 (2020).
Lei, Y. et al. Perovskite superlattices with efficient carrier dynamics. Nature 608, 317–323 (2022).
Qian, Q. et al. Photocarrier-induced persistent structural polarization in soft-lattice lead halide perovskites. Nat. Nanotechnol. 18, 357–364 (2023).
Fang, Y. et al. Micelle-enabled self-assembly of porous and monolithic carbon membranes for bioelectronic interfaces. Nat. Nanotechnol. 16, 206–213 (2021).
Parameswaran, R. et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 13, 260–266 (2018).
Jiang, Y. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat. Mater. 15, 1023–1030 (2016).
Rotenberg, M. Y. et al. Living myofibroblast–silicon composites for probing electrical coupling in cardiac systems. Proc. Natl Acad. Sci. 116, 22531–22539 (2019).
Rotenberg, M. Y. et al. Silicon nanowires for intracellular optical interrogation with subcellular resolution. Nano Lett. 20, 1226–1232 (2020).
Maya-Vetencourt, J. F. et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 15, 698–708 (2020).
Parameswaran, R. et al. Optical stimulation of cardiac cells with a polymer-supported silicon nanowire matrix. Proc. Natl Acad. Sci. 116, 413–421 (2019).
Nikolski, V. P., Sambelashvili, A. T. & Efimov, I. R. Mechanisms of make and break excitation revisited: paradoxical break excitation during diastolic stimulation. Am. J. Physiol. Heart Circ. Physiol. 282, H565–H575 (2002).
Sobie, E. A., Susil, R. C. & Tung, L. A generalized activating function for predicting virtual electrodes in cardiac tissue. Biophys. J. 73, 1410–1423 (1997).
Wikswo, J. P., Lin, S. F. & Abbas, R. A. Virtual electrodes in cardiac tissue: a common mechanism for anodal and cathodal stimulation. Biophys. J. 69, 2195–2210 (1995).
Gillis, A. M., Fast, V. G., Rohr, S. & Kléber, A. G. Spatial changes in transmembrane potential during extracellular electrical shocks in cultured monolayers of neonatal rat ventricular myocytes. Circ. Res. 79, 676–690 (1996).
Louch, W. E., Koivumäki, J. T. & Tavi, P. Calcium signalling in developing cardiomyocytes: implications for model systems and disease. J. Physiol. 593, 1047–1063 (2015).
Herring, N., Kalla, M. & Paterson, D. J. The autonomic nervous system and cardiac arrhythmias: current concepts and emerging therapies. Nat. Rev. Cardiol. 16, 707–726 (2019).
Vernooy, K., van Deursen, C. J. M., Strik, M. & Prinzen, F. W. Strategies to improve cardiac resynchronization therapy. Nat. Rev. Cardiol. 11, 481–493 (2014).
Haeberlin, A. et al. Conduction system pacing today and tomorrow. J. Clin. Med. 11, 7258 (2022).
Mulla, W. et al. Prominent differences in left ventricular performance and myocardial properties between right ventricular and left ventricular-based pacing modes in rats. Sci. Rep. 7, 5931 (2017).
Chen, R. et al. Deep brain optogenetics without intracranial surgery. Nat. Biotechnol. 39, 161–164 (2021).
Trafford, A. W., Díaz, M. E. & Eisner, D. A. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflüg. Arch. 437, 501–503 (1999).
Kleinman, L. I. & Radford, E. P. Ventilation standards for small mammals. J. Appl. Physiol. 19, 360–362 (1964).
Acknowledgements
We thank K. M. Watters for scientific editing of the manuscript. This work was supported by the National Institutes of Health (1R56EB034289-01), the U.S. Air Force Office of Scientific Research (FA9550-20-1-0387), the National Science Foundation (NSF CBET-2128140, NSF DMR-2105321 and NSF MPS-2121044) and the U.S. Army Research Office (W911NF-21-1-0090). We would like to thank the University of Chicago Animal Resources Center (RRID: SCR_021806), especially the Carlson Large Animal clinic staff, A. Ostdiek, D. Mailhiot, J. McGrath, A. Brown, E. Becerra and P. Latalladi, for their assistance with the animal surgery. This work made use of the Pritzker Nanofabrication Facility at the Pritzker School of Molecular Engineering at the University of Chicago, which receives support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-2025633), a node of the National Science Foundation’s National Nanotechnology Coordinated Infrastructure (RRID: SCR_022955). We thank C. He for providing us the use of RT-qPCR systems. We thank F. Shi, Y. Jiang and A. Prominski for the help on the STEM imaging; this work made use of instruments in the Electron Microscopy Service (Research Resources Center, University of Illinois Chicago).
Author information
Authors and Affiliations
Contributions
B.T. and N.H. supervised the research. P.L. and B.T. initiated and conceived the Si-based photostimulation device concept. P.L. conducted most of the data collection on materials synthesis, characterization and animal experiments. P.L. designed and fabricated all the devices. J.Z. assisted with in vitro and ex vivo experiments. H.H. assisted with in vivo pig experiments. J.Y. assisted with in vitro and in vivo rodent experiments. W.L., C.Y., C.S. and J.S. assisted with materials preparation and characterization. J.H.-S. assisted with in vitro experiments. P.L. conducted all the subsequent data analysis. P.L. and B.T. prepared the manuscript, with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The University of Chicago filed provisional patent applications for the Si-based photostimulation platform and applications in multisite and minimally invasive cardiac modulation. B. Tian, P. Li, N. Hibino and H. Hayashi are the inventors. All remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Igor Efimov, Francesco Lodola and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Characterization of photocurrent precision for the four Si-based devices.
The measurement includes nine-point photocurrent measurements at the centre, edge and corner of each device. Smaller variance in the nine-point results indicates more precise random-access photostimulation across different device locations. a, Three-by-three map showing the distribution of normalized averaged photocurrent peak value on each of the nine points. s.d. is calculated from the N = 9 point measurements for each device configuration b, Bar graph showcasing the normalized photocurrents on the edge, centre and corner points. Data are expressed as mean ± s.d. derived from measurements of four independent samples (N = 4) for each Si-based device configuration. N = 16 data points were included for corner and edge plot owing to the fourfold multiplication of locations. PIN-Si and Por-Si show high photocurrent precision, with uniform photocurrents on various device locations.
Extended Data Fig. 2 Characterization of photocurrent accuracy for the four Si-based devices.
Accuracy is evaluated by mapping the photocurrent profile on the monolithic device to determine photocurrent magnitude and polarity distributions relative to the illumination spot (centre). We calculate how accurately the photocurrent hotspot aligns with stimulation location. Green dot indicates light spot and location. Black cross represents photocurrent maximum. Error (Err) defines the extent to which the photocurrent maximum deviates from the illumination centre. a, sPN-Si showed a 75% error in edge illumination. b, sPN-Si showed a 100% error in corner illumination. Detailed calculations can be found in Methods.
Extended Data Fig. 3 Further characterizations on photocurrent localization profiles.
a, Normalized photocurrent profiles of various Si-based devices under different light-stimulation spot sizes. Data are expressed as mean ± s.d. of N = 3 independent samples for each spot size and device configuration. b, Photocurrents at FWHM for different spot sizes. Data are expressed as mean ± s.d. of N = 3 independent samples for each spot size and device configuration. The Por-Si device showcases superior photocurrent resolution and a tunable FWHM in response to changes in light-spot size.
Extended Data Fig. 4 Static and dynamic spatiotemporal photocurrent profiles of the four devices.
a, Two static heat maps demonstrating the photocurrent distribution of Por-Si on horizontal and vertical planes. b, Spatiotemporal photocurrent heat maps of monolithic Por-Si reveal bipolarity evolution and domain boundary diffusion during charge (light on) and discharge (light off) when illumination is directed to the device bottom-left corner. The photocurrent normalization methods are different for the static and dynamic profiles (see Methods).
Extended Data Fig. 5 Further in vivo ischaemic rat heart pacing with 10-ms light pulse duration.
a, Plot of ECG waveforms before and after LAD ligation. Acute heart ischaemia results in increased heart rate and stronger breathing cycles, indicated by periodic fluctuations of the baseline. b, Optoelectronic pacing on LAD-ligated heart with varying light intensities. Pacing frequency was 360 bpm and pulse duration was 10 ms. Plotted are representative data from N > 3 individual rat hearts. c, Photostimulation success rate at different intensities. 100% reliability was achieved for intensities above 0.84 mW mm−2. Data are expressed as mean ± s.d. measured from N = 3 independent rats and devices. For six the plotted intensities from 0.64 to 1.65 mW mm−2, the total number of QRS evaluated are as follows, respectively: 78, 90, 94, 89, 95 and 95. d, Stable and consistent synchronization was demonstrated at 0.73 mW mm−2 with 10-ms light duration. Plotted are representative data from N = 4 individual rat hearts. e, Photograph of biventricular photostimulation on a single monolithic Si device using two spatially separated laser sources with wavelengths of 635 nm and 473 nm. ECG traces show biventricular pacing following simultaneous optoelectronic pacing on the left and right ventricles. f, Comparison of QRS durations among sinus rhythm and various pacing conditions. N = 10 QRS durations were evaluated for each condition. Boxes bind the IQR divided by the median; whiskers extend 1.5 times the IQR. Statistics are calculated using one-way analysis of variance followed by post hoc Tukey’s honestly significant difference test. n.s. > 0.05. **P < 0.01, ****P < 0.0001. g, Rat heart optoelectronic pacing at 1.22 mW mm−2 for 5 min (approximately 1,800 paced QRS complexes) demonstrated a 100% success rate. Surface ECG data were recorded using an Arduino Uno board and the ECG sensor AD8232.
Extended Data Fig. 6 Optical pacing of in vivo mouse heart.
a, Open-thoracic pacing of mouse heart using a 10-ms pulse duration, achieving stable pacing at 360 bpm with optical intensities of 1.65 mW mm−2. b, Photograph illustrating the through-thoracic pacing of a mouse heart. c, Successful through-thoracic pacing at 360 bpm using optical intensities of 62.7 mW mm−2 and a 10-ms pulse duration. N = 1 mouse heart experiment was conducted. Mouse surface ECG signals were captured using an Arduino Uno board paired with the ECG sensor AD8232.
Extended Data Fig. 7 Further data on in vivo optical pacing of pig heart.
a, ECG grid paper illustrating the transition from normal to photostimulated ECG waveforms using 23 mW mm−2 intensity and 10-ms pulse. b, Heart-contraction events depicted as vertical displacement, synchronized with photostimulation at 120 bpm, 18 mW mm−2 and 40-ms pulse. c, Deterministic pig heart optical pacing at 120 bpm using 1-ms pulse and 25 mW mm−2 intensity. d, Comparison of photostimulated ECG waveforms with sinus rhythm, showing elongated QRS durations. Statistics was performed using a two-tailed independent-sample t-test. ****P < 0.0001. Box plots represent IQR divided by the median, with whiskers extending to 1.5 times the IQR from nine analysed waveforms. N = 9 QRS waveforms obtained from the same pig experiments were analysed. e–g, Multisite spatial modulation of pig heart tissues: horizontally on the left ventricle (e); longitudinally on the right ventricle (f); longitudinally on the left ventricle (g). All multisite modulations used 25 mW mm−2 intensity and 1-ms pulse duration. To improve the image clarity and remove sensitive content, an artificial white background was incorporated into images e–g.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Table 1, Supplementary Figs. 1–53 and legends for Supplementary Videos 1–14
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, P., Zhang, J., Hayashi, H. et al. Monolithic silicon for high spatiotemporal translational photostimulation. Nature 626, 990–998 (2024). https://doi.org/10.1038/s41586-024-07016-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07016-9
This article is cited by
-
Light can restore a heart’s rhythm
Nature (2024)
-
Photoelectrochemical stimulation of the heart
Nature Reviews Bioengineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.