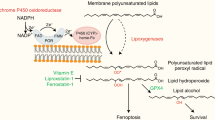
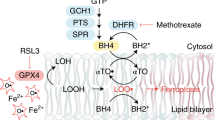
Abstract
Ferroptosis is a form of cell death that has received considerable attention not only as a means to eradicate defined tumour entities but also because it provides unforeseen insights into the metabolic adaptation that tumours exploit to counteract phospholipid oxidation1,2. Here, we identify proferroptotic activity of 7-dehydrocholesterol reductase (DHCR7) and an unexpected prosurvival function of its substrate, 7-dehydrocholesterol (7-DHC). Although previous studies suggested that high concentrations of 7-DHC are cytotoxic to developing neurons by favouring lipid peroxidation3, we now show that 7-DHC accumulation confers a robust prosurvival function in cancer cells. Because of its far superior reactivity towards peroxyl radicals, 7-DHC effectively shields (phospho)lipids from autoxidation and subsequent fragmentation. We provide validation in neuroblastoma and Burkitt’s lymphoma xenografts where we demonstrate that the accumulation of 7-DHC is capable of inducing a shift towards a ferroptosis-resistant state in these tumours ultimately resulting in a more aggressive phenotype. Conclusively, our findings provide compelling evidence of a yet-unrecognized antiferroptotic activity of 7-DHC as a cell-intrinsic mechanism that could be exploited by cancer cells to escape ferroptosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data and materials to draw the conclusions in this paper are presented in the main text, figures and the extended data figures. Raw data from the (epi)lipidomics experiments are available at the repository MASSIVE (https://doi.org/10.25345/C5F47H47Z). Further data can be received from the corresponding author on reasonable request. CRISPR analysis and uncropped blot are presented in the Supplementary Information. Source data are provided with this paper.
References
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions and therapeutic applications. Cell 185, 2401–2421 (2022).
Dos Santos, A. F., Fazeli, G., Xavier da Silva, T. N. & Friedmann Angeli, J. P. Ferroptosis: mechanisms and implications for cancer development and therapy response. Trends Cell Biol. 33, 1062–1076 (2023).
Korade, Z., Xu, L., Shelton, R. & Porter, N. A. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith–Lemli–Opitz syndrome. J. Lipid Res. 51, 3259–3269 (2010).
Yin, H., Xu, L. & Porter, N. A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 111, 5944–5972 (2011).
Angeli, J. P. F., Shah, R., Pratt, D. A. & Conrad, M. Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol. Sci. 38, 489–498 (2017).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008).
Yant, L. J. et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 (2003).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Ursini, F., Maiorino, M., Valente, M., Ferri, L. & Gregolin, C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta 710, 197–211 (1982).
Nishida Xavier da Silva, T., Friedmann Angeli, J. P. & Ingold, I. GPX4: old lessons, new features. Biochem. Soc. Trans. 50, 1205–1213 (2022).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Zou, Y. et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 10, 1617 (2019).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Shah, R., Shchepinov, M. S. & Pratt, D. A. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 4, 387–396 (2018).
Pedrera, L. et al. Ferroptotic pores induce Ca(2+) fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 28, 1644–1657 (2021).
Riegman, M. et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 22, 1042–1048 (2020).
Kandutsch, A. A. & Russell, A. E. Preputial gland tumor sterols. 3. A metabolic pathway from lanosterol to cholesterol. J. Biol. Chem. 235, 2256–2261 (1960).
Xu, L., Davis, T. A. & Porter, N. A. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 131, 13037–13044 (2009).
Li, Y. et al. 7-Dehydrocholesterol dictates ferroptosis sensitivity. Nature, https://doi.org/10.1038/s41586-023-06983-9 (2024).
Tzelepis, K. et al. A CRISPR dropout screen identifies genetic vulnerabilities and therapeutic targets in acute myeloid leukemia. Cell Rep. 17, 1193–1205 (2016).
Yuan, H., Li, X., Zhang, X., Kang, R. & Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 478, 1338–1343 (2016).
Dixon, S. J. et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015).
Zou, Y. et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585, 603–608 (2020).
Xu, L., Korade, Z. & Porter, N. A. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: product and mechanistic studies. J. Am. Chem. Soc. 132, 2222–2232 (2010).
Conrad, M. & Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019).
Garcia-Bermudez, J. et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122 (2019).
Westover, E. J. & Covey, D. F. The enantiomer of cholesterol. J. Membr. Biol. 202, 61–72 (2004).
Johnston, E. J., Moses, T. & Rosser, S. J. The wide-ranging phenotypes of ergosterol biosynthesis mutants and implications for microbial cell factories. Yeast 37, 27–44 (2020).
Shah, R., Farmer, L. A., Zilka, O., Van Kessel, A. T. M. & Pratt, D. A. Beyond DPPH: use of fluorescence-enabled inhibited autoxidation to predict oxidative cell death rescue. Cell Chem. Biol. 26, 1594–1607 (2019).
Zhang, X., Barraza, K. M. & Beauchamp, J. L. Cholesterol provides nonsacrificial protection of membrane lipids from chemical damage at air–water interface. Proc. Natl Acad. Sci. USA 115, 3255–3260 (2018).
McLean, L. R. & Hagaman, K. A. Effect of lipid physical state on the rate of peroxidation of liposomes. Free Radic. Biol. Med. 12, 113–119 (1992).
Do, Q. et al. Development and application of a peroxyl radical clock approach for measuring both hydrogen-atom transfer and peroxyl radical addition rate constants. J. Org. Chem. 86, 153–168 (2021).
Bacellar, I. O. L. et al. Photosensitized membrane permeabilization requires contact-dependent reactions between photosensitizer and lipids. J. Am. Chem. Soc. 140, 9606–9615 (2018).
Friedmann-Angeli, J. P., Miyamoto, S. & Schulze, A. Ferroptosis: the greasy side of cell death. Chem. Res. Toxicol. 32, 362–369 (2019).
Schmitz, R. et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490, 116–120 (2012).
Bonfiglio, F. et al. Inherited rare variants in homologous recombination and neurodevelopmental genes are associated with increased risk of neuroblastoma. EBioMedicine 87, 104395 (2023).
Eagle, K. et al. An oncogenic enhancer encodes selective selenium dependency in AML. Cell Stem Cell 29, 386–399 (2022).
Ubellacker, J. M. et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585, 113–118 (2020).
Zou, Y. et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 10, 1617 (2019).
Xu, L. & Porter, N. A. Reactivities and products of free radical oxidation of cholestadienols. J. Am. Chem. Soc. 136, 5443–5450 (2014).
Zilka, O. et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent. Sci. 3, 232–243 (2017).
Lu, Y. et al. MYCN mediates TFRC-dependent ferroptosis and reveals vulnerabilities in neuroblastoma. Cell Death Dis. 12, 511 (2021).
Floros, K. V. et al. MYCN-amplified neuroblastoma is addicted to iron and vulnerable to inhibition of the system Xc-/glutathione axis. Cancer Res. 81, 1896–1908 (2021).
Alborzinia, H. et al. MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat. Cancer 3, 471–485 (2022).
Alborzinia, H. et al. LRP8-mediated selenocysteine uptake is a targetable vulnerability in MYCN-amplified neuroblastoma. EMBO Mol. Med. 15, e18014 (2023).
Kim, H. Y. et al. Inhibitors of 7-dehydrocholesterol reductase: screening of a collection of pharmacologically active compounds in Neuro2a cells. Chem. Res. Toxicol. 29, 892–900 (2016).
Hall, P. et al. Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith–Lemli–Opitz syndrome. Mol. Genet. Metab. 110, 176–178 (2013).
Francis, K. R. et al. Modeling Smith–Lemli–Opitz syndrome with induced pluripotent stem cells reveals a causal role for Wnt/beta-catenin defects in neuronal cholesterol synthesis phenotypes. Nat. Med. 22, 388–396 (2016).
Sever, N. et al. Endogenous B-ring oxysterols inhibit the Hedgehog component Smoothened in a manner distinct from cyclopamine or side-chain oxysterols. Proc. Natl Acad. Sci. USA 113, 5904–5909 (2016).
Acknowledgements
J.P.F.A. acknowledges the support of the Junior Group Leader programme of the Rudolf Virchow Center, University of Würzburg, the Deutsche Forschungsgemeinschaft (DFG) WE 5719/2-1, FR 3746/3-1, FR 3746/5-1 and FR 3746/6-1. J.P.F.A, I.W. and M.K. acknowledge the DFG CRC205 (INST 269/886-1). J.P.F.A. and R.C.B. are grateful for the support provided by the Interdisziplinäres Zentrum für klinische Forschung (IZKF, B-424) and the Deutsche Jose Carreras Leukämie Stiftung (DJCLS 01 R/2022). R.C.B. acknowledges the DFG through grant BA 1596/7-1. A.T. acknowledges the DKTK joint funding project ‘RiskY-AML’; the ‘Integrate-TN’ Consortium funded by the Deutsche Krebshilfe, the Dietmar Hopp Foundation and the European Research Council (ERC; AdG-101055270). M.F. receives financial support from the German Federal Ministry of Education and Research (BMBF) in the framework of the e:Med research and funding concept for SysMedOS project and FERROPath (01EJ2205A), ‘Sonderzuweisung zur Unterstützung profilbestimmender Struktureinheiten 2021’ by the SMWK, TG70 by Sächsische Aufbaubank and SMWK, the measure is co-financed with tax funds on the basis of the budget passed by the Saxon state parliament, Deutsche Forschungsgemeinschaft (FE 1236/5-1); further thanks goes to R. Hoffmann (Institute of Bioanalytical Chemistry, University of Leipzig) for providing access to his laboratory. M.C. acknowledges support from the DFG CO 291/7-1, the DFG SPP 2306 (CO 291/9-1, CO 291/10-1), BMBF VIP+ program NEUROPROTEKT (03VP04260) and the ERC (grant no. GA 884754). D.B.K. is grateful to the Fonds of the Chemical Industry for a Liebig fellowship. D.A.P. would like to thank the Natural Sciences and Engineering Council of Canada and the Canada Foundation for Innovation for their support. S.M. and A.I. acknowledge support from the São Paulo Research Foundation (FAPESP) 2013/07937-8 (CEPID Redoxoma) and 2017/13804-1. T.C.G.-M. and K.M. received support from the National Institutes of Health NIMH R01 MH110636. J.K.S. acknowledges National Science Foundation grants HDR: DIRSE-IL 1940169 and RAPID 2031614. D.F.C. acknowledges the NIH through grant P50 MH122379 and R01 HL067773. Further support through the DFG priority program SPP 2306 is acknowledged by M.F., M.C., D.B.K., A.G.J.S., A.T. and J.P.F.A. We are grateful to excellent technical assistance of T. Henninger, Z. Donova and A. Haberberger.
Author information
Authors and Affiliations
Contributions
F.P.F. carried out most of the in vitro experiments with contributions from H.N., A.F.S., T.N.X.S., S.A., Z.C. S.M., and A.I. S.M.L. performed the CRISPR-based screen. Epilipidomics analysis were performed by P.N. and M.F. FENIX assays and corresponding LC/MS/MS and UV/Vis experiments were performed by O.Z., E.L.S. and I.E. with support from D.A.P. L.P. and A.G.S. contributed to the study of truncated vesicles permeabilization and studies using PhotoPC. Synthesis and characterization of PhotoPC was performed by M.B.S. and D.B.K. D.C. synthesized ent-cholesterol. F.G. and L.E.S.N performed and analysed the yeast spot assays. B.M. carried the analysis determining selenium content. H.A. designed and conducted in vivo experiments, followed by the implementation of related analyses. C.K., N.A., K.K. and B.K. assisted with in vivo experiments and subsequent analyses. F.P.F, V.K. and K.B. were responsible for performing and analysing the MM1S xenograft experiments. A.H. and P.I. synthesize and characterized the specificity of DHCR7 inhibitor RB38. T.C.G.M. and K.M. performed the quantification of 7-DHC oxidation products. W.S. contributed with lipidomics and sterol detections and analysis. L.K. and J.K.S. conducted structural modelling. M.C., G.W.B., A.T., R.C.B., S.D., S.M., A.W., M.K. and I.W. contributed with reagent, critical information and/or platforms. J.P.F.A. initiated, supervised the study and conceived the experimental plan. All authors contributed with discussion and data interpretation and read and agreed on the content of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Andreas Linkermann, Markus Müschen, Ned Porter and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Lipidomic characterization of DHCR7-deficient cells.
a, Lipidomics analysis of HT1080 cells expressing a Cas9 containing lentiviral vector co-expressing sgRNA targeting DHCR7 or EGFP as a control. Represented are the total amount of PE containing PUFA and the ratio of mono- to polyunsaturated fatty acids (MUFA/PUFA) in PE species. Data are represented as mean values ± s.d. of n = 3 technical replicates (from 10 cm plate) performed once. b, Fatty acid composition of PE species in the indicated cell lines. Data are representative of mean values ± s.d. of n = 3 technical replicates (6 cm plate) performed twice. c, Principal component analysis of PE composition data.
Extended Data Fig. 2 DHCR7 deficiency impact on ferroptosis and other cell death modalities.
a and b, Dose-dependent toxicity of RSL3 and FIN56 in DHCR7-Knockout clonal cell lines generated in the HT1080 (a) and PFa1 (b) cell lines. c, Levels of 7-DHC in MDA-MB-435 parental lines untreated and treated with a DHCR7 inhibitor (RB38 [500 nM]) and three independent DHCR7-KO clones. Data are represented as mean values ± s.d. of n = 2 technical replicates (from 10 cm plate) performed once. d, Dose-dependent toxicity of RSL3, FIN56, ML210, Erastin, Atheronal, Brefeldin-A, PLX-4032, Carfilzomib, TBOOH, Auranofin; Bortezomib and Docetaxel in MDA-MB-435 parental cells and the three DHCR7 knockout clones. Cell viability was assessed after 48 h using Alamar blue and data are representative of mean ± s.d. of n = 3 technical replicates of 2 independent experiments.
Extended Data Fig. 3 Characterization of HT1080 DHCR7-deficient clonal cell line.
a, Graphical representation of the strategy used to generate DHCR7-deficient cells with defined genomic alterations. b, Representative PCR of the pools and single clones derived thereof. c, Schematic representation of the sequencing results obtained from the PCR product (in blue) covering the edited region (in red) in comparison with the wild-type product. d, Sequencing chromatogram obtained from the edited allele. e, Relative quantification of 7-DHC and levels of Cholesterol in HT1080 Cas9 WT, DHCR7-Knockout clone (DHCR7-KO) and the corresponding DHCR7-KO reconstituted with an empty lentiviral vector (mock) or overexpressing DHCR7. Data are the mean ± s.d. of n = 3 wells of a 6-well plate from one representative experiment. f, Dose-dependent toxicity of RSL3 and FIN56 in HT1080 Cas9 DHCR7-KO clone and overexpressing DHCR7 or mock. g, Dose-dependent toxicity of ML210, Erastin, Carfilzomib, TBOOH, Atheronal B, Brefeldin-A, Auranofin and Docetaxel in HT1080 Cas9 DHCR7-KO clone transduced with a mock or a DHCR7 expressing vector. Cell viability was assessed after 48 h using Alamar blue and data are representative of mean ± s.d. of n = 3 technical replicates 2 independent experiments. *p < 0.05; two-way ANOVA (e, f).
Extended Data Fig. 4 Impact of 7-DHC accumulation on ferroptosis.
a, Schematic depiction of cholesterol biosynthesis, highlighting the pharmacological targets of the enzymes used in the present work. b, Dose-dependent toxicity of RSL3 in DHCR7 WT and knockout HT1080 cells in the presence of pharmacological agents modulating cholesterol biosynthesis. Concentrations for the different inhibitors are: atorvastatin [1 µM], Amorolfine [500 nM], Tasin-1 [500 nM], Tamoxifen [1 µM] and RB38 [500 nM]. c, Dose-dependent toxicity of Paclitaxel and Auranofin in HT1080 Cas9 DHCR7/SC5D knockout transduced with SC5D and/or DHCR7. d, Effect of sterols and squalene supplementation (10 µM) on RSL3 toxicity in cell expressing a control and two independent sgRNA targeting DHCR7. e, Effect of sterol supplementation on a genetic model of Gpx4 deficiency, i.e Pfa1 cells treated with TAM. f, Flow cytometry analysis of BODIPY 581/591 C11 oxidation in HT1080 cell line induced by RSL3 treatment ([100 nM], 5 h) in cells pretreated for 16 h with 10 µM of different sterols. g, Impact of exogenous free cholesterol and ent-cholesterol on the sensitivity of DHCR7-deficient cells to GPX4 inhibitors. h, Relative quantification of 7-DHC and Cholesterol levels in DHCR7-deficient cells treated with cholesterol and ent-cholesterol (8 µM). i, 7-DHC levels in HT1080 Cas9 DHCR7-KO clone and pool of HT1080 expressing two independent sgRNA targeting DHCR7 treated with Cholesterol (5 µM). Data are representative data of mean ± s.d. of n = 3 technical replicate of a 96-well plate (b-e) or 6-well (f-i) performed twice. Cell viability was assessed after 48 h (b-d) or 72 h (e, f) using Alamar blue and data are representative of mean ± s.d. of n = 3 technical replicates (96-well plate) performed three times.
Extended Data Fig. 5 Influence of cholesterol low conditions on the antiferroptotic activity of the 7-DHC/DHCR7 axis.
a, Quantification of cholesterol in FBS samples treated with fumed silica (20 g/L). Results are representative of one batch preparation used throughout this experiments. b, Relative quantification of 7-DHC and Cholesterol levels in HT1080 cells expressing a control and a DHCR7 targeting sgRNA grown in normal and delipidated FBS (dlFBS). c, Assessment of the response to RSL3 of DHCR7-deficient and proficient cells in FBS and dlFBS containing the indicated metabolites. d, Immunoblot analysis of ferroptosis regulators, FSP1, ACSL4 and GPX4 in cells grown in FBS and dlFBS. e, Immunoblot analysis of LRP8 and GPX4 in the indicated cell lines grown in FBS and dlFBS in the presence of the specified sterols (10 µM, 48 h). f, Total quantification of selenium by ICP-MS in FBS and dlFBS. g, Strategy and validation of A375 LDLR-KO cell lines using primers specific for the LDLR transcript. h, Assessment of SREBP2 target genes (DHCR7, HMGR, MSMO1 and MVK) in LDLR proficient and deficient cells. i, Immunoblot of LRP8 and GPX4 in the indicated cell lines. j, Assessment of uptake capacity of fluorescently labelled LDL in LDLR proficient and deficient cells. Visualization (j) and quantification of LDL (k) or cholesterol upon Tasin-1 (500 nM) treatment for 48 h (l) in LDLR proficient and deficient cells. m, Effect of LDLR loss on ferroptosis induction (ML210 + 2 µM iFSP1) in RB38 (500 nM) and Tasin-1 (500 nM) treated cells. Data are representative of mean ± s.d. of n = 2 (f, g), n = 3 (b, c, l, m), n = 4 (h) or n = 12 (k) technical replicates of a 96-well plate (c, m, j, k) or 6-well plate (b, g, h, l, m) performed twice. Cell viability was assessed after 72 h using Alamar blue (c, m).
Extended Data Fig. 6 Role of B-ring unsaturated sterol in ferroptosis.
a, Chemical structure of 7-DHC and ergosterol highlighting the conjugated double-bond. b, Effect of sterols and squalene supplementation (5 µM) on RSL3-induced cell death in the HT1080 cell line. Cell viability was assessed after 48 h using Alamar blue, data are representative of mean ± s.d. of n = 3 technical replicates from one representative of 2 independent experiments. c, Schematic representation of the ergosterol biosynthesis pathway in S. cerevisae, highlighting the major products reported to accumulate in these strains. d, Spot dilutions of the indicated strains of S. cerevisiae treated with the designated PUFAs (50 μM).
Extended Data Fig. 7 Impact and consequence of 7-DHC on phospholipid peroxidation.
a, Rate of initiation (Ri) in each soy PC liposome composition. b, Dynamic light scattering assessment of the impact of different sterols on the integrity of liposomes c, Scheme of the formation of PLPC-OOH, DLPC-OOH and DLPC-2OOH during autoxidation of soy PC that can be analysed by LC-MS/MS using MRM. d, The resulting profiles of PLPC-OOH, DLPC-OOH and DLPC-2OOH formation over time (integrations are relative to an internal standard (prostaglandin B2). e, Calculated rates from linear regression of the data related to d. f, Representative UV-Vis spectra obtained from a sample of soy PC with 8 mol% 7-DHC during autoxidation. Spectra were processed by subtracting the background trace of vehicle liposomes immediately after the addition of DTUN. Loss of 7-DHC was plotted from the 294 nm peak (inset) with concentrations determined from a standard curve from liposomes prepared with soy PC with inhibitor and added 7-DHC (see Supporting Information). g, Standard curve for 7-DHC prepared in either 95% EtOH or in soy PC liposomes with inhibitor. h, Time course of iron/ascorbate mediated oxidation of Egg-PC and sterol consumption in liposomes containing cholesterol, lathosterol or 7-DHC monitored via HPLC-UV detection (235 nm for PCOOH, 205 nm for cholesterol and lathosterol and 275 nm for 7-DHC). i, Quantification of 7-DHC and secondary oxidation products of 7-DHC in HT1080 SC5D/DHCR7 knockout cells expressing empty vector (black) and SC5D (red) upon 200 nM RSL3 with and without 500 nM Lip1 (6 h). Data are the mean ± s.d of n = 6 wells of a 10 cm plate from two independent experiments, *p < 0.05 two-way ANOVA (i). Each reaction (b, d, e, f, h) was repeated three times and is reported as the mean ± s.d for the kinetic plot (d) or error propagation from the slopes of d derived from linear regression.
Extended Data Fig. 8 Impact of ferroptosis inhibitors on oxidant mediated liposomal rupture.
a, Schematic representation of the CF/liposome assay used to monitor vesicle permeability. b, CF release from CF encapsulated liposomes generated using different sterols. CF release was stimulated using a mixture of iron and ascorbate (10 µM and 100 µM respectively). c, Impact of Lip1 on CF release from CF encapsulated liposomes containing cholesterol or 7-DHC. d, CF release in vesicles containing different ferroptosis inhibitors (10 µM) stimulated using a mixture of iron and ascorbate (20 µM and 200 µM respectively) in the absence (left panel) or in the presence of Ferrostatin-1 (Fer-1; right panel). Data are representative of mean ± s.d. of n = 3 technical replicates of 2 independent experiments.
Extended Data Fig. 9 Role of truncated phospholipid in membrane permeability.
a, Structure of selected truncated PC and related molecules tested. b Impact of native, peroxidised and truncated PC species in CF permeabilization. c, CF release in liposomes treated with different PC truncated species in a time and dose-dependent manner. d, Cell death induction by different PC truncated species in HT1080 cells in a time and dose-dependent manner. e, C50 of different truncated PL on CF release from liposomes containing 7-DHC or cholesterol. f, Schematic representation for the chemical basis of the PhotoPC probe: photoactivation leads to the generation of a defined mixture between the oxidized derivative PhotoOx-PC and the truncated derivative PhotoTrunc-PC. g, CF release in liposomes treated with PhotoPC before and after photoactivation in response to dose (fixed 1 h exposure) and time (fixed 365 µM). h, assessment of cell death (Draq7 positive) induced by equimolar concentrations of PhotoPC and PhotoTrunc-PC in HT1080 cells. Data are the mean ± s.d. of n = 6 wells of a 96-well plate from one representative of two independent experiments. i, Schematic representations of the events leading to the formation of truncated phospholipid species of formation of oxidatively truncated (phospho)lipid species and 7-DHC impact on it. Proferroptotic players are depicted in red and suppressing events are depicted in green.
Extended Data Fig. 10 Conjugation at the omega position affects ferroptosis sensitivity.
a, Lipidomics analysis of WT and ACSL4 KO HT1080 cell lines incubated for 16 h with αLNN and γLNN (20 µM). Presented are the total amount of PE containing PUFA and the ratio of mono- to polyunsaturated fatty acids (MUFA/PUFA) in PE species. Mean values ± s.d. of n = 3 technical replicates (10 cm plate) performed twice. b, Fatty acid composition of PE species in WT and ACSL4 KO HT1080 cell lines incubated for 16 h with αLNN and γLNN (20 µM). Mean values ± s.d. of n = 3 technical replicates (10 cm plate) performed twice. c, Assessment of the impact of αLNN and γLNN [20 µM] re-senitization on RSL3-induced ferroptosis. Cell viability was assessed after 24 h measuring PI incorporation. Mean values ± s.d. of n = 3 technical replicates (6 cm plate) performed twice are presented. d. Dose-dependent effect of α-LNN and γ-LNN on RSL3 mediated toxicity in HT1080 ACSL4 KO cell lines. Data are the mean ± s.d. of n = 6 wells of a 96-well plate from one representative of two independent experiments. *p < 0.05; two-way ANOVA.
Extended Data Fig. 11 Impact of 7-DHC accumulation on BL growth.
a, Impact of selected pharmacological inhibitors of DHCR7 (RB38) and Lip1 on Multiple Myeloma cell line OPM2 and the BL cell lines RAMOS, BL02 and BL30 grown in the absence of thiols and pyruvate. b, Relative quantification of 7-DHC and cholesterol levels in cell lines treated with RB38 and Tasin-1. Data are the mean ± s.d. of n = 3 wells, of a 6-well plate from one representative experiment.
Extended Data Fig. 12 Impact of DHCR7 loss in vivo.
a, Immunoblot for DHCR7 in MM1S cells DHCR7-proficient and deficient. Representative of n = 2. b, Dose-dependent toxicity of ML210 in DHCR7WT or DHCR7KO in the presence of indicated treatments. c, Tumour growth upon implantation of MM1S DHCR7WT (n = 10) or DHCR7KO (n = 10). Data are mean ± SEM; p value n.s., Mann–Whitney test one-tailed. In each box, horizontal lines denote mean values, while the box contains the 25th to 75th percentiles of dataset and whiskers mark the 5th and 95th percentiles. d, Representative luminescence images of mice from c. e, Schematic of tail vein injection of DHCR7WT (n = 5) or DHCR7KO (n = 5) BL41 cell in mice under selenium-adequate or -deprived diet. f, Immunoblot for GPX4 from tissues of animals related to e. g, Kaplan–Meier plot displaying tumour-free survival (TFS) for mice injected with DHCR7WT (n = 5) or DHCR7KO (n = 5) BL41 cells. Data represent the mean ± s.e.m.; Mann–Whitney test one-tailed; A Log-rank (Mantel–Cox) test was conducted for statistical analysis, p values are indicated. h, Luminescence images related to g. i, Immunoblot for DHCR7 in SK-N-DZ DHCR7KO and DHCR7WT. j, Relative quantification of cholesterol and 7-DHC levels in SK-N-DZ cells treated with RB38 and Tasin-1. Data are the mean ± s.d. of n = 3 wells from one representative experiment. k, Dose-dependent toxicity of ML210 in the indicated cells. l, Flow cytometry analysis of BODIPY 581/591 C11 oxidation in SK-N-DZ cells induced by RSL3 treatment ([100 nM], 3 h) and Lip1 500 nM. m, Schematic representation illustrates an orthotopic mouse model created by transplanting DHCR7WT or DHCR7KO SK-N-DZ cells into the right adrenal gland of NSG mice. n, Kaplan- Meier plot displaying TFS for mice injected orthotopically with DHCR7WT (blue, n = 6) or DHCR7KO (red, n = 9) SK-N-DZ cells; *p < 0.05, A Log-rank (Mantel–Cox) test was conducted for statistical analysis. o, Lung colonization was evaluated (left panel) in mice orthotopically transplanted with SK-N-DZ neuroblastoma cells, with DHCR7WT (red, n = 6) or DHCR7KO (blue, n = 9), using ex vivo lung bioluminescence analysis (right panel);. p, Representative examples of evidence of metastases from o (indicated by green circle lines), determined by Hematoxylin and Eosin staining from samples of n. Scale bar: 500 µM. Cell viabilities were assessed after 72 h using Alamar blue, data are mean ± s.d. of n = 3 replicates from one representative of three independent experiments (b, k). RB38 (500 nM) and Tasin-1 (500 nM) (b, j, k). Panels created with BioRender.com (e, m).
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Freitas, F.P., Alborzinia, H., dos Santos, A.F. et al. 7-Dehydrocholesterol is an endogenous suppressor of ferroptosis. Nature 626, 401–410 (2024). https://doi.org/10.1038/s41586-023-06878-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06878-9
This article is cited by
-
Cholesterol business: life or death by rust
Signal Transduction and Targeted Therapy (2024)
-
Natural inhibitor found for cell death by ferroptosis
Nature (2024)
-
B-ring sterols to the rescue
Nature Reviews Cancer (2024)
-
Enhancing 7-dehydrocholesterol suppresses brain ferroptosis and tissue injury after neonatal hypoxia–ischemia
Scientific Reports (2024)
-
Targeting ferroptosis for treating kidney disease
Clinical and Experimental Nephrology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.