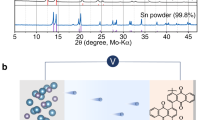
Abstract
Calcium–oxygen (Ca–O2) batteries can theoretically afford high capacity by the reduction of O2 to calcium oxide compounds (CaOx) at low cost1,2,3,4,5. Yet, a rechargeable Ca–O2 battery that operates at room temperature has not been achieved because the CaOx/O2 chemistry typically involves inert discharge products and few electrolytes can accommodate both a highly reductive Ca metal anode and O2. Here we report a Ca–O2 battery that is rechargeable for 700 cycles at room temperature. Our battery relies on a highly reversible two-electron redox to form chemically reactive calcium peroxide (CaO2) as the discharge product. Using a durable ionic liquid-based electrolyte, this two-electron reaction is enabled by the facilitated Ca plating–stripping in the Ca metal anode at room temperature and improved CaO2/O2 redox in the air cathode. We show the proposed Ca–O2 battery is stable in air and can be made into flexible fibres that are weaved into textile batteries for next-generation wearable systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. Source data are provided with this paper.
References
Liang, Y., Dong, H., Aurbach, D. & Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 5, 646–656 (2020).
Hou, S. et al. Solvation sheath reorganization enables divalent metal batteries with fast interfacial charge transfer kinetics. Science 374, 172–178 (2021).
Li, M. et al. Design strategies for nonaqueous multivalent-ion and monovalent-ion battery anodes. Nat. Rev. Mater. 5, 276–294 (2020).
Arroyo-de Dompablo, M. E., Ponrouch, A., Johansson, P. & Palacin, M. R. Achievements, challenges, and prospects of calcium batteries. Chem. Rev. 120, 6331–6357 (2020).
Song, H. & Wang, C. Current status and challenges of calcium metal batteries. Adv. Energy Sustain. Res. 3, 2100192 (2022).
Wang, D. et al. Plating and stripping calcium in an organic electrolyte. Nat. Mater. 17, 16–20 (2018).
Dong, H. et al. High-power Mg batteries enabled by heterogeneous enolization redox chemistry and weakly coordinating electrolytes. Nat. Energy 5, 1043–1050 (2020).
Sun, W. et al. A rechargeable zinc-air battery based on zinc peroxide chemistry. Science 371, 46–51 (2021).
Ponrouch, A., Frontera, C., Bardé, F. & Palacín, M. R. Towards a calcium-based rechargeable battery. Nat. Mater. 15, 169–172 (2016).
Lu, Y., Neale, A. R., Hu, C. & Hardwick, L. J. Divalent nonaqueous metal-air batteries. Front. Energy Res. 8, 602918 (2021).
Wang, M. et al. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 10, 667–672 (2018).
Forero-Saboya, J. et al. Understanding the nature of the passivation layer enabling reversible calcium plating. Energy Environ. Sci. 13, 3423–3431 (2020).
Zhao-Karger, Z. et al. Calcium-tin alloys as anodes for rechargeable non-aqueous calcium-ion batteries at room temperature. Nat. Commun. 13, 3849 (2022).
Jie, Y. et al. Electrolyte solvation manipulation enables unprecedented room-temperature calcium-metal batteries. Angew. Chem. Int. Ed. 59, 12689–12693 (2020).
Gao, X. et al. Alkoxy-functionalized ionic liquid electrolytes: understanding ionic coordination of calcium ion speciation for the rational design of calcium electrolytes. Energy Environ. Sci. 13, 2559–2569 (2020).
Asadi, M. et al. A lithium-oxygen battery with a long cycle life in an air-like atmosphere. Nature 555, 502–506 (2018).
Aurbach, D., McCloskey, B. D., Nazar, L. F. & Bruce, P. G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 1, 16128 (2016).
Chi, X. et al. A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 592, 551–557 (2021).
Pujare, N. U., Semkow, K. W. & Sammells, A. F. A calcium oxygen secondary battery. J. Electrochem. Soc. 135, 260–261 (1988).
Shiga, T., Kato, Y. & Hase, Y. Coupling of nitroxyl radical as an electrochemical charging catalyst and ionic liquid for calcium plating/stripping toward a rechargeable calcium-oxygen battery. J. Mater. Chem. A 5, 13212–13219 (2017).
Li, T., Zhang, X,-Q., Shi, P. & Zhang, Q. Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule 3, 2647–2661 (2019).
Li, Z., Fuhr, O., Fichtner, M. & Zhao-Karger, Z. Towards stable and efficient electrolytes for room-temperature rechargeable calcium batteries. Energy Environ. Sci. 12, 3496–3501 (2019).
Lai, J. et al. Electrolytes for rechargeable lithium–air batteries. Angew. Chem. Int. Ed. 59, 2974–2997 (2020).
Ye, L. et al. Stabilizing lithium into cross-stacked nanotube sheets with an ultra-high specific capacity for lithium oxygen batteries. Angew. Chem. Int. Ed. 58, 2437–2442 (2019).
Nishioka, K. et al. Isotopic depth profiling of discharge products identifies reactive interfaces in an aprotic Li–O2 battery with a redox mediator. J. Am. Chem. Soc. 143, 7394–7401 (2021).
Jung, H. et al. An improved high-performance lithium–air battery. Nat. Chem. 4, 579–585 (2012).
Kondori, A. et al. A room temperature rechargeable Li2O-based lithium-air battery enabled by a solid electrolyte. Science 379, 499–505 (2023).
Shyamsunder, A., Blanc, L. E., Assoud, A. & Nazar, L. F. Reversible calcium plating and stripping at room temperature using a borate salt. ACS Energy Lett. 4, 2271–2276 (2019).
Ko, S., Yamada, Y. & Yamada, A. An overlooked issue for high-voltage Li-ion batteries: suppressing the intercalation of anions into conductive carbon. Joule 5, 998–1009 (2021).
Yu, Y. et al. A renaissance of N,N-dimethylacetamide-based electrolytes to promote the cycling stability of Li–O2 batteries. Energy & Environ. Sci. 13, 3075–3081 (2020).
Feng, S. et al. Molecular design of stable sulfamide- and sulfonamide-based electrolytes for aprotic Li-O2 batteries. Chem 5, 2630–2641 (2019).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1998).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Liu, Q. et al. Interlocked CNT networks with high damping and storage modulus. Carbon 86, 46–53 (2015).
He, J. et al. Scalable production of high-performing woven lithium-ion fibre batteries. Nature 597, 57–63 (2021).
Liao, M. et al. Industrial scale production of fibre batteries by a solution-extrusion method. Nat. Nanotechnol. 17, 372–377 (2022).
Acknowledgements
This work was supported by MOST (2022YFA1203002, 2022YFA1203001), NSFC (T2321003, 22335003, 52122310, 22075050, 52222310, T2222005, 22175042, 22225201) and STCSM (21511104900, 20JC1414902). We thank A.-L. Chun of Science Storylab for reading and editing the paper.
Author information
Authors and Affiliations
Contributions
H.P., Y. Wang, H.Z., J.L. and B.W. conceived and designed the research project. L.Y., M.L. and K.Z. performed the experiments on Ca–O2 batteries, fibre batteries and the simulations of reaction pathways. M.Z., Y.J., X.C., C.T., P.L. and Y. Wen performed electrochemical measurements of Ca–O2 batteries. C.W. performed experiments on the textile batteries. Q.X. collected the TOF-SIMS characterization data. Y. Xu collected the cryo-TEM characterization data. X.S., P.C., H.S., Y.G., Y.Z., Y. Xia and X.X. analysed the data. All the authors discussed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Characterization of CNT air cathode and cycling performance of the Ca-O2 battery.
a, b, SEM (a) and TEM (b) images of CNT cathode. Scale bars, 1 μm (a), 5 nm (b). c, Nitrogen adsorption-desorption isotherm of the CNT, indicating a specific surface area of 172.5 m2/g. d, Galvanostatic discharge and charge curves of Ca-O2 battery at a current density of 1 A/g and a specific capacity of 500 mAh/g for 700 cycles.
Extended Data Fig. 2 Characterization of the discharge product in Ca-O2 batteries.
a, XRD patterns of the cathode after the 1st discharge and the pristine cathode (CNT) suggesting that the main discharge product of Ca-O2 batteries at room temperature is CaO2. References: CaO2 (mp-634859) and CaO (mp-2605) from the Materials Project database. b, TEM image and the corresponding selected area electron diffraction (SAED) pattern (inset) of the discharge product. Scale bar, 100 nm. c, Tof-SIMS depth profile of various secondary ion species obtained by sputtering the fully discharged cathode, indicating the formation of CaO2 in the discharge process. d, Tof-SIMS two-dimensional view images of CaO2− from the discharged cathode showing the uniform distribution of CaO2. e, Tof-SIMS depth profile of various secondary ion species obtained by sputtering the recharged cathode, indicating the decomposition of CaO2 in the charge process. f, Tof-SIMS two-dimensional view images of CaO2− from the recharged cathode showing the decomposition of CaO2.
Extended Data Fig. 3 Reversibility of CaO2 formation/decomposition in Ca-O2 cell chemistry.
a, The standard curve of the [Ti(O2)]2+ complex concentration obtained from the UV-vis spectrometer for the quantitative titration measurement of CaO2. b, The amount of cathodic products upon discharge/recharge derived from the titration measurements, using the optimized electrolyte (EMIM-BF4/DMSO, 50:50 vol%, red bars) and the contrast electrolyte (EMIM-BF4 without DMSO, blue bars). The results show that, in the optimized electrolyte (EMIM-BF4/DMSO, 50:50 vol%), 97.3% of the CaO2 discharge product can be decomposed upon recharge, pointing to a higher reversibility (97.3%) than that of the contrast electrolyte (<60%). Error bars represent the standard deviations of the results from five samples. c, DEMS profiles showing that the H2O signal during discharging and charging keep below 0.05 nmol/s, indicating the absence of the potential parasitic reaction between CaO2 and hydrofluoric acid (HF). d, DEMS profile at open circuit voltage showing the absence of O2 evolution (<0.05 nmol/s) from the potential CaO2 decomposition. e, Calculated Gibbs free energy of O2 release from CaO2 on the aligned CNT cathode, suggesting the feasibility of CaO2 decomposition during the charge process in Ca-O2 batteries. Note that an applied potential (U = 3.36 V versus Ca/Ca2+) is used in order to simulate the charging process, which, in turn, leads to different Gibbs free energy values for the intermediates CaO4* with respect to CaO2 here, as compared to those in the discharge process (Fig. 2d).
Extended Data Fig. 4 Characterization of Ca metal anode disassembled from Ca-O2 batteries.
a, XRD pattern of the Ca anode disassembled from a charged Ca-O2 battery showing the presence of metallic Ca and CaF2. b, c, TEM images and the corresponding SAED pattern (inset) of the cycled Ca metal anode. Scale bars, 20 nm (b), 3 nm (c). d, Fast Fourier transform (FFT) images of the crystal regions marked in Fig. 3b showing the presence of metallic Ca (cubic Fm3m crystal phase, (200) plane) and randomly dispersed CaF2 nanocrystals (cubic Fm3m crystal phase, (111) plane). e, XPS Ca 2p spectrum (after etching) of pure Ca metal displaying two peaks centered at 349.5 (Ca 2p1/2) and 346.0 eV (Ca 2p3/2). f, XPS C 1 s, F 1 s, and S 2p spectra of the cycled Ca metal anode, revealing a hybrid SEI composed of inorganic CaF2 and organic compounds.
Extended Data Fig. 5 Evaluation of oxidation stability of the electrolyte.
a, Linear sweep voltammetry curves of our ionic liquid-based electrolyte on CNT electrode at a scan rate of 1.0 mV/s. Inset, the zoomed-in plot. b, The corresponding current derivative from a. c, Potentiostatic polarization of the Ca | |CNT cell, suggesting an oxidation voltage over 4.1 V for our ionic liquid-based electrolyte.
Extended Data Fig. 6 Properties of the optimized electrolyte containing DMSO.
a, Dependence of apparent viscosity on the electrolyte formula containing different volume ratio of DMSO, demonstrating that the electrolyte of EMIM-BF4/DMSO (50:50 vol%) has the lowest apparent viscosity. b, Dependence of ionic conductivity on the electrolyte formula containing different volume ratio of DMSO, showing that the electrolyte of EMIM-BF4/DMSO (50:50 vol%) has the highest ionic conductivity (11.5 mS/cm). c, Steady-state current density of electrolytes containing different volume ratio of DMSO upon varying applied polarization potentials. The electrolyte of EMIM-BF4/DMSO (50:50 vol%) demonstrates the comparably higher steady-state current densities, pointing to the facilitated Ca2+ transportation. d–f, Potentiostatic polarization curves of the EMIM-BF4/DMSO (50:50 vol%) electrolyte (d), EMIM-BF4/DMSO (75:25 vol%) electrolyte (e), and EMIM-BF4 electrolyte without DMSO (f). Inset, corresponding Nyquist plots of the electrolyte. Among the various electrolytes, our optimized electrolyte (d) has the highest Ca2+ transference number \(({t}_{{{Ca}}^{2+}}=0.43)\). g, h, Linear sweep voltammetry curves of the O2 reduction reaction in electrolytes with DMSO (EMIM-BF4/DMSO, 50:50 vol%, in g) and without DMSO (only EMIM-BF4, in h) at a scan rate of 100 mV/s with rotating disc electrode rotation rates from 400 to 1,200 rpm and an increment of 200 rpm. The comparably higher limiting current densities in the electrolytes with DMSO (EMIM-BF4/DMSO, 50:50 vol%) indicates the improved O2 reduction reaction kinetics by the introduction of DMSO. The clear observation of O2 reduction at about 1.5 V originates from the maximized exposure of working electrode to the continuously applied O2 flux. i, The fitted linear Levich plots of the limiting current density (O2 reduction reaction) versus the square root of rotation rate (ω1/2), deducing a higher O2 diffusion coefficient of 1.69 × 10−6 cm2/s in the electrolyte with DMSO (EMIM-BF4/DMSO, 50:50 vol%) than 4.47 × 10−7 cm2/s in the electrolyte without DMSO. Error bars represent the standard deviations of the results from five samples.
Extended Data Fig. 7 Electrochemical performance of Ca metal anode in electrolytes with and without DMSO.
a, Cyclic voltammogram of Ca plating/stripping in electrolyte with DMSO (EMIM-BF4/DMSO (50:50 vol%)). Scan rate, 100 mV/s. Inset, passed charge during plating/stripping from the cyclic voltammogram. b, Cyclic voltammogram of Ca plating/stripping in electrolyte without DMSO (EMIM-BF4). Scan rate, 100 mV/s. Inset, passed charge during plating/stripping. c, Cyclic voltammogram of Ca plating/stripping in electrolyte with DMSO (EMIM-BF4/DMSO (50:50 vol%)) in the presence of O2 shows similar reversibility compared with that without O2 (a). Scan rate, 100 mV/s. Inset, passed charge during plating/stripping from the cyclic voltammogram. d, Nyquist plots of the symmetric battery after the 1st, 50th, and 100th Ca plating/stripping cycles.
Extended Data Fig. 8 Improved Ca2+ de-solvation and Ca plating/stripping.
a, Raman spectra of the electrolytes with different volume ratio of DMSO, suggesting the coordination of DMSO and Ca2+. b, Percentage of Ca2+-coordinated BF4−/TFSI− as determined by the ratio between the peak area of coordinated species and the total peak area from the Raman spectra. DMSO effectively reduces Ca2+-coordinated BF4−/TFSI−. c, 43Ca NMR spectra of electrolytes with DMSO (EMIM-BF4/DMSO, 50:50 vol%) and without DMSO (only EMIM-BF4) have different chemical shifts and shapes, suggesting that DMSO relieves the Ca2+ coordination by BF4−/TFSI− anions. d, e, Temperature-dependent Nyquist plots of symmetric batteries using the electrolytes with DMSO (EMIM-BF4/DMSO, 50:50 vol%, in d) and without DMSO (only EMIM-BF4, in e). f, Arrhenius plots of the resistance corresponding to Ca2+ de-solvation in electrolytes with DMSO (red) and without DMSO (blue), suggesting that the introduced DMSO can reduce the Ca2+ de-solvation energy barrier. g, Voltage curves of Ca | |Ca symmetric cells using electrolytes with DMSO (EMIM-BF4/DMSO, 50:50 vol%) and without DMSO (only EMIM-BF4) at a current density of 0.2 mA/cm2 and an areal capacity of 0.2 mAh/cm2. Inset, the enlarged voltage curves.
Extended Data Fig. 9 Electrochemical performance of Ca-O2 batteries under practical conditions.
a, Discharge and charge voltage plateaus of the Ca-O2 battery tested in air show a stable cycling performance for over 450 cycles at a current density of 1 A/g and a specific capacity of 500 mAh/g. b, Discharge and charge voltage plateaus at 1 A/g and specific capacity at 1 Ah/g of the Ca-O2 battery display stable cycling for over 270 cycles (top). Discharge and charge voltage plateaus at 1 A/g and specific capacity at 2 Ah/g of the Ca-O2 battery are stable for over 150 cycles (bottom). c, Discharge and charge voltage plateaus of the Ca-O2 battery with a CNT cathode mass loading of 1 mg/cm2 show a stable cycling performance for over 100 cycles at a current density of 100 mA/g and a specific capacity of 500 mAh/g (i.e., areal capacity of 0.5 mAh/cm2). d, Discharge and charge voltage curves of a Ca-O2 battery based on a thin metallic Ca anode (5 mAh/cm2) at an areal capacity of 0.5 mAh/cm2 and a current density of 0.25 mA/cm2. e, Evolution of the discharge and charge plateaus in d.
Extended Data Fig. 10 Fabrication and electrochemical performances of fibre Ca-O2 batteries.
a, Schematic illustration to the preparation procedures of the flexible fibre Ca-O2 batteries. b, SEM image of aligned CNT fibre as the current collector for fibre batteries. Scale bar, 50 μm. c, d, SEM and elemental mapping images of metallic Ca-coated fibre anode, respectively. Scale bars, 100 μm. e, SEM image of the fibre Ca-O2 battery wrapped by a layer of aligned CNT as the air cathode. Scale bar, 100 μm. f, Flexible fibre Ca-O2 battery showing a cycle life over 100 h at a current density of 500 mA/g and a specific capacity of 250 mAh/g. g, Galvanostatic discharge/charge curves of the fibre Ca-O2 battery under increasing bending angles (0 to 180°). Fibre batteries remain stable when deformed. h, Discharge curves of fibre Ca-O2 batteries connected in series.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, L., Liao, M., Zhang, K. et al. A rechargeable calcium–oxygen battery that operates at room temperature. Nature 626, 313–318 (2024). https://doi.org/10.1038/s41586-023-06949-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06949-x
This article is cited by
-
A rechargeable Ca/Cl2 battery
Science China Chemistry (2024)
-
Calcium–Oxygen Fiber Batteries for Next-Generation Wearables
Advanced Fiber Materials (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.