Abstract
Bacteria in the gastrointestinal tract produce amino acid bile acid amidates that can affect host-mediated metabolic processes1,2,3,4,5,6; however, the bacterial gene(s) responsible for their production remain unknown. Herein, we report that bile salt hydrolase (BSH) possesses dual functions in bile acid metabolism. Specifically, we identified a previously unknown role for BSH as an amine N-acyltransferase that conjugates amines to bile acids, thus forming bacterial bile acid amidates (BBAAs). To characterize this amine N-acyltransferase BSH activity, we used pharmacological inhibition of BSH, heterologous expression of bsh and mutants in Escherichia coli and bsh knockout and complementation in Bacteroides fragilis to demonstrate that BSH generates BBAAs. We further show in a human infant cohort that BBAA production is positively correlated with the colonization of bsh-expressing bacteria. Lastly, we report that in cell culture models, BBAAs activate host ligand-activated transcription factors including the pregnane X receptor and the aryl hydrocarbon receptor. These findings enhance our understanding of how gut bacteria, through the promiscuous actions of BSH, have a significant role in regulating the bile acid metabolic network.
Similar content being viewed by others
Main
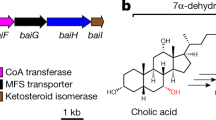
Bile acids are host–microbiota cometabolites that facilitate dietary lipid absorption and regulate lipid, glucose and xenobiotic metabolism7,8,9. In humans and rodents, the primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA) are synthesized in the liver from cholesterol. Most of the CA and CDCA is then conjugated with glycine or taurine in a two-step reaction catalysed by bile acid–coenzyme A (CoA) synthetase and bile acid–CoA:amino acid N-acyltransferase10 (Fig. 1a). Conjugated primary bile acids, predominantly glycocholic acid (GCA) and taurocholic acid (TCA), are stored in the gallbladder until the consumption of a meal triggers their secretion into the duodenum. Bile salt hydrolase (BSH), an enzyme commonly found in bacteria inhabiting the small intestine and colon, hydrolyses the amide linkage of conjugated bile acids11 (Fig. 1b). Posthydrolysis, bacteria further metabolize the bile acid backbone to generate secondary bile acids such as deoxycholic acid (DCA) and lithocholic acid, which are either excreted in the faeces or reabsorbed, conjugated and recycled by the host5,12. BSH activity has been associated with colonization resistance to Clostridium difficile13, weight gain14 and regulation of circadian rhythm15. Furthermore, the addition of a recombinant BSH cocktail changes the pathogenesis of C. difficile16, which was previously attributed to BSH deconjugation activity.
a, Bile acid–CoA:amino acid N-acyltransferase activity with the substrate depicted as a general bile acid structure modified with CoA. b, Deconjugation reaction by bacterial BSH of host-derived GCA or TCA. c, The BSH amino acid N-acyltransferase activity characterized in this study, with the conjugated bile acids GCA and TCA as examples. This activity leads to the biosynthesis of BBAAs. a–c, R1–R3 represent potential sites of backbone hydroxylation; RG/T represents glycine and taurine. d, Correlation between presence or absence of the bsh gene with the ability of various bacterial taxa to produce BBAAs. e, RT-qPCR of B. longum NCTC 11818 supplemented with 200 μM each of CA and DCA. Expression of bsh was compared to vehicle using the 2−∆∆Ct method normalized to the reference gene ldhl, and significance was determined using a two-tailed t-test (P = 0.0026**). Bar plots designate the mean ± s.d., n = 5.
Recent studies demonstrated that bacteria, in addition to the host, have bile acid amine N-acyltransferase activity, with dozens of bacterially conjugated amino acid amidates identified1,2,3,4,6,17. The enzymes responsible for these conjugation reactions are unknown. Furthermore, bacterial bile acid amidates (BBAAs) are abundant in the gastrointestinal tract18 and have been associated with human inflammatory bowel disease3. Although bacterial reconjugation of bile acids shapes the bile acid pool, how this process affects host–microbiota communication is not understood. Some BBAAs activate host bile acid nuclear receptors like the farnesoid X receptor (FXR) and pregnane X receptor (PXR)1,3, but an assessment of other receptors has not been completed. We demonstrate herein that bacterial BSHs re-amidate bile acids to produce BBAAs in addition to their known role in hydrolysing conjugated bile acids (Fig. 1c). We found that BBAAs are produced in early life during expansion of the BSH-expressing gut microbiota. In addition, we report that in cell culture models, unique collections of BBAAs activate host ligand-activated receptors to potentially mediate host–microbiota communication.
BSH correlates with BBAA production
Diverse bacteria from the human gastrointestinal tract produce BBAAs2,3. We therefore set out to discover the gene(s) responsible for the production of BBAAs. It was recently demonstrated that gut bacteria can amidate CA with phenylalanine, tyrosine and leucine1. Additionally, in a screen of 202 human-associated bacterial isolates, production of BBAAs from a mixture of CA and DCA was reported3. Another study screened 72 bacterial isolates and found that 25 strains were able to conjugate DCA, CDCA or CA to produce at least one BBAA2. We compared the genomes of the isolates involved in these two studies with their production of BBAAs to identify possible candidate gene(s) responsible for BBAA production. We found that the bile salt hydrolase (bsh) gene was significantly associated (P = 9.4 × 10−6, phylogenetic linear regression) with BBAA production (Fig. 1d and Extended Data Fig. 1). The association is exemplified in the genera Bifidobacterium and Enterococcus, which are efficient producers of BBAAs and also widely harbour the bsh gene, whereas Fusobacterium spp., which lack the bsh gene, do not make BBAAs. Overall, with few exceptions, bacterial isolates that produce at least one BBAA have a bsh homologue.
To further explore the involvement of bsh and other genes in bile acid conjugation, we performed RNA sequencing on the BBAA-producing strain Bifidobacterium longum, National Collection of Type Cultures (NCTC) 11818, treated with 100 μM unconjugated bile acids (CA and DCA, equimolar). Global transcriptional profiling identified 1,929 genes, of which 89 were differentially expressed (adjusted P ≤ 0.05 and log2FC ≤ −1.5 or ≥ 1.5), including bsh, which was significantly upregulated (log2FC = 2.14, adjusted P = 6.52 × 10−85) (Extended Data Fig. 2 and Supplementary Table 1). We also validated the RNA sequencing results with quantitative reverse transcription polymerase chain reaction (RT-qPCR), confirming that bsh messenger RNA was increased 2.7-fold (P = 0.0026) (Fig. 1e). Previously, bsh expression was reported to be upregulated in response to a mixture of conjugated and unconjugated bile acids, but the response was attributed solely to the presence of conjugated bile acids19,20. Therefore, we sought next to examine the possibility that BSH has amine N-acyltransferase activity with unconjugated bile acids as the substrate.
BSH is a bacterial N-acyltransferase
We hypothesized that pharmacologic inhibition of the BSH enzyme would eliminate the production of BBAAs from unconjugated bile acids. The pan-BSH inhibitor gut restricted-7 (GR-7) (ref. 21) was used to inhibit BSH of B. longum NCTC 11818 treated with equimolar 100 μM CA and DCA. We monitored the production of BBAAs from 0 to 24 h with and without GR-7. Addition of 100 μM GR-7 resulted in a significant decrease in the production of BBAAs, particularly phenylalanine- and alanine-conjugates, as compared to vehicle-treated controls (Fig. 2a). The attenuated BBAA synthesis was not due to bacterial growth inhibition because colony-forming units (CFUs) were equal with or without GR-7 (Extended Data Fig. 3).
a, The pan-BSH inhibitor GR-7 attenuates conjugated bile acid production in B. longum NCTC 11818 cultures. Mean BBAA concentrations, quantified by targeted liquid chromatography with tandem mass spectrometry (LC–MS/MS), are shown with vertical lines indicating s.d. (n = 3 biologically independent bacterial cultures per treatment group). b, Purified BlBSH enzyme synthesizes BBAAs from 100 µM CA or TCA in vitro. c, E. coli transformed with a BlBSH expression vector produces BBAAs from 1 mM CA or TCA. d, BSH knockout in B. fragilis ablates the aminotransferase activity observed in wild-type (WT) B. fragilis NCTC 9343 with 1 mM CA or 1 mM TCA supplementation. Bars in b–d represent individual biological replicates (n = 4), with heights indicating BBAA concentrations. e, E. coli BL21(DE3) expressing mutated BlBSH at key active site residues exhibited altered deconjugation and conjugation activities. Deconjugation, indicated by the CA:TCA ratio, was assessed in M9 media with 1 mM TCA. Conjugation, measured through Ala-CA production, was evaluated in Luria–Bertani media with 1 mM TCA using LC–MS/MS. Controls included untransformed E. coli (Unt) and E. coli expressing unmutated BlBSH (WT). Bars represent mean values ± s.d. (n = 3 biologically independent bacterial cultures). f–i, Bile acid profile of germ-free C57BL/6 J mice monocolonized with B. fragilis WT or Δbsh strains (n = 7 for WT, n = 8 for Δbsh groups). CFUs per gram of B. fragilis in faeces after 7 days (f). Ileal contents primary bile acids quantified by LC–MS/MS (CA P = 2.3 × 10−4***, βMCA P = 1.3 × 10−4***, αMCA P = 3.7 × 10−4***, CDCA P = 1.1 × 10−4, UDCA P = 2.7 × 10−5****) (g). Free taurine levels in caecal contents quantified using 1H nuclear magnetic resonance (NMR) (P = 4.27 × 10−4***) (h). Sum of peak areas for all BBAAs in ileal contents measured by LC–MS/MS (P = 3.1 × 10−4***) (i). f–i, box plots depict first quartile and third quartile with median as centre and min–max values for whiskers, except for outliers calculated as data points ±1.5× interquartile range. Significant differences (P < 0.001***, P < 0.0001****) were determined by two-tailed t-tests. MCA, muricholic acid; UDCA, ursodeoxycholic acid.
Given that Bifidobacterium strains are difficult to manipulate genetically22, we investigated the necessity of BSH using Bacteroides fragilis NCTC 9343, which has been extensively studied for its role in secondary bile acid metabolism and is more compatible with genetic manipulation. We generated a complete bsh deletion strain (Δbsh) and another strain that complemented the bsh knockout in trans (Δbsh bsh+). We then treated the bacterial strains with 1 mM CA or 1 mM TCA for 16 h to assess whether differences could be observed in BBAA production by BSH from unconjugated or conjugated bile acid substrates. Deletion of bsh eliminated the production of BBAAs from both CA and TCA, whereas complementation rescued BBAA production (Fig. 2d). These observations support our hypothesis that BSH has amine N-acyltransferase activity.
To test whether BSH is sufficient for the production of BBAAs, we cloned the bsh gene from B. longum NCTC 11818 into the pET-28b(+) vector (BlBSH) and overexpressed in Escherichia coli BL21(DE3). Heterologous expression of the enzyme in E. coli, which lacks endogenous bsh, confers amine N-acyltransferase activity. Incubation of the E. coli-BlBSH with 1 mM CA or 1 mM TCA produced BBAAs, whereas the untransformed E. coli did not produce BBAAs (Fig. 2c).
Next, we purified BlBSH and assayed for BBAA production from 100 μM CA or TCA and a 250 μM equimolar mixture of amino acids. Both CA and TCA treatment yielded BBAAs, but not all amino acids were conjugated, demonstrating substrate selectivity by BSH. The large proportion of alanine and serine conjugates produced by B. fragilis (Fig. 2d), BlBSH-expressing E. coli (Fig. 2c) and purified BlBSH (Fig. 2b) indicate that the enzyme prefers amino acids with smaller side chains. However, the greater diversity of BBAAs produced by purified BlBSH in vitro (Fig. 2b) compared to BlBSH-expressing E. coli (Fig. 2c) is probably a result of the different amino acid profiles in E. coli (alanine is present at about a 100-fold greater concentration than serine)23.
Although we observed BSH amine N-acyltransferase activity with both CA and TCA, the conjugation mechanism is unclear. On the basis of the known mechanistic details of BSH’s hydrolytic activities and it belonging to the N-terminal nucleophile superfamily of enzymes, the terminal cysteine Cys1 of BlBSH is predicted to be the primary catalytic residue, although other active site residues (for example, Thr2, Trp21, Thr171, Asn172 and Arg225) may also be important for BSH activity or substrate specificity24. We performed site-directed mutagenesis on nine residues proximal to the active site of BlBSH to identify which are necessary for N-acyltransferase activity in BSH (Fig. 2e). The N-acyltransferase activity of BSH closely mirrored the deconjugation activity with each mutation, with C1A, R17A, N172A and R225A all being essential for its function. None of the mutations inhibited expression of the BlBSH protein (Extended Data Fig. 4).
To support the relevance of bacterial BSH in producing BBAAs in vivo, we colonized germ-free mice with the wild-type or Δbsh strain of B. fragilis described above. Both strains equally colonized the mouse gastrointestinal tract seven days after gavage (Fig. 2f), and there was no change in body weight between the groups (Extended Data Fig. 5). The bile acid pool of Δbsh-colonized mice reflected a perturbation in bile acid hydrolysis, as the release of unconjugated primary bile acids was reduced and the production of BBAAs attenuated (Fig. 2g,i). Similarly, free taurine was also significantly higher in caecal contents of wild-type-colonized mice compared to Δbsh-colonized mice (Fig. 2h). This observation is consistent with hydrolysis of taurine-conjugated bile acids and the formation of BBAAs. Importantly, we demonstrate that colonization with wild-type B. fragilis is key to the production of BBAAs, as BBAA levels are significantly depleted in Δbsh-colonized mice (Fig. 2i). Therefore, in the mouse gastrointestinal tract, colonization with BSH-positive bacteria is required for bile acid hydrolysis and re-amidation.
BBAA levels coincide with microbiota development
During the first year of life, the gut microbiota undergoes rapid expansion and maturation. To understand the biological relevance of BBAAs, we quantified bile acids including BBAAs from the stool of infants followed from birth to 12 months25. Metagenomic sequencing showed an increase in microbial diversity, measured by the Shannon Index, from birth through 12 months (Fig. 3a). Variation in sample diversity between 0 and 4 days is probably due to the rapid maturation of the gut microbiota. Principal coordinates analysis of Bray–Curtis dissimilarity supports the differences in beta-diversity between one-month- and 12-month-old infants (Fig. 3b, permutational multivariate analysis of variance (PERMANOVA) P = 0.001). The Bacteroides population expanded from one to 12 months, whereas the relative abundance of Bifidobacterium was reduced (Fig. 3c). As the microbiota evolved with age, we observed a shift from host-conjugated to unconjugated primary bile acids due to the colonization of bsh-positive bacteria (Fig. 3d). The levels of BBAAs also increased from 0–4 days to 12 months after birth (Fig. 3d). Further stratifying the samples collected at 0–4 days, the levels of several BBAAs (Ala-CA, Glu-CDCA, Tyr-CA, His-CA, Ile/leu-CA, Trp-CA, Ser-CA and Tyr-CDCA) were positively correlated with the hours elapsed after birth (Extended Data Fig. 6). We correlated the abundance of Bacteroides, Bifidobacterium and Enterococcus with levels of host-conjugated (Gly and Tau) bile acids and BBAAs. Bacteroides abundance was not correlated with BBAA production. In 12-month-old infants, Enterococcus abundance was positively correlated with host-conjugated bile acids and BBAAs including Ala, Ile/Leu, Phe, Ser and Tyr (Fig. 3e). Bifidobacterium abundance was negatively correlated with host-conjugated bile acids in one-month-old infants and positively correlated with bile acid conjugates of His and Thr (Fig. 3e). These data support our findings that BSHs from Bifidobacterium species are amine N-acyltransferases that produce BBAAs in humans. To our knowledge, this is the first demonstration connecting BBAA production with BSH-expressing bacteria during human infant development.
a, Microbiota diversity measured by Shannon diversity index of infant faecal samples acquired at 0–4 days (0–4D), one month (1M) and 12 months (12M) after birth, n = 108. Significant differences between time points were determined by linear mixed effects model (0–4D versus 12M P = 4.4 × 10−7***, 1M versus 12M P = 2 × 10−5***). b, Principal coordinate analysis plot of the Bray–Curtis dissimilarity of infants 1M and 12M old. Significant differences were determined by permutational multivariate analysis of variance (PERMANOVA) test on Bray–Curtis distances (P = 0.001***). c, Barplot depicting faecal microbiota composition of 1M and 12M infants, including all genera greater than 5% mean relative abundance in either time point. d, Faecal concentrations of total unconjugated, host glycine and taurine-conjugated and BBAAs from 0–4D to 12M, quantified using LC–MS/MS. Significant differences between time points were determined by linear mixed effects models, with log(bile acids) as outcomes, time as predictor and subject information as random effect (P < 0.001***). Several comparisons were adjusted using the Benjamini–Hochberg method. e, Associations of BBAAs and glycine-/taurine-conjugated BAs with relative Enterococcus, Bifidobacterium and Bacteroides abundances using Spearman correlation (P < 0.05*, P < 0.01**, P < 0.001***). NS, not significant.
BBAAs activate host transcription factors
To demonstrate potential biological relevance of BSH-mediated BBAA production, we assessed in cell culture models the activation of VDR, CAR3, PXR and AHR in a cell-based reporter assay (Extended Data Fig. 7). Most of the BBAA activators of the human bile acid receptor FXR were conjugates of CDCA (5 of 8, P < 0.0001), probably reflecting the potency of CDCA as an FXR agonist26. Bile acids conjugated with glutamate activated human FXR, CAR3, PXR, AHR and PPARα (P < 0.0001). Therefore, we selected Glu-CA and Glu-CDCA for further exploration in organoid models. We treated organoids cultured from mouse and human small intestinal crypts with 50 µM or 100 µM Glu-CA or Glu-CDCA and measured the expression of FXR, CAR, PXR and AHR target genes. In mouse organoids, Glu-CA and Glu-CDCA upregulated Cyp3a11, Cyp1a1 and Bsep expression, indicating that PXR and AHR were transcriptionally activated (Extended Data Figs. 8a and 9a). Responses in human organoids were less pronounced, but CYP1A1 expression was also upregulated with 100 µM Glu-CDCA treatment (P < 0.01, Extended Data Figs. 8b and 9b). We previously showed that Glu-conjugated bile acids were activators of human PXR in vitro14, and herein, we observed activation of AHR by both bacterially conjugated and conventional bile acids in vitro. Further studies, both in vitro and in vivo, are required to demonstrate whether BBAAs affect the metabolism of substrates, drugs and carcinogens regulated by PXR26 and AHR27.
Conclusion
We identified a previously undescribed role for BSH as an amine N-acyltransferase that produces BBAAs in mice and humans. These BBAAs may facilitate communication between the microbiota and host through the activation of the human ligand-activated transcription factors. As there are hundreds of different BSHs found in microbiota that synthesize different BBAAs3, BSHs may be instrumental in fine tuning different pathways that promote health or disease. Historically, BSH knockout or inhibition has been associated with modulation of inflammation, cancer and liver disease without knowledge of BSH amine N-acyltransferase activity. Future studies must re-evaluate the contribution of BBAA production on disease outcomes related to BSH activity.
Methods
Correlation of BSH and ability to produce BBAAs
To check for the association of BSH and BBAA production, we leveraged liquid chromatography with tandem mass spectrometry (LC–MS/MS) data from two published datasets involving 202 Human Microbiome Project (HMP) bacterial isolates in ref. 3 and 69 representative gut bacteria members in ref. 2. We combined the two datasets. For comparison between bacteria from the studies, we normalized the dataset on the basis of the proportion of BBAAs detected from the total number of BBAAs tested in each study. Next, we downloaded the assembled genomes of the bacteria from the National Center for Biotechnology Information (NCBI) genome database. A phylogenetic tree was obtained from the NCBI taxonomy database using taxids in the study and visualized using the ggtree28 package in R. We annotated the genomes using Prokka29 and checked for the presence or absence of the bsh gene. Briefly, Prokka uses several databases (Uniprot, RefSeq and Pfam) in a hierarchical manner to annotate coding sequences in the genome. Genomes with coding sequences annotated as choloylglycine hydrolase (cbh) or having its domain were deemed as having the bsh gene or its homologue, whereas genomes lacking these annotations were deemed lacking the gene. To test for correlation, we used the phylolm30 package in R to fit a following phylogenetic linear regression model:
where BBAAs is the vector of proportions of the BBAAs detected for bacteria in each study and bsh is the vector of presence or absence of the bsh gene.
RNA sequencing sample preparation and analysis
B. longum subsp. longum NCTC 11818 was acquired from ATCC, and 30 µl of an overnight culture of B. longum was inoculated into 3 ml of brain–heart infusion (BHI) broth (BD Difco) supplemented with 0.05% w/v cysteine, 5 µg ml−1 hemin and 1 µg ml−1 vitamin K (Sigma). Equivalent volumes of 1 M NaOH (control) or CA and DCA in 1 M NaOH at a final concentration of 200 µM were added. Cultures were grown to mid-exponential phase (approximately 6 h) at 37 °C in an anaerobic chamber filled with 20% CO2 and 5% H2 in N2 gas. Then 4 ml of RNAprotect (Qiagen) was added to 2 ml culture and incubated for 5 min at room temperature. Samples were centrifuged at 5,000g for 5 min at 4 °C, and the bacterial pellets were flash-frozen in liquid nitrogen and stored at −80 °C. Pellets were resuspended in 1 ml TRIzol Reagent and transferred to Lysing Matrix E tubes for homogenization at 2 × 20 s cycles at 6,500g. Next, 200 µl chloroform was added, vortexed for 15 s and incubated at room temperature for 10 min. Samples were centrifuged for 15 min at 16,000g, 4 °C, and 500 µl upper aqueous phase was transferred to a fresh tube containing 500 µl of 100% ethanol. Samples were then transferred to a Purelink spin column, centrifuging for 15 s at 12,000g to elute flowthrough. Columns were washed with 350 µl Wash Buffer I and removed by centrifugation for 15 s at 12,000g. Contaminating DNA was degraded by adding 80 µl of deoxyribonuclease (DNase) solution (10 µl DNase in 1× reaction buffer) and incubating at room temperature for 15 min. A second wash with Wash Buffer I was then performed. Columns were washed twice with 500 µl Wash Buffer II, eluting each time by centrifugation for 15 s at 12,000g. Purified RNA was eluted with 30 µl RNase-free water by centrifugation for 1 min at 12,000g into a fresh tube.
RNA samples were submitted to the Microbial Genome Sequencing Center for sequencing. Samples were DNase treated with Invitrogen DNase (RNAse-free). We prepared the library using Illumina’s Stranded Total RNA Prep Ligation with Ribo-Zero Plus Kit and 10 bp IDT for Illumina indices. Sequencing was done on a NextSeq2000 to yield 2 × 50 bp reads. Demultiplexing, quality control and adaptor trimming were performed with bcl-convert (v.3.9.3).
For the analysis, the complete reference genome and annotation of B. longum NCTC 11818 were obtained from the NCBI Assembly database. Obtained demultiplexed reads were checked for quality and filtered using fastp (v.0.12.4) (ref. 31). Filtered reads were aligned with the reference genome using bowtie2 (v.2.2.5) (ref. 32). Obtained alignments were sorted using samtools (v.1.10) (ref. 33). Qualimap (v.2.2.2) (ref. 34) was used to check for the quality of alignments. featureCounts (v.2.0.1) (ref. 35) was used to generate counts of reads uniquely mapped to annotated genes. These raw counts were then normalized and analysed for differential gene expression using the DESeq2 package (v.1.35.0) (ref. 36) in RStudio.
qScript cDNA SuperMix (Quantabio) was used for the reverse transcription of 250 ng RNA template in a 20 µl reaction for RT-qPCR. Primer sequences were designed using PrimerBlast for B. longum bsh and lactate dehydrogenase (ldhl) (Supplementary Table 2). We performed the RT-qPCR with 1 µl complementary DNA (cDNA) template and 0.4 µM of each primer on a QuantStudio 3 using fast cycling parameters with PowerUp SYBR detection reagent.
B. longum BSH inhibition
B. longum subsp. longum NCTC 11818 was acquired from ATCC, and 30 µl of an overnight culture of B. longum was inoculated into 3 ml of BHI broth (BD Difco) supplemented with 0.05% w/v cysteine, 5 µg ml−1 hemin and 1 µg ml−1 vitamin K (Sigma). Next, 2 µl of the overnight culture was added to 196 µl fresh supplemented-BHI media, and 1 µl of 1 M NaOH (vehicle) or 20 mM CA and DCA in 1 M NaOH at a final concentration of 100 µM were added to the plate. To inhibit BSH, 1 µl of 20 mM GR-7 in dimethyl sulfoxide (DMSO) was added to the treatment wells to a final concentration of 100 µM. Equivalent volume of DMSO was added to the control wells. To monitor the production of BBAAs, samples were taken out at 4, 6, 8, 10, 14, 16 and 24 h after incubation and flash-frozen in liquid nitrogen until extraction for LC–MS/MS quantification of bile acids.
Heterologous expression and purification of B. longum BSH in E. coli
The gene for B. longum BSH (NC_015067.1, codon-optimized) cloned into pET-28b(+) using NdeI and XhoI restriction sites was synthesized by GenScript. The resulting plasmid was transformed into E. coli Tuner (DE3) cells (Novagen) by means of electroporation at 2,500 V, and positive transformants were selected on Luria–Bertani media containing 50 µg ml−1 kanamycin. Expression strains were grown in yeast extract media (45 g yeast extract (Research Products Int.), 1.6 g KH2PO4 (Research Products Int.), 13.0 g K2HPO4 (Research Products Int.) and 1% glycerol (v/v) (Sigma-Aldrich) per 1 l) at 37 °C to late exponential phase (optical density at 600 nm (OD600) = 0.8). The cells were cooled to 18 °C before induction with 0.1 mM isopropyl β-d-1-thiogalactopyranoside (Research Products Int.) and allowed to express BSH for 18–20 h before being collected by centrifugation (4,000g at 4 °C, 20 min). The resulting cell pellets were frozen at −80 °C until use.
The cells were resuspended in buffer A (50 mM Tris (Research Products Int.), 300 mM NaCl (Research Products Int.), 20 mM imidazole (Sigma-Aldrich), pH 7.4) with protease inhibitors benzamidine HCl (Research Products Int.) and Pefabloc SC (Sigma-Aldrich) and disrupted by means of sonication (QSonica Sonicator Q500) for 6 min on ice (30 s pulse, 30 s pause, at 60% amplitude). The resulting lysate was centrifuged at 130,000g in a Beckman Optima L-90X ultracentrifuge at 4 °C for 1 h.
The following purification steps were performed at 4 °C. Supernatant was applied to a HisPur Ni-column (Fisher Scientific) pre-equilibrated with buffer A. The column was washed with 20 column volumes of buffer, and then the BSH protein was eluted with 5 column volumes of buffer B (50 mM Tris, 300 mM NaCl, 250 mM imidazole, pH 7.4). The eluent was dialysed into the final storage buffer (50 mM Tris, 50 mM NaCl, 5% glycerol (v/v), pH 7.0) overnight at 4 °C. The resulting protein solutions were concentrated using Amicon Ultra 15 ml centrifugal filters (Millipore) with a molecular weight cutoff of 10 kDa, flash-frozen in liquid nitrogen and stored at −80 °C.
In vitro purified enzyme assay
Purified BlBSH enzyme was diluted to 10 mg ml−1 in phosphate buffer (pH 7.0). An equimolar mixture of 21 amino acids was prepared in H2O (5 mM each of l-alanine, l-arginine, l-asparagine, l-aspartic acid, l-cysteine, l-glutamine, l-glutamic acid, glycine, l-histidine, l-isoleucine, l-leucine, l-lysine, l-methionine, l-phenylalanine, l-proline, l-serine, l-threonine, l-tryptophan, l-tyrosine, l-taurine and l-valine). Each reaction mixture contained 178 µl phosphate buffer (pH 6.0), 10 µl of 5 mM amino acids mixture, 2 µl of 100 mM bile acid (CA or TCA in DMSO) or vehicle (DMSO) and 10 µl of the 10 mg ml−1 enzyme. The reaction mixture was incubated for 90 min at 37 °C. The reaction was stopped by adding 800 µl ice-cold 100% methanol containing 0.5 µM deuterated bile acid internal standards.
Construction of B. fragilis NCTC 9343 Δbsh mutant
A 1,797 bp DNA fragment containing the N-terminal region of the BSH gene was PCR amplified from the B. fragilis NCTC 9343 using primers BSH-NT-FOR and BSH-NT-REV. The N-terminal fragment was cloned into the SphI/BamHI sites of the pFD516 vector. Then a 1,734 bp DNA fragment containing the C-terminal region was PCR amplified with primers BSH-CT-FOR and BSH-CT-REV2 and cloned into the SacI site of pFD516. The new construct, pER363, contains a deletion of 765 bp DNA fragment from the bsh gene. A 3,533 bp BglII/SalI DNA fragment from pER363 containing the Δbsh construct was cloned into the BamHI/SalI sites of the suicide vector for allelic replacement in Bacteroides, pLGB13 to construct pER-366. pER-366 was mobilized from E. coli S17-1 λpir into B. fragilis NCTC 9343 by biparental mating as described previously. Transconjugants were selected on brain–heart infusion supplemented (BHIS) plus 0.5% yeast extract plates containing 200 μg ml−1 gentamycin and 10 μg ml−1 erythromycin. Four transconjugant colonies were grown overnight on BHIS broth with 200 μg ml−1 gentamycin (erythromycin was omitted). Then 10 μl of an overnight culture was spread on BHIS with 0.5% yeast extract plates containing 200 μg ml−1 gentamycin and 100 ng ml−1 anhydrotetracycline. Transconjugant colonies were streaked on fresh BHIS with 0.5% yeast extract plates containing 200 μg ml−1 gentamycin and 100 ng ml−1 anhydrotetracycline. PCR amplification using BSH-mutcheck-FOR and BSH-mutcheck-REV primers was used to identify chromosomal double-crossed over genetic recombination. The B. fragilis NCTC 9343 Δbsh mutant isolates (BER-279) were also tested for loss of erythromycin resistance and respiratory test.
Genetic complementation of Δbsh mutant
pER-300 containing bsh operon in the pNBU2-bla-ermGb vector was mobilized into BER-279 by biparental mating. Transconjugants were selected on BHIS plus 0.5% yeast extract plates containing 200 μg ml−1 gentamycin and 10 μg ml−1 erythromycin (BER-280).
Site-directed mutagenesis of BlBSH active site amino acid residues
The pET-28b(+)-BlBSH vector constructed above was mutated using the Q5 Site-Directed Mutagenesis Kit according to the manufacturer’s instructions (New England Biolabs). The NEBaseChanger tool was used to design primers (Supplementary Table 2) and calculate the appropriate annealing temperatures for PCR. Following PCR mutagenesis and treatment with kinase, ligase and DpnI, the products were transformed into NEB 5-alpha-competent E. coli by heat shock at 42 °C, and positive transformants were selected on Luria–Bertani media containing 50 µg ml−1 kanamycin. Individual colonies were selected, and successful mutations were confirmed by QIAprep Spin Miniprep kit (Qiagen) preparation of plasmid DNA followed by Sanger sequencing from the T7 promoter region. Positively identified plasmid DNA was stored at −20 °C. To express the proteins, mutant pET-28B(+)-BlBSH plasmids were transformed into E. coli BL21(DE3) cells by electroporation at 2,500 V, and positive transformants were selected on Luria–Bertani media containing 50 µg ml−1 kanamycin. To measure TCA deconjugation, transformed E. coli were cultured in M9 media supplemented with 1 mM TCA. To measure CA conjugation, transformants were cultured in Luria–Bertani media supplemented with 1 mM CA. All transformants were grown at 37 °C and 250 rpm until OD600 ≈ 0.6 was reached; then protein was induced by the addition of 0.1 M isopropyl β-d-1-thiogalactopyranoside and grown for a further 4 h. E. coli were extracted for LC–MS/MS according to the protocol below.
Extraction and LC–MS/MS quantification of bile acids from bacterial cultures
For bacterial cultures, 200 µl of the culture following growth was lysed by three freeze–thaw cycles in liquid nitrogen. Next, 150 µl of each lysate was transferred to a 1 ml capacity 96-well plate, and 600 µl of ice-cold 100% high-performance liquid chromatography (HPLC)-grade methanol containing 0.5 µM deuterated bile acid internal standards was added and mixed thoroughly by pipetting. The plate was then incubated on ice for 20 min. Samples were sonicated for 10 min in a sonicating water bath and then centrifuged for 15 min at 2,000 rpm at 4 °C. Then 200 µl of each supernatant was transferred to a fresh 1.5 ml microcentrifuge tube and dried using a speedvac. Dried extracts were resuspended in 200 µl HPLC-grade methanol and sonicated for 10 min before transfer to autosampler vials for LC–MS/MS.
Quantitative LC–MS/MS analyses were performed using a Waters ACQUITY Ultra-performance liquid chromatography (UPLC) system coupled with a Waters Xevo TQ-S Triple Quadrupole mass spectrometer (Waters). Chromatographic separation was achieved using an ACQUITY BEH C8 (2.1 × 100 mm, 1.7 µm) UPLC column (Waters) heated to 60 °C. Samples (1 µl) were injected into the column and eluted with 90% mobile phase A (1 mM ammonium acetate in 9% acetonitrile, pH 4.15) and 10% mobile phase B (1:1 acetonitrile:isopropanol) for 0.1 min. Mobile phase B was increased in consecutive linear gradients from 10% to 35% over 9.15 min, 35% to 85% B over 2.25 min and 85% to 100% B over 0.3 min. Then 100% B was held for 0.6 min followed by a linear gradient to 10% B over 0.1 min and held for 2.5 min, for a total of 15 min. The flow rate was modified throughout the run starting with 300 µl per min for 9.25 min and then increasing to 325 µl per min over 2.25 min and 500 µl per min over 0.6 min. The flow rate was held at 500 µl per min for 0.3 min and then reduced to 300 µl per min over 0.4 min and held for 2.2 min. MS analyses were carried out using electrospray ionization in positive ion mode using the following settings: capillary voltage 3.5 kV, cone voltage 20 V, source temperature 150 °C, desolvation temperature 300 °C, desolvation gas flow 540 l h−1 and cone gas flow 150 l h−1. BBAAs were identified by multiple reaction monitoring transitions from parent [M + H]+ ions to daughter [M + H]+ ions corresponding to the amino acid fragment (Supplementary Table 3). Peak identification and processing was done using MassLynx software (Waters). Peak areas for each conjugated bile acid were normalized to peak areas from deuterated internal standards of GCA-d4 or GDCA-d4, matching the bile acid. Quantification was performed against a standard curve of authentic standards at eight concentrations ranging from 7.81 nM to 1 µM.
Monocolonization of GF mice
Fifteen male 8- to 10-week-old C57BL/6 J mice were maintained under germ-free conditions in positive-pressure isolators (Class Biologically Clean). Gnotobiotic experiments were conducted under the Pennsylvania State University Institutional Animal Care and Use Committee approved protocol 202101826. Mice were singly housed with ad libitum access to water and food. Sterility of mice was ensured by qPCR and aerobic/anaerobic culture with rich media. Mice were administered 108 CFUs of either B. fragilis NCTC 9343 or the ∆bsh mutant, with mice monitored daily. No adverse effects on mouse weight or behaviour were observed. After 7 days of colonization, mice were euthanized and ileal and caecal tissue and contents collected. To quantify B. fragilis colonization, faecal samples were resuspended in phosphate-buffered saline (PBS) and serially diluted before quantification on BHI plates using the drop-plate method.
Extraction and untargeted LC–MS/MS profiling of monocolonized mice
Bile acids were extracted from mouse ileal contents according to ref. 37. Briefly, 25 mg of ileal contents were weighed in 1.5 ml screw-cap homogenization tubes, and approximately 50 µl of 0.1 mm zirconia beads were added. Next, 1 ml of ice-cold methanol containing 0.5 µM deuterated internal bile acid standards was added. Samples were then thoroughly homogenized in Precellys 24 tissue homogenizer at 20 s × 2 at 6,500 rpm. Homogenized samples were then freeze–thawed three times in liquid nitrogen and centrifuged at 15,000 rpm at 4 °C. Then 200 µl of the supernatant was transferred to autosampler vials for LC–MS/MS analysis.
We performed LC–MS/MS analyses using a Thermo Fisher Scientific Orbitrap Exploris 120 Mass Spectrometer system coupled with a Vanquish ultra-high-performance liquid chromatography system (Thermo Fisher Scientific). Chromatographic separation was carried out using an ACQUITY BEH C18 UPLC column (2.1 × 100 mm, 1.7 µm; Waters). The column was maintained at a temperature of 60 °C. Mobile phase solvent A was a mixture of 9% acetonitrile and 1 mM ammonium acetate with a pH of 4.15, and solvent B was a 1:1 ratio of acetonitrile and isopropanol. Samples (5 µl) were injected into the column, and the initial mobile phase was 10% B, kept for 0.1 min. Mobile phase B increased in consecutive linear gradients to 35% at 9.25 min and to 85% B at 11.5 min and was held at 85% for 3.4 min. It then decreased to 10% B at 15.1 min and was held at 10% B for 2.9 min. The flow rate of the mobile phase was 0.3 ml min−1. Heated electrospray ionization was used with the following settings: spray voltages of 3.5 kV for positive charge and 2.5 kV for negative charge, sheath gas at 50 Arb, auxiliary gas at 10 Arb, sweep gas at 1 Arb, ion transfer tube temperature 325 °C and vaporize temperature 350 °C. MS settings included an Orbitrap resolution of 120,000, a scan range (m/z) from 150 to 1,200 and an RF lens of 60%. MS2 spectra were data-dependently acquired using higher-energy collisional dissociation with normalized collision energies of 20%, 35% and 50% and a resolution of 60,000. Data were analysed using MS-DIAL (v.4.9.221218).
Amino acid quantification of caecal content by 1H NMR
Roughly 50 mg of caecal content was weighed in a 2.0 ml screw-cap microcentrifuge tube and mixed with 1.2 ml of 0.1 M phosphate buffer (K2HPO4:NaH2PO4 = 4:1, pH = 7.4) containing 50% D2O and 0.005% (w/v) of sodium 3-(trimethylsilyl) propionate-2,2,3,3-d4 as internal standard. Samples were homogenized and then subjected to two freeze–thaw cycles in liquid nitrogen. After centrifuging at 17,000g for 10 min at 4 °C, 550 µl of clear supernatant was transferred to 5 mm NMR tubes for NMR analysis.
The one-dimensional 1H spectra of caecal content extracts were recorded at 298 K by using a Bruker Avance NEO 600 MHz NMR spectrometer (Bruker Biospin) equipped with a 5 mm TCI cryoprobe with enhanced sensitivity for 1H. The noesygppr1d pulse sequence was used, and spectral acquisition parameters were as follows: 64 scans were collected into 64,000 data points using 90° pulses, four dummy scans, 20 ppm spectral width. All spectra were analysed with Chenomx NMR Suite (v.9.02). After automatic processing, the phase, baseline and internal standard were checked and modified manually for each spectrum for quality assurance. The amino acids were identified and quantified using a built-in metabolite library.
Cell-based reporter assays
Reporter assay kits for human farnesoid X receptor (hFXR, cat. no. IB00601); vitamin D receptor (hVDR, IB00701); constitutive androstane receptor variant 3 (hCAR3, IB00901); pregnane X receptor (hPXR, IB07001); aryl hydrocarbon receptor (hAhR, IB06001); peroxisome proliferator-activated receptors alpha (hPPARα, IB0011), beta/delta (hPPARβ/δ, IB0012) and gamma (hPPARγ, IB0010); and mouse FXR (mFXR, M0060) and PPAR alpha (mPPARα, M0011), beta/delta (mPPARβ/δ, M0012) and gamma (mPPARγ, MR0010) were purchased from INDIGO Biosciences, Inc. Agonism assays were performed with test compounds at a single concentration (50 µM) with a concurrent dose-response of reference ligands following the manufacturer’s instructions. Briefly, each reporter assay cell suspension was thawed by the addition of cell recovery medium (CRM), and 100 µl of the cell suspension were used per well. Then 100 µl of compound screening medium supplemented with test compounds were added and incubated at 37 °C, 5% CO2 for 24 h. The next day, the medium was removed, detection reagents were added and luminescence was measured (Synergy H4 Hybrid Multi-Mode Microplate Reader). Data were expressed relative to vehicle-treated cells and log10-transformed for visualization. Differences between treatments were determined using analysis of variance (ANOVA) followed by Dunnett’s post hoc test (JMP Pro 15, SAS Institute). Significant differences were determined when P < 0.05. Non-linear regression and EC50 calculations were performed with Prism v.6.0 (GraphPad Software, Inc.).
Enteroid model
Normal (de-identified) human small intestinal samples were established and cultured in high-Wnt-containing medium (human colonoid medium) as previously described38. Enteroids were treated with 50 µM and 100 µM Glu-CA or Glu-CDCA for 18 h, the medium was removed, and enteroids were washed twice and collected in PBS. Matrigel and PBS were carefully removed, and enteroids were lysed and extracted using a PureLink RNA mini kit according to the manufacturer’s instructions. qScript cDNA SuperMix (Quantabio) was used for the reverse transcription of 500 ng RNA template in a 40 µl reaction for RT-qPCR. We performed the RT-qPCR with 1 µl cDNA template and 0.4 µM of each primer (Supplementary Table 2) on a QuantStudio 3 using fast cycling parameters with PowerUp SYBR detection reagent.
IGRAM cohort
African American women in their third trimester of pregnancy were enroled as part of a prospective, longitudinal, observational maternal–infant cohort study (Infant Growth and Microbiome (IGRAM))25. The study was approved by the Institutional Review Board at Children’s Hospital of Philadelphia. Stool samples from infants (56 male and 52 females) were collected at birth, 1 month and 12 months of life.
LC–MS/MS quantification of bile acids in IGRAM stool samples
A separate method was used for the quantification of bile acids in the IGRAM cohort. Bile acids were extracted from infant stool according to ref. 37. Briefly, 25 mg of stool was weighed in 1.5 ml screw-cap homogenization tubes, and approximately 50 µl of 0.1 mm zirconia beads were added. Next, 1 ml of ice-cold methanol containing 0.5 µM deuterated internal bile acid standards was added. Samples were then thoroughly homogenized in Precellys 24 tissue homogenizer at 20 s × 2 at 6,500 rpm. Homogenized samples were then freeze–thawed three times in liquid nitrogen and centrifuged at 15,000 rpm at 4 °C. Then 200 µl of the supernatant was transferred to autosampler vials for LC–MS/MS analysis in a Thermo Fisher Scientific TSQ Quantis Plus mass spectrometer.
UPLC parameters were modified from the method of ref. 39. LC–MS/MS analyses were performed using a Vanquish ultra-high-performance liquid chromatography system coupled with a TSQ Quantis Plus mass spectrometer (Thermo Fisher Scientific). Chromatographic separation was achieved using a BEH C18 (2.1 × 100 mm, 1.7 µm) UPLC column (Waters) heated to 60 °C. Then 2 µl of samples was injected into the column with a flow rate of 0.4 ml min−1. Mobile phase A was 1 mM ammonium acetate and 0.1% formic acid in water, and mobile phase B was 1 mM ammonium acetate and 0.1% formic acid in 225:25:3 methanol:acetonitrile:water. The chromatographic gradient for a total 22 min method was 65% A at 0 min, 65% A at 1 min, 40% A at 2 min, 35% A at 9 min, 0% A at 15 min, 0% A at 20 min and 65% A at 20.5 min until 22 min. MS analyses were carried out using a triple quadrupole mass spectrometer with electrospray ionization in negative ion mode using the following settings: spray voltage 3.5 kV, sheath gas-flow rate 45 arb, aux gas-flow rate 10 arb, sweep gas-flow rate 1 arb, ion transfer tube temperature 325 °C and vaporizer temperature 350 °C. BBAAs were identified by selected reaction monitoring (SRM) mode with a chromatographic peak width of 12 s, Q1 and Q3 resolution of 1.2 full-width at half-maximum and collision gas pressure of 1.5 mTorr. SRM transitions from parent [M-H]− ions to daughter [M-H]− ions corresponded to the loss of bile acids (Supplementary Table 4). Peak areas for each conjugated bile acid were normalized to peak areas from deuterated internal standards of GCA-d4, GUDCA-d4 or GDCA-d4, matching the bile acid. Quantification was performed against a standard curve of authentic standards at eight concentrations ranging from 7.81 nM to 1 µM.
Shotgun metagenomics of IGRAM stool samples
DNA was extracted from faecal samples and from negative controls using the PowerSoil-htp kit (MO BIO Laboratories) following the manufacturer’s instructions, with the optional heating step included (note that MO BIO has since been purchased by QIAGEN; the extraction kit is now sold as the DNeasy PowerSoil HTP 96 Kit). Shotgun libraries were generated from 1 ng of DNA using the NexteraXT kit (Illumina). Libraries were sequenced on the Illumina HiSeq using 2 × 125 bp chemistry in High Output mode. In each plate, two positive controls containing a mix of Vibrio campbellii and PhiX lambda phage were added.
Shotgun metagenomic data were analysed using Sunbeam v.2.0.1 (ref. 40). Low-quality sequences were trimmed using Trimmomatic with default parameters41. Sequences with low complexity and sequences that align to the human and phiX genomes were removed from further analysis. The abundance of bacteria was estimated using Kraken42. Sample similarity was assessed by Bray–Curtis dissimilarity and visualized on a principal coordinate plot. Difference in community composition was assessed using the PERMANOVA test. The Shannon diversity metric was used to calculate the alpha diversity. Differences in bile acid levels across the time points were calculated using linear mixed effects models. Log-transformed bile acid levels were used as the outcome, the time points were modelled as the predictor, and the subject information was added as the random effect.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA sequencing datasets are deposited in NCBI SRA Database under bioproject PRJNA878764. BBAA quantification data were obtained from earlier published studies (https://doi.org/10.21203/rs.3.rs-820302/v1 and https://doi.org/10.1128/mSystems.00805-21), and corresponding bacterial genomes for comparative genomics were obtained from NCBI RefSeq. Peak areas from bile acid quantification are found in the Supplementary Information. For the human study, raw triple quadrupole MS data have been deposited in the Mass Spectrometry Interactive Virtual Environment (MassIVE) under accession no. MSV000093144. Structures of B. longum and Clostridium perfringens BSH were acquired from the Protein Database (2HF0 and 2BJF, respectively). The IGRAM study enroled pregnant African American mothers and their newborn infants. The purpose of the research study was to learn more about the bacteria normally living in the child’s gut, how it is transferred from mother to child and whether it affects the child’s growth in the first three years of life. The IGRAM data used in this publication were consistent with the stated purpose of the research. The Institutional Review Board-approved consent documents included language that allowed participants to indicate whether they would like to have their information included in future research. Subjects may participate in the original research without their information (even if de-identified) being included in future research. Therefore, the data submitted to the repository (SRA accessions PRJNA1042647 and PRJNA557731) were limited to those individuals who consented to future use of their data and are not the entire dataset used in the analyses presented here. To request the complete dataset, contact B. Zemel (zemel@chop.edu) or K. Bittinger (bittingerk@chop.edu) with a summary of how the data will be used and how it is consistent with the goals of the study. The request will be reviewed by the study team at Children’s Hospital of Philadelphia. If approved, the data will be made available within 1 month at no cost. Source data are provided with this paper.
Code availability
The code used for analysis and generating the figures are found at GitHub (https://github.com/PattersonLab-PSU/BBAAs-paper) and Zenodo (https://doi.org/10.5281/zenodo.10072966).
References
Quinn, R. A. et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 579, 123–129 (2020).
Lucas, L. N. et al. Dominant bacterial phyla from the human gut show widespread ability to transform and conjugate bile acids. mSystems 6, e00805–e00821 (2021).
Gentry, E. C. et al. Reverse metabolomics for the discovery of chemical structures from humans. Nature https://doi.org/10.1038/s41586-023-06906-8 (2023).
Hoffmann, M. A. et al. High-confidence structural annotation of metabolites absent from spectral libraries. Nat. Biotechnol. 40, 411–421 (2022).
Funabashi, M. et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 582, 566–570 (2020).
Aatsinki, A.-K. et al. Dynamics of gut metabolome and microbiome maturation during early life. Preprint at medRxiv https://doi.org/10.1101/2023.05.29.23290441 (2023).
Angelin, B., Einarsson, K., Hellström, K. & Leijd, B. Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J. Lipid Res. 19, 1017–1024 (1978).
Stayrook, K. R. et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology 146, 984–991 (2005).
Handschin, C. et al. Cholesterol and bile acids regulate xenosensor signaling in drug-mediated induction of cytochromes P450 *. J. Biol. Chem. 277, 29561–29567 (2002).
Falany, C. N., Johnson, M. R., Barnes, S. & Diasio, R. B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 269, 19375–19379 (1994).
Grill, J., Schneider, F., Crociani, J. & Ballongue, J. Purification and characterization of conjugated bile salt hydrolase from Bifidobacterium longum BB536. Appl. Environ. Microbiol. 61, 2577–2582 (1995).
Sato, Y. et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 599, 458–464 (2021).
Thanissery, R., Winston, J. A. & Theriot, C. M. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe 45, 86–100 (2017).
Joyce, S. A. et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl Acad. Sci. USA 111, 7421–7426 (2014).
Yao, L. et al. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 7, e37182 (2018).
Foley, M. H. et al. Bile salt hydrolases shape the bile acid landscape and restrict Clostridioides difficile growth in the murine gut. Nat. Microbiol. 8, 611–628 (2023).
Wang, M. et al. Mass spectrometry searches using MASST. Nat. Biotechnol. 38, 23–26 (2020).
Shalon, D. et al. Profiling the human intestinal environment under physiological conditions. Nature 617, 581–591 (2023).
Pavlović, N., Stankov, K. & Mikov, M. Probiotics—interactions with bile acids and impact on cholesterol metabolism. Appl. Biochem. Biotechnol. 168, 1880–1895 (2012).
Koskenniemi, K. et al. Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol. Cell. Proteomics 10, M110.002741 (2011).
Adhikari, A. A. et al. Development of a covalent inhibitor of gut bacterial bile salt hydrolases. Nat. Chem. Biol. 16, 318–326 (2020).
Brancaccio, V. F., Zhurina, D. S. & Riedel, C. U. Tough nuts to crack. Bioengineered 4, 197–202 (2013).
Bennett, B. D. et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 (2009).
Kumar, R. S. et al. Structural and functional analysis of a conjugated bile salt hydrolase from Bifidobacterium longum reveals an evolutionary relationship with penicillin V acylase. J. Biol. Chem. 281, 32516–32525 (2006).
Bittinger, K. et al. Bacterial colonization reprograms the neonatal gut metabolome. Nat. Microbiol. 5, 838–847 (2020).
Li, T. & Chiang, J. Y. L. Nuclear receptors in bile acid metabolism. Drug Metab. Rev. 45, 145–155 (2013).
Rothhammer, V. & Quintana, F. J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 19, 184–197 (2019).
Yu, G. Data Integration, Manipulation and Visualization of Phylogenetic Trees (Chapman and Hall/CRC, 2022); https://doi.org/10.1201/9781003279242
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Tung Ho, L. S. & Ané, C. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408 (2014).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 4, 357–359 (2012).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, giab008 (2021).
Okonechnikov, K., Conesa, A. & García-Alcalde, F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 32, 292–294 (2016).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Tian, Y., Cai, J., Allman, E. L., Smith, P. B. & Patterson, A. D. Quantitative analysis of bile acid with UHPLC-MS/MS. Methods Mol. Biol. 2194, 291–300 (2021).
Dame, M. K. et al. Human colonic crypts in culture: segregation of immunochemical markers in normal versus adenoma-derived. Lab Invest. 94, 222–234 (2014).
Zhang, Y. et al. Expanding the scope of targeted metabolomics by one-pot microscale synthesis and tailored metabolite profiling: investigation of bile acid–amino acid conjugates. Anal. Chem. 94, 16596–16603 (2022).
Clarke, E. L. et al. Sunbeam: an extensible pipeline for analyzing metagenomic sequencing experiments. Microbiome 7, 46 (2019).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Wood, D. E. & Salzberg, S. L. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 15, R46 (2014).
Acknowledgements
We thank the Michigan Medicine Translational Tissue Modeling Laboratory for providing enteroids and enteroid growth medium. The Translational Tissue Modeling Laboratory is a University of Michigan funded initiative (Center for Gastrointestinal Research, Office of the Dean, Comprehensive Cancer Center, Departments of Pathology, Pharmacology and Internal Medicine) with support by the Endowment for Basic Sciences. B.R. was supported by Penn State University (PSU)/National Institutes of Health (NIH) grant no. T32GM102057. S.L.C. and E.R.R. were supported by Rosalind E. Franklin Science Achievement Graduate Fellowships from the Eberly College of Science at PSU. Y.M.S. was supported by NIH grant nos. R01CA148828, R01CA245546 and R01DK095201, the University of Michigan Comprehensive Cancer Center Core (grant no. P30CA046592), the GI SPORE Molecular Pathology and Bio Sample Core (grant no. P50CA130810) and the Center for Gastrointestinal Research (grant no. DK034933). P.C.D. was supported by the Collaborative Microbial Metabolite Center (grant no. 1U24DK133658-01). B.S.Z. and G.D.W. are supported by NIH grant no. R01DK107565. The IGRAM study was also supported by an unrestricted donation from the American Beverage Foundation for a Healthy America to the Children’s Hospital of Philadelphia to support the Healthy Weight Program and the NIH National Center for Research Resources Clinical and Translational Science Program (grant no. UL1TR001878). G.D.W. is supported by services provided by the H-MARC Core for the Center for Molecular Studies in Digestive and Liver Diseases (P30 P30DK050306) as well as the Penn Center for Nutritional Science and Medicine. C.E.T., K.B. and G.D.W. are supported by the PennCHOP Microbiome Program. P.C.D. was further supported by grant no. R01DK136117-01. P.C.D. and S.S. were supported by Crohn’s and Colitis Foundation awards (grant nos. 675191 and 623914) and the American Heart Association postdoctoral fellowship (19 POST34380588). E.R.R. and M.S.K. were supported by PSU/National Institute of Diabetes and Digestive and Kidney Diseases (grant no. T32DK120509). J.P.Z. was supported by NIH grant no. R35GM138369). J.E.B. was supported by NIH grant nos. R00AI147165 and R35GM151045. A.D.P. was supported by NIH grant nos. U01DK119702, S10OD021750 and R35ES035027 and the Pennsylvania Department of Health using Tobacco CURE funds. A.D.P. and J.P.V.H. were supported by United States Department of Agrigulture National Institute of Food and federal appropriations under project no. PEN047702. T.Y., K.W.K. and F.J.G. are supported by the National Cancer Institute Intramural Research Program. N.J.H. was supported by PSU. E.E.W. was supported by NIH grant no. R01GM125842 and PSU.
Author information
Authors and Affiliations
Contributions
B.R., S.L.C. and A.D.P. conceived the project. P.C.D. and E.C.G. provided standards, suggestions and feedback for the duration of the project. M.A.G. and J.P.V.H. performed luciferase reporter assays and edited the manuscript. E.R.R. and J.P.C. generated bsh mutants. S.S. and Y.M.S. performed organoid experiments. N.J.H. and E.E.W. helped with BSH purification and provided suggestions and feedback for the duration of the project. T.Y., K.W.K. and F.J.G. helped with bile acid analysis and interpretation. B.R., S.L.C., M.S.K. and J.E.B. performed gnotobiotic experiments. I.K., J.E.B., C.E.T., K.B., B.S.Z., G.D.W. and J.P.Z. provided data analysis and interpretation. D.D. and S.A. synthesized BBAA standards. B.R., S.L.C., N.J.H., E.E.W., P.C.D. and A.D.P. wrote the manuscript. All authors edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
P.C.D. is an advisor to and holds equity in Cybele and is a scientific cofounder of, is an advisor to and holds equity in Ometa, Armome and Enveda, with previous approval by the University of California San Diego. J.V.H. is cofounder and chief scientific officer of INDIGO Biosciences, Inc., with previous approval from Penn State University. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jason Ridlon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 An extended version of correlation plot.
Plot of bsh presence in human-associated bacteria and BBAA production showing all the taxa names. Taxa-names are trimmed for readability. Two species of genera, Cutibacterium and Gardnerella, are condensed to their parent genus group.
Extended Data Fig. 2 Differential gene expression analysis of Bifidobacterium longum NCTC 11818 supplemented with 200 μM each of CA and DCA.
Genes with absolute log2 fold-change of 1 and FDR-adjusted p ≤ 0.05 are represented in red (based on two-sided Wald tests as implemented in DESeq2); n = 5.
Extended Data Fig. 3 Colony-forming units of Bifidobacterium longum NCTC 11818 with or without GR-7.
The bounds of the box plot represent Q1–Q3 with median as center and min-max values for whiskers. n = 4 biologically independent bacterial cultures per group.
Extended Data Fig. 4 Heterologous protein expression of BlBSH with active site residue substitutions.
SDS-PAGE from single experimental replicates showing BlBSH expression at ~35 kDa, 4 h post-induction, in (a) LB media or (b) M9 minimal media. (c) Structure of B. longum BSH (PDB: 2HF0) highlighting active site residues mutated in this study in grey. Cholate and taurine (green) were fit using Discovery Studio software from the structure of Clostridium perfringens BSH (PDB: 2BJF) to demonstrate ligand binding location.
Extended Data Fig. 5 Body weights and ileal bile acid concentrations in B. fragilis monocolonized mice.
(a) Body weights relative to weight at inoculation on day 0. Data are presented as mean values ± SD. Ileal concentrations of (b) secondary, (c) glycine-conjugated, and (d) taurine-conjugated bile acids. Significant differences were determined by two-tailed t-test between WT (pink) and Δbsh (blue) colonized mice (HDCA p = 5.2e-6****, 3αOH-KetoLCA p = 1.1e-7****, MuroCA p = 1.4e-4***). For (b-d), the bounds of the box plots represent Q1–Q3 with median as center and min-max values for whiskers. Biological replicates were n = 7 for WT and n = 8 for Δbsh.
Extended Data Fig. 6 Levels of BBAAs correlate with the time elapsed after birth until sample collection within 0–4 day sampling group.
Spearman’s rank correlation was used to calculate significant correlations between individual bile acids quantified by LC-MS/MS and hours after birth the initial sampling occurred.
Extended Data Fig. 7 Regulation of human and murine ligand-activated transcription factors by bile acids.
(a) Luciferase reporter assays for activation of human transcription factors FXR, VDR, CAR3, PXR, AHR, and PPARα, β/δ, and γ by a 50 µM dose of conventional and bacterially conjugated bile acids. (b) Luciferase reporter assays for activation of mouse nuclear receptors FXR and PPARα, β/δ, and γ by a 50 µM dose of conventional and bacterially conjugated bile acids. Differences between treatments and vehicle-treated control were determined using ANOVA with Dunnett’s post-hoc test (p < 0.05*), n = 3 per group. Color gradient is on a log scale.
Extended Data Fig. 8 Regulation of ligand-activated transcription factors by BBAAs in organoids.
RT-qPCR of target genes for FXR (Fgf15/FGF19), CAR3 (Cyp2b10/CYP2B6), PXR (Cyp3a11/CYP3A4), and AHR (Cyp1a1/CYP1A1) in (a) mouse and (b) human small intestinal organoids treated with Glu-CA or Glu-CDCA. Expression was calculated using the 2−ΔΔCt method, normalized to Gapdh/GAPDH. Error bars represent SD. (a) Biological replicates were n = 3 for vehicle and n = 4 for treatments (Cyp3a11 p = 4.8e-3**, p = 1.5e-2*, Cyp1a1 Glu-CA-50 p = 4.7e-4***, Glu-CA-100 p = 3.3e-4***, Glu-CDCA-100 p = 2.3e-3). (b) Biological replicates were n = 8 for vehicle and n = 6 for treatments across two independent experiments (p = 3.3e-3**). All significant differences between treatments and vehicle were determined using one-way ANOVA with Dunnett’s post-hoc test.
Extended Data Fig. 9 RT-qPCR of additional receptor target genes in organoids.
(a) Relative mRNA expression of Bsep, Adrp, S100g, and Acox1 in mouse intestinal organoids. Biological replicates were n = 3 for vehicle and n = 4 for treatments. (b) Relative mRNA expression of FXR, PXR, CAR, and the FXR target genes short heterodimeric partner (SHP), bile salt export protein (BSEP), organic solute transporter-α (OSTα) and -β (OSTβ), and ileal bile acid binding protein (IBABP) in human intestinal organoids. Biological replicates were n = 8 for vehicle and n = 6 for treatments. Differences between treatments and vehicle were determined using one-way ANOVA with Dunnett’s post-hoc test (p = 0.011*).
Supplementary information
Supplementary Table 1
RNA sequencing analysis for B. longum treated with bile acids. Gene identities and statistical results related to Extended Data Fig. 2.
Supplementary Tables 2–4
Supplementary Table 2 contains a list of PCR primers used in this study, Table 3 contains multiple reaction monitoring method parameters for conjugated bile acid LC–MS/MS analysis on the Waters TQ-S instrument (retention times, mass-to-charge ratios (m/z) of multiple reaction monitoring transitions and collision energies for conjugated bile acids measured using the Waters TQ-S mass spectrometer; corresponding deuterium-labelled spiked internal standards used for each analyte are listed), and Table 4 contains SRM method parameters for LC–MS/MS of infant stool on the Thermo Fisher Scientific TSQ instrument (retention times, mass-to-charge ratios (m/z) of multiple reaction monitoring transitions and collision energies for bile acids measured in Fig. 3 as part of the infant microbiome study; corresponding deuterium-labelled spiked internal standards used for each analyte are listed).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rimal, B., Collins, S.L., Tanes, C.E. et al. Bile salt hydrolase catalyses formation of amine-conjugated bile acids. Nature 626, 859–863 (2024). https://doi.org/10.1038/s41586-023-06990-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06990-w
This article is cited by
-
Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets
Signal Transduction and Targeted Therapy (2024)
-
The changing metabolic landscape of bile acids – keys to metabolism and immune regulation
Nature Reviews Gastroenterology & Hepatology (2024)
-
Another renaissance for bile acid gastrointestinal microbiology
Nature Reviews Gastroenterology & Hepatology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.