Abstract
An ideal vaccine both attenuates virus growth and disease in infected individuals and reduces the spread of infections in the population, thereby generating herd immunity. Although this strategy has proved successful by generating humoral immunity to measles, yellow fever and polio, many respiratory viruses evolve to evade pre-existing antibodies1. One approach for improving the breadth of antiviral immunity against escape variants is through the generation of memory T cells in the respiratory tract, which are positioned to respond rapidly to respiratory virus infections2,3,4,5,6. However, it is unknown whether memory T cells alone can effectively surveil the respiratory tract to the extent that they eliminate or greatly reduce viral transmission following exposure of an individual to infection. Here we use a mouse model of natural parainfluenza virus transmission to quantify the extent to which memory CD8+ T cells resident in the respiratory tract can provide herd immunity by reducing both the susceptibility of acquiring infection and the extent of transmission, even in the absence of virus-specific antibodies. We demonstrate that protection by resident memory CD8+ T cells requires the antiviral cytokine interferon-γ (IFNγ) and leads to altered transcriptional programming of epithelial cells within the respiratory tract. These results suggest that tissue-resident CD8+ T cells in the respiratory tract can have important roles in protecting the host against viral disease and limiting viral spread throughout the population.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All RNA-sequencing data is available from the NCBI Gene Expression Omnibus (GEO) under accession GSE247464 (Fig. 2) and GSE247376 (Fig. 4). Source data are provided with this paper.
References
Viana, R. et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 603, 679–686 (2022).
Zheng, M. Z. M. & Wakim, L. M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 15, 379–388 (2022).
Nelson, S. A. & Sant, A. J. Potentiating lung mucosal immunity through intranasal vaccination. Front. Immunol. 12, 808527 (2021).
Mettelman, R. C., Allen, E. K. & Thomas, P. G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 55, 749–780 (2022).
Hayward, S. L. et al. Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8+ T cells. Nat. Immunol. 21, 309–320 (2020).
Grau-Exposito, J. et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 12, 3010 (2021).
Dejnirattisai, W. et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 185, 467–484.e415 (2022).
Smith, D. J. et al. Mapping the antigenic and genetic evolution of influenza virus. Science 305, 371–376 (2004).
Valkenburg, S. A. et al. The hurdles from bench to bedside in the realization and implementation of a universal influenza vaccine. Front. Immunol. 9, 1479 (2018).
Auladell, M. et al. Recalling the future: immunological memory toward unpredictable influenza viruses. Front. Immunol. 10, 1400 (2019).
Hassert, M. & Harty, J. T. Tissue resident memory T cells—A new benchmark for the induction of vaccine-induced mucosal immunity. Front. Immunol. 13, 1039194 (2022).
Rimmelzwaan, G. F., Kreijtz, J. H., Bodewes, R., Fouchier, R. A. & Osterhaus, A. D. Influenza virus CTL epitopes, remarkably conserved and remarkably variable. Vaccine 27, 6363–6365 (2009).
Li, Z. T. et al. Why are CD8 T cell epitopes of human influenza A virus conserved? J. Virol. 93, e01534-18 (2019).
Bhatt, S., Holmes, E. C. & Pybus, O. G. The genomic rate of molecular adaptation of the human influenza A virus. Mol. Biol. Evol. 28, 2443–2451 (2011).
Zens, K. D., Chen, J. K. & Farber, D. L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1, e85832 (2016).
Wu, T. et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95, 215–224 (2014).
McMaster, S. R., Wilson, J. J., Wang, H. & Kohlmeier, J. E. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J. Immunol. 195, 203–209 (2015).
Tang, J. et al. Respiratory mucosal immunity against SARS-CoV-2 following mRNA vaccination. Sci. Immunol. 7, eadd4853 (2022).
Pizzolla, A. et al. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2, eaam6970 (2017).
Lowen, A. C., Bouvier, N. M. & Steel, J. Transmission in the guinea pig model. Curr. Top. Microbiol. Immunol. 385, 157–183 (2014).
Burke, C. W., Bridges, O., Brown, S., Rahija, R. & Russell, C. J. Mode of parainfluenza virus transmission determines the dynamics of primary infection and protection from reinfection. PLoS Pathog. 9, e1003786 (2013).
Burke, C. W. et al. Illumination of parainfluenza virus infection and transmission in living animals reveals a tissue-specific dichotomy. PLoS Pathog. 7, e1002134 (2011).
Liang, S., Mozdzanowska, K., Palladino, G. & Gerhard, W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 152, 1653–1661 (1994).
McMaster, S. R. et al. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal. Immunol. 11, 1071–1078 (2018).
Gooch, K. E. et al. Heterosubtypic cross-protection correlates with cross-reactive interferon-gamma-secreting lymphocytes in the ferret model of influenza. Sci. Rep. 9, 2617 (2019).
Piet, B. et al. CD8+ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J. Clin. Invest. 121, 2254–2263 (2011).
Topham, D. J., Tripp, R. A. & Doherty, P. C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159, 5197–5200 (1997).
Schenkel, J. M., Fraser, K. A., Vezys, V. & Masopust, D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 14, 509–513 (2013).
Uddback, I. et al. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol. 14, 92–99 (2021).
Slutter, B., Pewe, L. L., Kaech, S. M. & Harty, J. T. Lung airway-surveilling CXCR3hi memory CD8+ T cells are critical for protection against influenza A virus. Immunity 39, 939–948 (2013).
Gilchuk, P. et al. A distinct lung-interstitium-resident memory CD8+ T cell subset confers enhanced protection to lower respiratory tract infection. Cell Rep. 16, 1800–1809 (2016).
McMahan, K. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 590, 630–634 (2021).
Bertoletti, A., Le Bert, N. & Tan, A. T. SARS-CoV-2-specific T cells in the changing landscape of the COVID-19 pandemic. Immunity 55, 1764–11778 (2022).
Clemens, E. B., van de Sandt, C., Wong, S. S., Wakim, L. M. & Valkenburg, S. A. Harnessing the power of T Cells: the promising hope for a universal influenza vaccine. Vaccines 6, 18 (2018).
Gotch, F., McMichael, A., Smith, G. & Moss, B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J. Exp. Med. 165, 408–416 (1987).
Boon, A. C. et al. The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype. J. Virol. 76, 582–590 (2002).
Koutsakos, M. et al. Human CD8+ T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 20, 613–625 (2019).
Richard, M. et al. Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat. Commun. 11, 766 (2020).
Yang, W. & Shaman, J. Viral replication dynamics could critically modulate vaccine effectiveness and should be accounted for when assessing new SARS-CoV-2 variants. Influenza Other Respir. Viruses 16, 366–367 (2022).
Jacobs, S., Zeippen, C., Wavreil, F., Gillet, L. & Michiels, T. IFN-λ decreases murid herpesvirus-4 infection of the olfactory epithelium but fails to prevent virus reactivation in the vaginal mucosa. Viruses 11, 757 (2019).
Lobby, J. L. et al. Persistent antigen harbored by alveolar macrophages enhances the maintenance of lung-resident memory CD8+ T cells. J. Immunol. 209, 1778–1787 (2022).
Upadhyay, R. et al. A critical role for fas-mediated off-target tumor killing in T-cell immunotherapy. Cancer Discov. 11, 599–613 (2021).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9, e1003118 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
We acknowledge the NIH tetramer core facility at Emory University for providing Sendai NP324–332, influenza NP366–374 and influenza PA224–233 tetramers (NIAID contract 75N93020D00005). We thank the Emory+Children’s Flow Cytometry Core for cell sorting and the Emory Integrated Genomics Core for assistance with preparation of RNA-sequencing libraries. This work was supported by NIH grants R35 HL150803 and U01 HL139483 (J.E.K.). I.U. was supported by Lundbeck Foundation and Carlsberg Foundation. C.M. was supported by F31 HL164049. S.E.M. was supported by F31 HL168914.
Author information
Authors and Affiliations
Contributions
I.U., S.E.M. and J.E.K. conceived the study. I.U., S.E.M., A.R.T., J.P.C. and J.E.K. designed experiments. I.U., S.E.M., C.M., K.N.K., L.A.L. and S.L.H. performed infection, transmission and RNA-sequencing experiments. I.U., S.E.M., A.S., M.E.W., H.A., C.D.S., K.K. and R.A analysed data. A.C.L., C.J.R. and J.M.B. provided critical reagents and technical insight. I.U., S.E.M. and J.E.K. wrote the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Tao Dong, Katherine Kedzierska and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Distribution and characterization of tissue-resident Sendai-specific CD8+ T cells limit following intranasal and intraperitoneal infection with recombinant influenza virus.
(a) Schematic of experiment for investigating immunization route on protection from direct infection. (b) Representative plots of tetramer staining at day 35 in immunized mice. (c) Absolute numbers of Sendai NP324-332/Kb+ CD8 T cells in spleen, BAL, lung, and nasal cavity of immunized mice (n = 9 for PR8 SenNP i.p. and PR8 SenNP i.n.). (d) Representative plots of staining for TRM markers CD69 and CD103 on antigen-specific cells. (e) Absolute numbers of CD69+CD103+ Sendai NP324-332/Kb+ CD8+ T cells in BAL (n = 9 for PR8-SenNP i.n. and n = 8 for PR8-SenNP i.p.), lung and nasal cavity (n = 10 for PR8-SenNP i.n. and n = 9 for PR8-SenNP i.p.). (f) Representative histograms of CD49a, CXCR6, and CXCR3 expression gated on nasal cavity Sendai NP324-332/Kb+ CD8+ T cells following i.n. or i.p. immunization based on TRM marker expression (CD69+CD103+, red; CD69+CD103−, black; CD69−CD103−, green). (g) Frequency of CD49a, CXCR6, and CXCR3 expression on Sendai NP324-332/Kb+ CD69+CD103+ CD8+ T cells in the nasal cavity (n = 5 per group) following i.n. or i.p. immunization. Data are representative of two individual experiments. Lines represent mean values (c, e, g). Statistical significance was determined using two-sided Mann Whitney test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns: non-significant.
Extended Data Fig. 2 Sendai-luciferase bioluminescence strongly correlates with viral titer.
(a) Bioluminescence in Sendai-Luc infected mice combined with viral titer from the total respiratory tract (nasal cavity, trachea, and lungs) on days 2, 4 and 6 post-infection. Black dots represent viral titer, grey curves represent bioluminescence of individual mice, and dotted black line represents background bioluminescence. (b) Correlation of bioluminescence with viral titers. Data shown are from two independent pooled experiments with 10 mice per timepoint.
Extended Data Fig. 3 Contact mice that show no indication of transmission by bioluminescence also fail to develop a Sendai-specific T cell response.
(a) Schematic of experiment investigating the cellular immune response in bioluminescence-positive versus bioluminescence-negative contact mice. (b) Representative flow cytometry plots of Sendai NP324-332Kb+ tetramer staining gated on CD8+ T cells in the BAL, lung, nasal cavity, and spleen. (c) Number of total Sendai NP324-332Kb+ CD8 T cells in the BAL, lung, nasal cavity, and spleen in contact mice 35 after co-housing with PR8-SenNP or WT PR8 immunized index mice. (d) Number of CD69+CD103+ Sendai NP324-332Kb+ CD8 TRM in the BAL, lung, and nasal cavity in contact mice 35 days after co-housing with PR8-SenNP or WT PR8 immunized index mice. Data are representative of two individual experiments with n = 7 mice per group. Lines represent mean values and error bars represent standard deviation (c, d).
Extended Data Fig. 4 Number of SenNP-specific CD8+ TRM in knockout mouse strains following immunization.
(a) Experimental schematic for immunizing WT and knockout mouse strains. (b) Number of CD69+ CD103+ Sendai NP324-332/Kb+ CD8 TRM in the BAL, lung, and nasal cavity 35 days after immunization with LAIV-SenNP for WT (n = 15), Prf−/− (n = 12 for BAL, n = 13 for lungs and nasal cavity), Ifng−/− (n = 18), and Ifngr−/− (n = 16 for lungs and nasal cavity, n = 15 for BAL). Data shown are from 3 independent pooled experiments. (c) Experimental schematic for immunizing WT and knockout mouse strains. (d) Number of natural killer (NK) cells (left graph) and inflammatory monocytes (right graph) in the nasal cavity 35 days after immunization with LAIV WT (n = 10) or LAIV-SenNP (n = 10 for WT, n = 9 for Prf−/−, n = 7 for Ifngr−/−). Data shown are from 2 pooled independent experiments. Lines represent means (b, d). Statistical significance was determined using two-sided Mann Whitney test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns: non-significant.
Extended Data Fig. 5 Immunization does not alter influx of NK cells and monocytes following Sendai virus transmission.
(a) Experimental schematic where immunized contact mice were cohoused with a Sendai-Luc infected index mouse and tissues analyzed for innate immune populations at the time of cohousing (D30) and two days after cohousing (D30 + 2). (b) Number of NK cells in nasal cavity (D30: n = 11 for PR8 WT i.n., n = 15 for PR8-SenNP i.n., n = 14 for PR8-SenNP i.p.) (D30 + 2: n = 11 for PR8 WT i.n., n = 12 for PR8-SenNP i.n., n = 12 for PR8-SenNP i.p.) and BAL (D30: n = 10 for PR8 WT i.n., n = 14 for PR8-SenNP i.n., n = 14 for PR8-SenNP i.p.) (D30 + 2: n = 11 for PR8 WT i.n., n = 11 for PR8-SenNP i.n., n = 12 for PR8-SenNP i.p.). (c) Number of inflammatory monocytes in nasal cavity and BAL (same n as (b), except n = 12 for D30 + 2 PR8-SenNP i.n. in BAL). Data shown are from 3 independent experiments. Lines represent means and error bars represent 95% confidence interval (b, c). Statistical significance was determined using two-sided Mann Whitney test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns: non-significant.
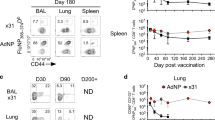
Extended Data Fig. 6 Sendai-specific TRM numbers and assessment of transmission under different immunization strategies at 1- and 6-months post-immunization.
(a) Experimental schematic where contact mice, immunized as indicated, were cohoused with a Sendai-Luc infected index mouse at 35- or 180-days post-immunization. (b) Number of CD69+CD103+ Sendai NP324-332/Kb+ CD8 TRM in the BAL, lung, and nasal cavity at 1 month or six months post-immunization with x31-SenNP i.n. (n = 10 per timepoint except n = 9 for 1 month BAL), PR8-SenNP i.p. (n = 10 per timepoint except n = 9 for 1 month BAL), PR8-SenNP i.n. (1 month n = 9 for lung and nasal cavity and n = 6 for BAL, 6 month n = 10 for lung and nasal cavity and n = 9 for BAL), LAIV-SenNP i.n. (1 month n = 8, 6 month n = 10), and Ad-SenNP i.n. (1 month n = 9 for lung and nasal cavity and n = 8 for BAL, 6 month n = 10 for nasal cavity and BAL, n = 9 for lung). (c and d) Bioluminescence curves of immunized contact mice following exposure to an infected index mouse at 1 month (c) or 6 months (d) post-immunization with x31 WT (1 month n = 14, 6 month n = 16), x31-SenNP (1 month n = 16, 6 month n = 15), PR8-SenNP i.p. (1 month n = 16, 6 month n = 16), PR8-SenNP i.n. (1 month n = 16, 6 month n = 16), LAIV-SenNP i.n. (1 month n = 15, 6 month n = 16), and Ad-SenNP i.n. (1 month n = 15, 6 month n = 16). Solid dark lines represent means, solid pale lines represent individual mice, dashed grey line represents background bioluminescence, and dashed red line represents the limit of infection (c, d). Solid lines (b) and error bars (b-d) represent mean with 95% confidence interval. Statistical significance was determined using two-sided Mann Whitney test. Data are combined from two independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns: non-significant.
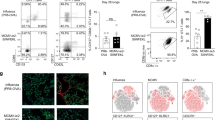
Extended Data Fig. 7 High viral burden in index mice does not correlate with increased viral burden in contact mice.
Contact mice were infected intranasally with WT x31 (n = 14), x31-SenNP (n = 16), PR8-SenNP (n = 16), or Ad-SenNP (n = 15) and cohoused with a Sendai-Luc infected index mouse at day 35 post-immunization. n = 4 index mice per immunization group are plotted. Total viral burden (AUC) of the co-housed index and contact mice were plotted for each cage. Data were fitted to a generalized linear model with gaussian family for each immunization group to investigate the relationship between AUCs of the index mice and contact mice. Data are combined from two independent experiments.
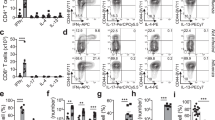
Extended Data Fig. 8 Pre-existing immunity to related influenza strains limits the efficacy of protective T cell immunity induced by LAIV-SenNP immunization but can be overcome by Ad-SenNP immunization.
(a) Experimental schematic for testing the impact of pre-existing influenza immunity on the ability of LAIV-SenNP to protect against transmission. (b) Bioluminescence curves of A/Cal/09 i.p. & PR8 LAIV WT (n = 16), PBS i.p. & PR8 LAIV-SenNP (n = 16), A/Cal/09 i.p. & PR8 LAIV-SenNP (n = 20), and A/Cal/09 i.p. & Ad-SenNP (n = 16) immunized contact mice following exposure to an infected index mouse 30 days after the second immunization. Solid dark lines represent means, solid pale lines represent individual mice, dashed grey line represents background bioluminescence, and dashed red line represents the limit of infection. (c) AUC of bioluminescence in immunized contact mice that become infected following co-housing with an infected index mouse. (d) Probability of infection for immunized contact mice calculated as the proportion of contact mice that became infected. Bars represent 95% binomial confidence intervals (d). Lines represent means (b-d) and error bars represent 95% confidence intervals (b, c). Data are combined from two independent experiments. Statistical significance was determined using two-sided Mann Whitney test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns: non-significant.
Extended Data Fig. 9 Heterologous influenza prime-boost does not improve the durability of respiratory tract TRM.
(a) Experimental schematic to assess the durability of respiratory tract TRM following heterologous PR8-SenNP or Ad-SenNP boosting. (b) Number of CD69+CD103+ Sendai NP324-332Kb+ CD8 TRM in the BAL, lung, and nasal cavity at day 120. Data are combined from two independent experiments with n = 10 mice for PR8 WT i.n., PR8-SenNP i.n., and Ad-SenNP i.n. secondary immunization groups. Lines represent means. Statistical significance was determined using two-sided Mann Whitney test. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns: non-significant.
Supplementary information
Supplementary Information
This file includes Supplementary Fig. 1 containing flow cytometry sorting gating strategies for RNA-sequencing analysis, Supplementary Fig. 2 flow cytometry gating strategies, and Supplementary Table 1—a list of specificities, conjugated fluorophores, clone numbers, catalogue numbers, and vendors for flow cytometry reagents used in this study.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uddbäck, I., Michalets, S.E., Saha, A. et al. Prevention of respiratory virus transmission by resident memory CD8+ T cells. Nature 626, 392–400 (2024). https://doi.org/10.1038/s41586-023-06937-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06937-1
This article is cited by
-
Silent battles: immune responses in asymptomatic SARS-CoV-2 infection
Cellular & Molecular Immunology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.