Abstract
Humans and animals exhibit various forms of prosocial helping behaviour towards others in need1,2,3. Although previous research has investigated how individuals may perceive others’ states4,5, the neural mechanisms of how they respond to others’ needs and goals with helping behaviour remain largely unknown. Here we show that mice engage in a form of helping behaviour towards other individuals experiencing physical pain and injury—they exhibit allolicking (social licking) behaviour specifically towards the injury site, which aids the recipients in coping with pain. Using microendoscopic imaging, we found that single-neuron and ensemble activity in the anterior cingulate cortex (ACC) encodes others’ state of pain and that this representation is different from that of general stress in others. Furthermore, functional manipulations demonstrate a causal role of the ACC in bidirectionally controlling targeted allolicking. Notably, this behaviour is represented in a population code in the ACC that differs from that of general allogrooming, a distinct type of prosocial behaviour elicited by others’ emotional stress. These findings advance our understanding of the neural coding and regulation of helping behaviour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data and analyses necessary to understand the conclusions of the manuscript are presented in the main text and in Extended Data. Source data are provided with this paper.
Code availability
Code for behavioural analysis (https://github.com/pdollar/toolbox and https://github.com/hongw-lab/Behavior_Annotator), animal pose tracking (https://github.com/murthylab/sleap/releases/tag/v1.2.9), analysis of mouse vocalizations (https://github.com/rtachi-lab/usvseg)47, microendoscopic imaging data analysis (https://github.com/etterguillaume/MiniscopeAnalysis, https://github.com/zhoupc/CNMF_E and https://github.com/flatironinstitute/NoRMCorre), ROC and SVM decoding analysis is available (https://github.com/hongw-lab/Code_for_2024_ZhangM) on GitHub.
References
Wu, Y. E. & Hong, W. Neural basis of prosocial behavior. Trends Neurosci. 45, 749–762 (2022).
de Waal, F. B. M. & Preston, S. D. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 18, 498–509 (2017).
Keysers, C., Knapska, E., Moita, M. A. & Gazzola, V. Emotional contagion and prosocial behavior in rodents. Trends Cogn. Sci. 26, 688–706 (2022).
Ferretti, V. & Papaleo, F. Understanding others: emotion recognition in humans and other animals. Genes Brain Behav. 18, e12544 (2019).
Sterley, T.-L. & Bains, J. S. Social communication of affective states. Curr. Opin. Neurobiol. 68, 44–51 (2021).
Melis, A. P. The evolutionary roots of prosociality: the case of instrumental helping. Curr. Opin. Psychol. 20, 82–86 (2018).
Dunfield, K. A. A construct divided: prosocial behavior as helping, sharing, and comforting subtypes. Front. Psychol. 5, 958 (2014).
Lim, K. Y. & Hong, W. Neural mechanisms of comforting: prosocial touch and stress buffering. Horm. Behav. 153, 105391 (2023).
Bartal, I. B.-A., Decety, J. & Mason, P. Empathy and pro-social behavior in rats. Science 334, 1427–1430 (2011).
Li, A. K., Koroly, M. J., Schattenkerk, M. E., Malt, R. A. & Young, M. Nerve growth factor: acceleration of the rate of wound healing in mice. Proc. Natl Acad. Sci. USA 77, 4379–4381 (1980).
Berckmans, R. J., Sturk, A., Tienen, L. M., van, Schaap, M. C. L. & Nieuwland, R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood 117, 3172–3180 (2011).
Day, B. J. The science of licking your wounds: function of oxidants in the innate immune system. Biochem. Pharmacol. 163, 451–457 (2019).
Lu, J. et al. Somatosensory cortical signature of facial nociception and vibrotactile touch–induced analgesia. Sci. Adv. 8, eabn6530 (2022).
Huang, T. et al. Identifying the pathways required for coping behaviours associated with sustained pain. Nature 565, 86–90 (2019).
Hutson, J. M., Niall, M., Evans, D. & Fowler, R. Effect of salivary glands on wound contraction in mice. Nature 279, 793–795 (1979).
Dittus, W. P. J. & Ratnayeke, S. M. Individual and social behavioral responses to injury in wild toque macaques (Macaca sinica). Int. J. Primatol. 10, 215–234 (1989).
Li, C.-L. et al. Validating rat model of empathy for pain: effects of pain expressions in social partners. Front. Behav. Neurosci. 12, 242 (2018).
Lariviere, W. R. & Melzack, R. The bee venom test: a new tonic-pain test. Pain 66, 271–277 (1996).
Wu, Y. E. et al. Neural control of affiliative touch in prosocial interaction. Nature 599, 262–267 (2021).
Mogil, J. S. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 10, 283–294 (2009).
Duan, B., Cheng, L. & Ma, Q. Spinal circuits transmitting mechanical pain and itch. Neurosci. Bull. 34, 186–193 (2018).
Smith, M. L., Asada, N. & Malenka, R. C. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 371, 153–159 (2021).
Carrillo, M. et al. Emotional mirror neurons in the rat’s anterior cingulate cortex. Curr. Biol. 29, 1301–1312 (2019).
Allsop, S. A. et al. Corticoamygdala transfer of socially derived information gates observational learning. Cell 173, 1329–1342 (2018).
Hernandez-Lallement, J. et al. Harm to others acts as a negative reinforcer in rats. Curr. Biol. 30, 949–961 (2020).
Lockwood, P. L. The anatomy of empathy: vicarious experience and disorders of social cognition. Behav. Brain Res. 311, 255–266 (2016).
Johansen, J. P., Fields, H. L. & Manning, B. H. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl Acad. Sci. USA 98, 8077–8082 (2001).
Sato, N., Tan, L., Tate, K. & Okada, M. Rats demonstrate helping behavior toward a soaked conspecific. Anim. Cogn. 18, 1039–1047 (2015).
Ueno, H. et al. Rescue-like behaviour in mice is mediated by their interest in the restraint tool. Sci. Rep. 9, 10648 (2019).
Burkett, J. P. et al. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378 (2016).
Langford, D. J. et al. Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970 (2006).
Bernhardt, B. C. & Singer, T. The neural basis of empathy. Annu. Rev. Neurosci. 35, 1–23 (2012).
Phillips, H. L. et al. Dorsomedial prefrontal hypoexcitability underlies lost empathy in frontotemporal dementia. Neuron 111, 797–806 (2023).
Gangopadhyay, P., Chawla, M., Monte, O. D. & Chang, S. W. C. Prefrontal–amygdala circuits in social decision-making. Nat. Neurosci. 24, 5–18 (2021).
Haroush, K. & Williams, Z. M. Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell 160, 1233–1245 (2015).
Paulus, M., Kühn-Popp, N., Licata, M., Sodian, B. & Meinhardt, J. Neural correlates of prosocial behavior in infancy: different neurophysiological mechanisms support the emergence of helping and comforting. Neuroimage 66, 522–530 (2013).
Paxinos, G. & Franklin, K. B. J. The Mouse brain in Stereotaxic Coordinates, 3rd edn (Academic Press, 2008).
Tjølsen, A., Berge, O.-G., Hunskaar, S., Rosland, J. H. & Hole, K. The formalin test: an evaluation of the method. Pain 51, 5–17 (1992).
Chen, J., Guan, S.-M., Sun, W. & Fu, H. Melittin, the major pain-producing substance of bee venom. Neurosci. Bull. 32, 265–272 (2016).
Kingsbury, L. et al. Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell 178, 429–446 (2019).
Zhou, T., Sandi, C. & Hu, H. Advances in understanding neural mechanisms of social dominance. Curr. Opin. Neurobiol. 49, 99–107 (2018).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Pereira, T. D. et al. SLEAP: a deep learning system for multi-animal pose tracking. Nat. Methods 19, 486–495 (2021).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: An online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017).
Zhou, P. et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 7, e28728 (2018).
Kingsbury, L. et al. Cortical representations of conspecific sex shape social behavior. Neuron 107, 941–953 (2020).
Tachibana, R. O., Kanno, K., Okabe, S., Kobayasi, K. I. & Okanoya, K. USVSEG: a robust method for segmentation of ultrasonic vocalizations in rodents. PLoS ONE 15, e0228907 (2020).
Acknowledgements
We thank M. Ma, S. Chaudhry, X. Zhang, L. Gu and S. Kim for technical assistance; C. Cahill for suggestions on pain-related experimental procedures; and members of the laboratory of W.H. for valuable comments. Schematics in Figs. 1a, 2a,m,p, 3a,d,i,n and 4a,f and Extended Data Figs. 4a, 9a and 10g,i were created with BioRender.com. This work was supported in part by National Institutes of Health grants (R01 MH130941, R01 NS113124, R01 MH132736, RF1 NS132912 and UF1 NS122124), a Packard Fellowship in Science and Engineering, a Keck Foundation Junior Faculty Award, a Vallee Scholar Award and a Mallinckrodt Scholar Award (to W.H.).
Author information
Authors and Affiliations
Contributions
M.Z., Y.E.W. and W.H. designed the study. M.Z. carried out all experiments. Y.E.W. and M.Z. carried out computational data analysis. M.J. assisted in some experiments. Y.E.W., M.Z. and W.H. wrote the manuscript. W.H. supervised the entire study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Steve Chang, Christian Keysers, Ewelina Knapska and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Behavioral responses of demonstrators and observers following melittin injection.
(a) Example images showing saline- or melittin-injected paws. (b) Example raster plots showing self-licking behavior directed towards the melittin-injected paw or other paws in demonstrator animals. Each row indicates an individual demonstrator animal. (c) Time courses of the cumulative duration of self-licking behavior towards the melittin-injected paw and other paws. (d) Duration of self-licking behavior towards the melittin-injected paw and other paws during 5-minute intervals throughout the interaction period. (e) Total duration of self-licking behavior towards the melittin-injected paw and other paws. (f) Duration of allolicking towards the uninjected forepaws of demonstrators that were injected with either melittin or saline in the hind paw during 5-minute sliding windows throughout the interaction period. (g) Time courses of the cumulative duration of allolicking towards the uninjected forepaws. (h, i) Total duration (h) and number of bouts (h) of allolicking towards the uninjected forepaws of melittin- and saline-injected demonstrators. (j-m) Total duration of different behaviors displayed by dominant observers when interacting with subordinate demonstrators in pain and by subordinate observers when interacting with dominant demonstrators in pain, including investigation (j), general allogrooming (k), allolicking towards injured paws and uninjured paws (l), and general allogrooming and targeted allolicking combined (m). In (c, d), data are mean ± s.e.m. In (e, h, i, j-m), the center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate the 10th and 90th percentiles. n = 24 mice in (c-e), 12 mice per group in (f-i), and 13 mice per group in (j-m). (e, h, i) Wilcoxon signed-rank test. (j, k, m) Unpaired t-test. (l) Two-way repeated measures ANOVA with post hoc Bonferroni’s multiple comparisons test. All statistical tests are two-sided. ****P < 0.0001, **P < 0.01, *P < 0.05. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 2 Behaviors of female observers towards female demonstrators in pain.
(a) Example raster plots showing general allogrooming and targeted allolicking behaviors towards demonstrators injected with either melittin or saline (control). Each row indicates an individual observer animal, and the same observers were plotted for the control and melittin-injected groups. (b) Time courses of the cumulative duration of different behaviors towards demonstrators in pain and control animals, including investigation, general allogrooming, targeted allolicking towards injured paws, allolicking towards uninjured paws, and general allogrooming and targeted allolicking combined. Data are mean ± s.e.m. (c) Duration of various behaviors during 5-minute sliding windows throughout the interaction period. (d-k) Quantification of the duration (d-g) and bout number (h-k) of behaviors towards demonstrators in pain and control animals. In (d-k), the center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate the minimum and maximum values. n = 18 mice per group in (b-k). (d, e, g, h, i, k) Wilcoxon signed-rank test. (f, j) Two-way repeated measures ANOVA with post hoc Bonferroni’s multiple comparisons test. All statistical tests are two-sided. ***P < 0.001, **P < 0.01, *P < 0.05. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 3 Observers’ behaviors towards demonstrators experiencing pain induced by formalin injection.
(a) Example raster plots showing general allogrooming and targeted allolicking behaviors towards formalin- and saline-injected demonstrators. Each row indicates an individual observer animal, and the same observers were plotted for the control and formalin-injected groups. (b) Time courses of the cumulative duration of different behaviors towards formalin- and saline-injected demonstrators, including investigation, general allogrooming, targeted allolicking towards injured paws, allolicking towards uninjured paws, and general allogrooming and targeted allolicking combined. Data are mean ± s.e.m. (c) Duration of various behaviors during 5-minute intervals throughout the interaction period. (d-k) Quantification of the duration (d-g) and bout number (h-k) of various behaviors towards formalin- and saline-injected demonstrators. In (d-k), the center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate the minimum and maximum values. n = 16 mice per group in (b-k). (d, e, g, h, i, k) Wilcoxon signed-rank test. (f, j) Two-way repeated measures ANOVA with post hoc Bonferroni’s multiple comparisons test. All statistical tests are two-sided. ***P < 0.001, **P < 0.01, *P < 0.05. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1.
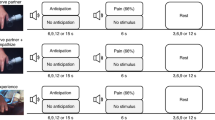
Extended Data Fig. 4 Observers display general allogrooming but not targeted allolicking towards demonstrators in a stress state induced by acute restraint.
(a) Schematic of the behavioral protocol for examining interaction between observer mice and demonstrators in stress. Created with BioRender.com. (b) Time courses of the cumulative duration of investigation, general allogrooming, and allolicking of paws towards stressed demonstrators and controls. Data are mean ± s.e.m. (c) Duration of various behaviors during 5-minute intervals throughout the interaction period. (d) Quantification of the duration of various behaviors towards stressed demonstrators and controls. The center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate the minimum and maximum values. n = 12 mice per group in (b-d). (d) Two-sided Wilcoxon signed-rank test. **P < 0.01. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 5 Allolicking assists others in coping with pain.
(a, b) Duration of self-licking behavior exhibited by demonstrators and combined duration of self-licking by demonstrators and targeted allolicking by observers. The demonstrators were either isolated or housed with cage mates after receiving melittin injection. The demonstrators were divided into a “low prosocial” group (a) and a “high prosocial” group (b) according to the level of targeted allolicking and general allogrooming behaviors exhibited by the cage mates. The combined duration of allolicking and allogrooming by cage mates were <50 s in the “low prosocial” group, and ≥ 50 s in the “high prosocial” group. Grey bar: the amount of time that demonstrators spent self-licking when they were alone; blue bar: the amount of time that demonstrators spent self-licking when they were together with observers; red bar: the combined duration of self-licking and allolicking in a social setting. (c, d) Example spectrograms overlaid with behavior annotations showing the lack of ultrasonic vocalizations (USV) during interaction between observers and demonstrators in pain, as well as prior to the onset of allolicking or allogrooming behavior (c). This contrasts with frequent vocalizations emitted by pups (d). (e-g) Correlations between the duration of self-licking by demonstrators and allolicking (e) or allogrooming (f) by observers or the two behaviors combined (g). Solid lines represent linear regression lines and dashed lines indicate 95% confidence intervals. (h) Raster plots showing targeted allolicking by observers towards the melittin- and saline-injected paws of sedated demonstrators. (i) Onset latency of allolicking towards the injured paw of awake and sedated demonstrators. (j, k) The fraction of targeted allolicking towards the injured paw and allolicking toward the uninjured paw of awake (j) and sedated (k) demonstrators during different interaction periods. In (a, b, i-k), data are mean ± s.e.m. n = 6 mice per group in (a), 18 mice per group in (b), 16 mice in (e-g), and 12 mice in the awake group and 18 mice in the sedated group in (i-k). (a, b) Friedman test with post hoc Dunn’s multiple comparisons test. (e-g) Linear regression. (i) Wilcoxon rank-sum test. (j, k) Two-way repeated measures ANOVA with post hoc Bonferroni’s multiple comparisons test. All statistical tests are two-sided. ****P < 0.0001, **P < 0.01, *P < 0.05. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 6 Response of ACC neurons to different states of others across demonstrators.
(a, b, f, g, k) Pearson correlation of AUROC values (reflecting cells’ tuning properties) with respect to investigation (“inv”) towards others in neutral (a), pain (b, g, k) or stress (f) state between pairs of demonstrators. AUROC values were derived using data from each demonstrator and Pearson correlation coefficient was calculated for cells defined as significantly responsive using data pooled from all demonstrators. Each dot represents correlation between a pair of demonstrators. P values less than 10−10 are plotted as 10−10 for visualization purposes. (c, h, l) Correlation between AUROC values for the same state of others (neutral, pain, or stress) across pairs of demonstrators (“dem”): groups 1 and 3 in (c, h), group 1 in (l). Correlation derived from randomly shuffled data (grey bars): groups 2 and 4 in (c, h), group 2 in (l). Correlation between AUROC values for different states of others within the same demonstrators: groups 5 and 6 in (c, h), groups 3 and 4 in (l); for these groups, correlation was calculated separately for cells responsive to either state. (d, i, m) Overlap between activated cells in the same or different response types across pairs of demonstrators. (e, j, n) Fraction of cells from each response type that overlap with the other response type within the same demonstrators. Activated cells were defined using AUROC values derived from data from each individual demonstrator. In (c-e, h-j, l-n), the center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate data within 1.5× interquartile range. Data were from 11 mice in (a-e), 10 mice in (f-j), 6 mice in (k-n). (c, d) Kruskal-Wallis test with post-hoc Dunn’s multiple comparison test. (h, i, l) One-way ANOVA test with with post hoc Bonferroni’s multiple comparisons test. (m) Wilcoxon rank-sum test. All statistical tests are two-sided. ****P < 0.0001, **P < 0.01. Details of statistical analyses and sample sizes are provided in Supplementary Table 1.
Extended Data Fig. 7 Single-cell- and population-level representations of prosocial behaviors and different states of demonstrators.
(a) Schematics illustrating dissociable and shared aspects in the neural representations of different states or behaviors at the single-cell and population levels. (b-j) Decoding performance using all cells and after removing significantly responsive cells in different groups of decoding analysis. Data in the “All cells” groups in (b-j) are the same as presented in Figs. 3h, 3p, 3s (left), 3t (left), 5e, 5 l, 5p, 5 m, and Extended Data Fig. 8n, respectively. (k, l) Fraction of variance explained by the first three PC (k) and PLS (l) components in the data used for decoding of others’ neutral versus pain state (Fig. 3h). In (b-l), the center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate the minimum and maximum values (b-j) or data within 1.5× interquartile range (k, l). n = 11 mice in (b, h, j-l), 6 mice in (c-e, g), 8 mice in (f), 12 mice in (i). (b-j) Two-sided Wilcoxon signed-rank test. ***P < 0.001, **P < 0.01, *P < 0.05. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 8 Response of ACC neurons to others’ pain and stress states and during prosocial behaviors.
(a, b) Schematic timelines showing the order of the presentation of different types of demonstrators and self-pain experiences for examining the neural representations of others’ stress versus pain state (a) and self-pain versus others’ pain (b). (c, e) Heatmaps showing average responses of all recorded ACC neurons during the 5 s before and after the onset of close investigation of demonstrators in neutral, stress, or pain state (c) as well as allolicking and allogrooming (e). Each row represents the activity of an individual cell aligned to the onset of close investigation, allolicking, or allogrooming towards demonstrators (time 0). Cells are clustered using K-means clustering using their activity dynamics. Clusters are separated by dashed horizontal lines. (d, f) Cells in clusters showing a trend of increased activity preferentially in response to one type of demonstrator or behavior in (c, e) are ordered by the time each cell takes to reach 50% of its maximum activity. (g) The expected and observed percentages of neurons activated by all three demonstrator types (naïve, stress, and pain) among the neurons activated by both stressed and pain-experiencing demonstrators. (h, i) Pair-wise distances between cells activated by demonstrators in stress or pain (h), or between cells activated during allolicking or allogrooming (i) within the field of view. Distances between cells within the same response type or from different response types are compared. Grey boxes show distances calculated after cell type identities were randomly shuffled. (j) Venn diagram showing the overlap between neurons activated during the observers’ self-licking after receiving melittin injection or when observing self-licking of melittin-injected demonstrators. (k) Fraction of variance accounted for by the first three PCs in the PCA analysis of population activity associated with allolicking and allogrooming as presented in Fig. 5g–i. (l) Venn diagram and example calcium traces of cells selectively activated during either allogrooming or investigation, but not both, towards demonstrators in pain or stress. (m) Heatmaps showing average responses of example cells (each row) activated selectively by either allogrooming or investigation (but not both) aligned to the onset of each type of behavior (time 0). (n) Performance of decoders trained on population activity in classifying allogrooming versus investigation. In (h, i, k, n), the center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate the minimum and maximum values. n = 4388 cells from 10 mice in (c, d, g), 5080 cells from 12 mice in (e, f), 10 mice per group in (h), 12 mice per group in (i), 2399 cells from 6 mice in (j), 6 mice per group in (k), 5406 cells from 13 mice in (l), 11 mice per group in (n). (h, i) Friedman test. (n) Wilcoxon signed-rank test. All statistical tests are two-sided. ***P < 0.001. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 9 Behavioral effects of DREADD inhibition of ACC neurons.
(a) Schematic of viral injection and experimental paradigm for DREADD inhibition experiments in mCherry-expressing control animals. Created with BioRender.com. (b) Time courses of the cumulative duration of general allogrooming, targeted allolicking, allogrooming and allolicking combined, and social investigation towards pain-experiencing demonstrators by observers that were injected with either CNO or saline. The observers expressed mCherry but not hM4Di. Data are mean ± s.e.m. (c, d) Quantification of the total duration (c) and bout number (d) of general allogrooming, targeted allolicking, allogrooming and allolicking combined, and social investigation towards pain-experiencing demonstrators by mCherry-expressing observers that were injected with either CNO or saline. (e, f) Correlation between the duration of investigation and allolicking (e) or allogrooming (f) directed towards demonstrators in pain during chemogenetic inhibition of ACC neurons. Solid lines: linear regression lines, dashed lines: 95% confidence intervals. (g) Schematic of the three-chamber social preference test. (h) Total time spent in the “social” and “non-social” zones in hM4Di-expressing animals injected with CNO or saline. (i) Sociability scores of hM4Di-expressing animals injected with CNO or saline. (j, k) Duration and bout number of allolicking (j) or investigation (k) displayed by hM4Di-expressing observers injected with CNO or saline towards melittin-injected demonstrators that were under sedation. In (c, d, h-k), the center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate the 10th and 90th percentiles (h, i) or the minimum and maximum values (c, d, j, k). n = 10 mice per group in (b-d), 16 mice in (e, f), 14 mice per group in (h, i), and 11 mice per group in (j, k). (c, d, i) Paired t-test. (e, f) Linear regression. (h) Two-way repeated measures ANOVA followed by post hoc Bonferroni’s multiple comparisons test. (j, k) Wilcoxon signed-rank test. All statistical tests are two-sided. **P < 0.01. *P < 0.05. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1.
Extended Data Fig. 10 Optogenetic activation of ACC neurons and control experiments.
(a) Example raster plots showing an overall increase in allolicking/allogrooming during the 3-minute laser-on periods in ACC optogenetic activation experiments, compared to the 1.5-minute laser-off periods immediately before and after stimulation. (b) Duration of investigation behavior towards demonstrators in pain during periods of optogenetic activation of ACC neurons (laser-on phases) compared to laser-off phases. (c, d) Correlation between the duration of investigation and allolicking (c) or allogrooming (d) during optogenetic activation. Solid lines: linear regression lines, dashed lines: 95% confidence intervals. (e, f) The probability of allolicking (e) and investigation (f) during the 30 s before and after the onset of laser stimulation in experiments where stimulations were initiated after the first three minutes of the interaction. (g) Schematic of viral injection and experimental paradigm for light-stimulation experiments the ACC in EYFP-expressing control animals. (h) Quantification of the total duration of general allogrooming, targeted allolicking, and allogrooming and allolicking combined towards pain-experiencing demonstrators by EYFP-expressing observers during laser-on and laser-off periods. (i) Schematic of viral injection and experimental paradigm for optogenetic activation of excitatory neurons in the prelimbic cortex (PrL). (j) Example image showing ChR2-EYFP expression. Scale bar, 500 μm. IL, infralimbic cortex. (k) Quantification of the total duration of general allogrooming, targeted allolicking of the injured paw, allolicking of uninjured paws, and allogrooming and targeted allolicking combined towards pain-experiencing demonstrators by observers during laser-on and laser-off periods. (l, m) Comparison of the duration (l) and bout number (m) of self-licking behavior displayed by melittin-injected subject animals during optogenetic activation of ACC neurons versus periods without laser stimulation. In (e, f), data are mean ± s.e.m. In (h, k), the center line in the boxplots indicates the median, the box limits indicate the upper and lower quartiles, and the whiskers indicate the minimum and maximum values. n = 18 mice per group in (b), 18 mice in (c, d), 80 trials from 16 mice per group in (e, f), 22 mice per group in (h), 11 mice per group in (l, m), and 8 mice per group in (k). (b, e, f, h, k, l, m) Wilcoxon signed-rank test. (c, d) Linear regression. All statistical tests are two-sided. **P < 0.01. ns, not significant. Details of statistical analyses are provided in Supplementary Table 1. g,i, Created with BioRender.com.
Supplementary information
Supplementary Information
Supplementary Table 1 and Notes 1–6.
Supplementary Video 1
Mice exhibit affiliative allogrooming towards partners in pain. An observer mouse exhibits affiliative allogrooming towards a demonstrator experiencing pain.
Supplementary Video 2
Mice exhibit targeted allolicking towards partners in pain. An observer mouse exhibits targeted allolicking specifically directed towards the injured paw of a demonstrator.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Wu, Y.E., Jiang, M. et al. Cortical regulation of helping behaviour towards others in pain. Nature 626, 136–144 (2024). https://doi.org/10.1038/s41586-023-06973-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06973-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.