Abstract
Ageing is a critical factor in spinal-cord-associated disorders1, yet the ageing-specific mechanisms underlying this relationship remain poorly understood. Here, to address this knowledge gap, we combined single-nucleus RNA-sequencing analysis with behavioural and neurophysiological analysis in non-human primates (NHPs). We identified motor neuron senescence and neuroinflammation with microglial hyperactivation as intertwined hallmarks of spinal cord ageing. As an underlying mechanism, we identified a neurotoxic microglial state demarcated by elevated expression of CHIT1 (a secreted mammalian chitinase) specific to the aged spinal cords in NHP and human biopsies. In the aged spinal cord, CHIT1-positive microglia preferentially localize around motor neurons, and they have the ability to trigger senescence, partly by activating SMAD signalling. We further validated the driving role of secreted CHIT1 on MN senescence using multimodal experiments both in vivo, using the NHP spinal cord as a model, and in vitro, using a sophisticated system modelling the human motor-neuron–microenvironment interplay. Moreover, we demonstrated that ascorbic acid, a geroprotective compound, counteracted the pro-senescent effect of CHIT1 and mitigated motor neuron senescence in aged monkeys. Our findings provide the single-cell resolution cellular and molecular landscape of the aged primate spinal cord and identify a new biomarker and intervention target for spinal cord degeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw sequencing data for monkey spinal cord tissues have been deposited at the Genome Sequence Archive (GSA) database under accession number CRA005773. Sequencing raw data for hMNs are deposited in the GSA-Human database under the accession of HRA001774. Source data are provided with this paper.
Code availability
The code used to perform bioinformatics analysis in this study is available at GitHub (https://github.com/Jiam1ng/spinal_cord_aging).
References
Roberts, E. A systems approach to aging, Alzheimer’s disease, and spinal cord regeneration. Prog. Brain Res. 86, 339–355 (1990).
Leiras, R., Cregg, J. M. & Kiehn, O. Brainstem circuits for locomotion. Annu. Rev. Neurosci. 45, 63–85 (2022).
Arber, S. Motor circuits in action: specification, connectivity, and function. Neuron 74, 975–989 (2012).
Tindle, J. & Tadi, P. Neuroanatomy, Parasympathetic Nervous System. StatPearls [internet] https://www.ncbi.nlm.nih.gov/books/NBK553141 (31 Oct 2022).
Wagner, J. U. G. et al. Aging impairs the neurovascular interface in the heart. Science 381, 897–906 (2023).
Blum, J. A. et al. Single-cell transcriptomic analysis of the adult mouse spinal cord reveals molecular diversity of autonomic and skeletal motor neurons. Nat. Neurosci. 24, 572–583 (2021).
Zhang, C., Goto, N., Suzuki, M. & Ke, M. Age-related reductions in number and size of anterior horn cells at C6 level of the human spinal cord. Okajimas Folia Anat. Jpn. 73, 171–177 (1996).
Piekarz, K. M. et al. Molecular changes associated with spinal cord aging. Geroscience 42, 765–784 (2020).
Maxwell, N. et al. α-Motor neurons are spared from aging while their synaptic inputs degenerate in monkeys and mice. Aging Cell 17, e12726 (2018).
Butt, A. M., Fern, R. F. & Matute, C. Neurotransmitter signaling in white matter. Glia 62, 1762–1779 (2014).
Zhang, H. et al. Nuclear lamina erosion-induced resurrection of endogenous retroviruses underlies neuronal aging. Cell Rep. 42, 112593 (2023).
Zhang, H. et al. Single-nucleus transcriptomic landscape of primate hippocampal aging. Protein Cell 12, 695–716 (2021).
Aguzzi, A., Barres, B. A. & Bennett, M. L. Microglia: scapegoat, saboteur, or something else? Science 339, 156–161 (2013).
Andreasson, K. I. et al. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J. Neurochem. 138, 653–693 (2016).
Mayne, K., White, J. A., McMurran, C. E., Rivera, F. J. & de la Fuente, A. G. Aging and neurodegenerative disease: is the adaptive immune system a friend or foe? Front. Aging Neurosci. 12, 572090 (2020).
Sathyamurthy, A. et al. Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep. 22, 2216–2225 (2018).
Rayon, T., Maizels, R. J., Barrington, C. & Briscoe, J. Single-cell transcriptome profiling of the human developing spinal cord reveals a conserved genetic programme with human-specific features. Development 148, dev199711 (2021).
Ma, S. et al. Decoding aging hallmarks at the single-cell level. Ann. Rev. Biomed. Data Sci. 6, 129–152 (2023).
Ma, Z. et al. A controlled spinal cord contusion for the rhesus macaque monkey. Exp. Neurol. 279, 261–273 (2016).
Courtine, G. et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 13, 561–566 (2007).
Sengupta, A. et al. Functional networks in non-human primate spinal cord and the effects of injury. NeuroImage 240, 118391 (2021).
Nout, Y. S. et al. Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury. Neurotherapeutics 9, 380–392 (2012).
Ye, Y. et al. SIRT2 counteracts primate cardiac aging via deacetylation of STAT3 that silences CDKN2B. Nature Aging 3, 1269–1287 (2023).
Wang, S. et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell 180, 585–600 (2020).
Sun, G. Q. et al. Single-cell transcriptomic atlas of mouse cochlear aging. Protein Cell 14, 180–201 (2023).
Cai, Y. et al. The landscape of aging. Sci. China Life Sci. 65, 2354–2454 (2022).
Aging Biomarker Consortium et al. Biomarkers of aging. Sci. China Life Sci. 66, 893–1066 (2023).
Alkaslasi, M. R. et al. Single nucleus RNA-sequencing defines unexpected diversity of cholinergic neuron types in the adult mouse spinal cord. Nat. Commun. 12, 2471 (2021).
Wang, S. et al. Deciphering primate retinal aging at single-cell resolution. Protein Cell 12, 889–898 (2020).
Takamori, Y. et al. Differential expression of nuclear lamin subtypes in the neural cells of the adult rat cerebral cortex. IBRO Rep. 5, 99–109 (2018).
Oyston, L. J. et al. Neuronal lamin regulates motor circuit integrity and controls motor function and lifespan. Cell Stress 2, 225–232 (2018).
Liang, K. et al. The joint detection and classification model for spatiotemporal action localization of primates in a group. Neural Comput. Appl. 35, 18471–18486 (2023).
Hubler, Z. et al. Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature 560, 372–376 (2018).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271 (2019).
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014).
Bisht, K. et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat. Commun. 12, 5289 (2021).
Ruscitti, P. et al. Pro-inflammatory properties of H-ferritin on human macrophages, ex vivo and in vitro observations. Sci. Rep. 10, 12232 (2020).
Kiskinis, E. et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 14, 781–795 (2014).
Lee, J. K. & Bou Dagher, J. Regulator of G-protein signaling (RGS)1 and RGS10 proteins as potential drug targets for neuroinflammatory and neurodegenerative diseases. AAPS J. 18, 545–549 (2016).
Olah, M. et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11, 6129 (2020).
Sun, S. et al. A single-cell transcriptomic atlas of exercise-induced anti-inflammatory and geroprotective effects across the body. Innovation 4, 100380 (2023).
Mifflin, L. et al. A RIPK1-regulated inflammatory microglial state in amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 118, e2025102118 (2021).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Dong, Y. et al. Single-cell and spatial RNA sequencing identify perturbators of microglial functions with aging. Nat. Aging 2, 508–525 (2022).
Young, A. M. H. et al. A map of transcriptional heterogeneity and regulatory variation in human microglia. Nat. Genet. 53, 861–868 (2021).
Paolicelli, R. C. et al. Microglia states and nomenclature: a field at its crossroads. Neuron 110, 3458–3483 (2022).
Lee, C.-M. et al. Chitinase 1 regulates pulmonary fibrosis by modulating TGF-β/SMAD7 pathway via TGFBRAP1 and FOXO3. Life Sci. Alliance 2, e201900350 (2019).
Fitch, P. M., Howie, S. E. & Wallace, W. A. Oxidative damage and TGF-β differentially induce lung epithelial cell sonic hedgehog and tenascin-C expression: implications for the regulation of lung remodelling in idiopathic interstitial lung disease. Int. J. Exp. Pathol. 92, 8–17 (2011).
Patel, R. K., Prasad, N., Kuwar, R., Haldar, D. & Abdul-Muneer, P. M. Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury. Brain Behav. Immun. 64, 244–258 (2017).
Katsuno, M. et al. Transforming growth factor-β signaling in motor neuron diseases. Curr. Mol. Med. 11, 48–56 (2011).
Liu, Z. et al. Cross-species metabolomic analysis identifies uridine as a potent regeneration promoting factor. Cell Discov. 8, 6 (2022).
Geng, L. et al. Low-dose quercetin positively regulates mouse healthspan. Protein Cell 10, 770–775 (2019).
Geng, L. et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell 10, 417–435 (2019).
Li, Y. et al. Vitamin C alleviates aging defects in a stem cell model for Werner syndrome. Protein Cell 7, 478–488 (2016).
Geng, L. et al. A comparative study of metformin and nicotinamide riboside in alleviating tissue aging in rats. Life Med. https://doi.org/10.1093/lifemedi/lnac045 (2022).
Li, J. et al. Determining a multimodal aging clock in a cohort of Chinese women. Med 4, 825–848.e13 (2023).
Aging Biomarker Consortium et al. A framework of biomarkers for brain aging: a consensus statement by the Aging Biomarker Consortium. Life Med. https://doi.org/10.1093/lifemedi/lnad017 (2023).
Coverdell, T. C., Abraham-Fan, R. J., Wu, C., Abbott, S. B. G. & Campbell, J. N. Genetic encoding of an esophageal motor circuit. Cell Rep. 39, 110962 (2022).
Furlan, A. et al. Visceral motor neuron diversity delineates a cellular basis for nipple- and pilo-erection muscle control. Nat. Neurosci. 19, 1331–1340 (2016).
Philippidou, P., Walsh, C. M., Aubin, J., Jeannotte, L. & Dasen, J. S. Sustained Hox5 gene activity is required for respiratory motor neuron development. Nat. Neurosci. 15, 1636–1644 (2012).
Navarro, A. I. & Mandyam, C. D. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience 293, 35–44 (2015).
Wallin-Miller, K., Li, G., Kelishani, D. & Wood, R. I. Anabolic-androgenic steroids decrease dendritic spine density in the nucleus accumbens of male rats. Neuroscience 330, 72–78 (2016).
Chow, H. M. et al. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat. Neurosci. 22, 1806–1819 (2019).
Obernosterer, G., Martinez, J. & Alenius, M. Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat. Protoc. 2, 1508–1514 (2007).
Sun, S. et al. WNT signaling represses astrogliogenesis via Ngn2-dependent direct suppression of astrocyte gene expression. Glia 67, 1333–1343 (2019).
Dai, Z. M. et al. Stage-specific regulation of oligodendrocyte development by Wnt/β-catenin signaling. J. Neurosci. 34, 8467–8473 (2014).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Lee, C. M. et al. Chitinase 1 regulates pulmonary fibrosis by modulating TGF-β/SMAD7pathway via TGFBRAP1 and FOXO3. Life Sci. Alliance 2, e201900350 (2019).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Fleming, S. J. et al. Unsupervised removal of systematic background noise from droplet-based single-cell experiments using CellBender. Nat. Methods 20, 1323–1335 (2023).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337 (2019).
Russ, D. E. et al. A harmonized atlas of mouse spinal cord cell types and their spatial organization. Nat. Commun. 12, 5722 (2021).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Murchison, E. P. et al. The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science 327, 84–87 (2010).
Dzwonek, J. & Wilczynski, G. M. CD44: molecular interactions, signaling and functions in the nervous system. Front. Cell Neurosci. 9, 175 (2015).
Acknowledgements
We thank Y. Liu, D. Li, A. Wang and P. Yang for their help in experiments; Y. Yang for her help in MN differentiation; C. Zheng and G. Ren for their help in the CSF acquisition from monkeys; L. Wang, K. Liang and S. Yang for their help in mouse and monkey behavioural analysis; W. Li and Y. Huang for their help in monkey spinal cord acquisition; J. Jia for his help in FACS; J. Hao, H. Qin and X. Zhu for their help in image scanning; and L. Bai, Q. Chu, J. Lu, Y. Yang, R. Bai, L. Tian, X. Li and J. Chen for administrative assistance. This work was supported by the National Key Research and Development Program of China (2022YFA1103700, 2020YFA0804000, the STI2030-Major Projects-2021ZD0202400, 2020YFA0112200, 2019YFA0110100, 2020YFA0112200, 2022YFA1103800, 2019YFA0802202, 2020YFA0803401, 2021YFF1201000, 2020YFA0113400, 2021YFA1101401), the National Science Foundation of China (grant nos 82122024, 81921006, 82125011, 92149301, 92168201, 91949209, 92049304, 92049116, 32121001, 82192863, 82071588, 31970597, 82271600, 32000500, 32100937, 82090051), the CAS Project for Young Scientists in Basic Research (YSBR-076, YSBR-012 and YSBR-036), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16000000), the Program of the Beijing Natural Science Foundation (Z190019), the Informatization Plan of Chinese Academy of Sciences (CAS-WX2021SF-0301, CAS-WX2022SDC-XK14 and CAS-WX2021SF-0101), New Cornerstone Science Foundation through the XPLORER PRIZE (2021-1045), the Youth Innovation Promotion Association of CAS (E1CAZW0401, 2023092, 2020085, 2022083, Y201930), the Excellent Young Talents Program of Capital Medical University (12300927), The Project for Technology Development of Beijing-affiliated Medical Research Institutes (11000023T000002036310), the Excellent Young Talents Training Program for the Construction of Beijing Municipal University Teacher Team (BPHR202203105), the Young Elite Scientists Sponsorship Program by CAST (YESS20210002) and the CAS Special Research Assistant (SRA) Program.
Author information
Authors and Affiliations
Contributions
G.-H.L., W.Z. and J.Q. conceived and designed the study. S.S., Jingyi Li and XuebaoWang performed single-nucleus isolation for 10x Genomics and library construction for STRT-seq. Jiaming Li performed all bioinformatic analysis with assistance from S.M. S.S., M.W., Y. Yu, Xuebao Wang, Y. Yang and M.M. optimized and performed immunofluorescence imaging, immunohistochemistry experiments, in situ hybridizations and quantification, and generated the data shown in this manuscript. Le Sun and S.S. performed the electrophysiological assay in the hMNs, mice and monkeys. X.M., Z.C. and H.M. performed behavioural analysis of the monkeys. F.Z. performed lateral ventricle injection in mice. S.S, M.W. and Y. Yu performed human MN differentiation, human microglial culture and cell treatment experiments with assistance from H.Z., Z.-Q.T., C.-M.L. and Y.-J.W. Z.B., Liang Sun and T.J. contributed to human tissue procurement and ethics expertise. S.S., Jingyi Li, M.W., Y. Yu, Xuebao Wang, J. Lei, Z.W., K.Y., Y.Z. and S.Z. contributed to monkey and mouse spinal cord procurement and ethics expertise. M.M. and Q.W. drew the diagram graphs in the figures. G.B., Y.-J.W., Jian Li, Xiaoqun Wang, G.Z., T.J. and J.C.I.B. helped with the supervision of the study. S.S., Jiaming Li, S.W. and W.Z. wrote the first draft of the manuscript, with major input from J.R. All of the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.C.I.B. is an employee of Altos Labs.
Peer review
Peer review information
Nature thanks V. Wee Yong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
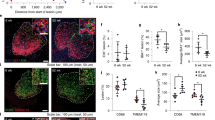
Extended Data Fig. 1 Aging-related phenotypes of spinal cords from cynomolgus monkeys.
a, Hematoxylin (DNA) staining of spinal cords from young and aged monkeys. Representative images (left) and quantifications of the area of total tissue (the transverse of total spinal cord parenchyma), white matter (WM), ventral grey matter (vGM), and dorsal grey matter (dGM) in spinal cord (right) are shown. Scale bars, 1 mm. b, Schematic diagram showing the statistical area of WM, dGM, and vGM in the spinal cord. c, Representative zoomed-out images of Fig. 1b. The arrows show the SA-β-Gal+ areas. Scale bars, 100 μm. d-e, Immunohistochemical staining of γ-H2A.X in the spinal cords of young and aged monkeys. Representative images (d) and the γ-H2A.X+ cells to each indicated zone cells in spinal cord (e) are shown. The arrows show the γ-H2A.X+ cells. Scale bars, 50 μm. Scale bars (zoomed in), 10 μm. f, Bar plot showing the percentage of γ-H2A.X+ MNs or γ-H2A.X+ non-MN cells in total spinal vGM cells from young and aged monkeys as shown in Extended Data Fig. 1d. A cell larger than 30 μm in cell soma diameter was considered a motor neuron (MN) in spinal vGM. g, Bar plot showing the percentage of γ-H2A.X+ MNs in total MNs from young and aged monkeys as shown in Extended Data Fig. 1d. h-i, Immunofluorescence staining of 4-HNE in the spinal WM, dGM, and vGM regions of young and aged monkey spinal cords. Representative images (h) and the mean intensity of 4-HNE fluorescence in each indicated zone (i) are shown. Scale bars, 50 μm. j-k, Immunofluorescence staining of 4-HNE and GFAP or P2RY12 in the spinal vGM region of young and aged monkey spinal cords. Representative images (j) and the mean intensity of 4-HNE fluorescence per MN or that per non-MN in spinal vGM (k) are shown. A cell larger than 30 μm in cell soma diameter was considered a MN in spinal vGM. All MNs and 300 non-MN cells (in the vGM region) from three discontinuous cross-sectional slices per monkey were detected. Scale bars, 10 μm. l-m, Oil Red O staining of the WM, dGM, and vGM regions of young and aged monkey spinal cords. The arrows indicate Oil Red O+ area. Representative images (l) and the percentage of Oil Red O+ area in total area of each indicated zone (m) are shown. Scale bars, 50 μm. Scale bars (zoomed in), 10 μm. n, Bar plot showing the percentage of Oil Red O+ MNs and Oil Red O+ non-MN cells in total spinal vGM cells from young and aged monkeys. A cell larger than 30 μm in cell soma diameter was considered a MN in spinal vGM. A signal of Oil Red O with more than six points in the MN was considered a positive MN. All MNs and 300 non-MN cells (from the vGM region) from three discontinuous cross-sectional slices per monkey were detected. The sum of the statistic values in MNs and non-MN cells was equal to the statistic of vGM in the Extended Data Fig. 1m. o, Bar plot showing the percentage of Oil Red O+ MNs in total MNs from young and aged monkeys, as shown in Extended Data Fig. 1l. p, Bar plot showing the percentage of ChAT+ cells in total spinal cells from young and aged monkeys as shown in Fig. 1c. q, RNA in situ hybridization (RNA-ISH) with TNS1 riboprobe in the spinal vGM region from young and aged monkeys. The arrows indicate TNS1+ MNs. Representative images (left) and the number of TNS1+ MNs (right) in each cross-sectional spinal cord slice (35 μm thickness) are shown. The dashed lines indicate the boundary of vGM. Scale bars, 100 μm. Data (a, e-g, i, k, and m-q) are shown as the mean ± s.e.m. P values were calculated using two-tailed unpaired t-tests. a.u., arbitrary unit. NA, not applicable. Three discontinuous cross-sectional slices per monkey (a, c-q). n = 8 monkeys per group (a, c-q).
Extended Data Fig. 2 Immunohistochemical staining of MN in spinal cords and schematic illustration of precise and fast motion analysis.
a, Immunohistochemical staining of NeuN or ChAT in adjacent sections from monkey spinal cords. Representative images of the vGM region (left) and the percentage of MNs or INs in total vGM neurons (right) are shown. The dashed lines show the boundary of the vGM region. NeuN, total neurons; ChAT, MNs; INs, NeuN-positive neurons minus ChAT-positive MNs. Scale bars, 100 μm. b, Immunohistochemical staining of ChAT or γ-H2A.X in the adjacent sections from young and aged monkey spinal cords. Representative images of the vGM region (left) and the percentage of γ-H2A.X+ MNs in total MNs (right) are shown. The arrows show the γ-H2A.X+ MNs. Scale bars, 100 μm. Scale bars (zoomed in), 20 μm. c, Immunofluorescence staining for 4-HNE and ChAT in the spinal vGM region from young and aged monkeys. Representative images (left) and the mean intensity of 4-HNE fluorescence per (ChAT+) MN (right) are shown. Scale bars, 20 μm. d, Immunofluorescence staining for H3K9me3 and ChAT in the spinal vGM region of young and aged monkeys. Representative images (left) and the mean intensity of H3K9me3 fluorescence per (ChAT+) MN (right) are shown. The dashed lines indicate the nuclei of MNs. Scale bars, 10 μm. e-f, Immunohistochemical staining of NeuN in spinal cords from young and aged monkeys. Representative images (e) and the percentage of total NeuN+ (left) or NeuN+ in dGM (right) in total spinal GM cells (f) are shown. The dashed lines show the boundary of the vGM and dGM regions. Scale bars, 200 μm. g, Bar plots showing the percentage of NeuN+ in vGM (left) in total spinal GM cells, as well as the percentage of NeuN+ in vGM (middle), and MNs (right) in total spinal vGM cells. A cell larger than 30 μm in cell soma diameter was considered a motor neuron in the spinal vGM. h, Co-staining of BTX and NF in neuromuscular junctions (NMJs) in skeletal muscle from young and aged monkeys. Representative images (left) and the percentage of nerve terminal area (BTX-positive) per 0.2 cm2 or the percentage of axon area (NF-positive) per 0.2 cm2 (right) are shown. Scale bars, 50 μm. i, Schematic illustration of precise and fast motion analysis with the joint detection and classification model with middle fusion approach (JDC-MF) network. A monkey was captured by three cameras. Deep learning algorithms and a 3D modelling approach were employed to accurately detect the monkey’s position, and to classify its actions into two types, analyse its upper limb activity, and estimate its motion in 3D space. j, Bar plot showing movement distance in four hours of young and aged monkeys. Data (a-h, and j) are shown as the mean ± s.e.m. P values were calculated using two-tailed unpaired t-tests. a.u., arbitrary unit. Three discontinuous cross-sectional slices per monkey (a-h). n = 8 monkeys per group (b-h). n = 16 monkeys per group (a). n = 8 in the young monkey group and n = 4 in the aged monkey group (j).
Extended Data Fig. 3 Transcriptomic analysis of young and aged monkey spinal cords.
a, Bar plot showing the numbers of detected genes for each sample of bulk RNA-seq. b, GSEA results for bulk RNA-seq of young and aged monkey spinal cords. c, Quantitative data for Fig. 2b. Left, the percentage of IBA1+ cells in total cells in spinal cords. Right, the percentage of P2RY12− IBA1+ cells in total spinal cells from young and aged monkeys. d, Quantitative data for Fig. 2b. Bar plot showing the number of P2RY12+ cells in spinal cords from young and aged monkeys, and the number of IBA1+ cells in spinal cords from young and aged monkeys. e, Immunofluorescence staining of MS4A7 and IBA1 in both AMF and spinal cords of young and aged monkeys. AMF, anterior median fissure. Scale bars, 100 μm. f, The percentage of MS4A7− cells or MS4A7+ cells in total spinal IBA1+ cells, the percentage of MS4A7+ IBA1+ cells in total spinal cells, and MS4A7− IBA1+ cells in total spinal cells. g, Immunofluorescence staining of CD68 and P2RY12 in spinal cords from young and aged monkeys. Representative images (left) and the percentage of CD68+ cells in total spinal P2RY12+ microglia in the spinal cord (right) are shown. Scale bars, 50 μm. h, Expression levels of CHIT1, GPNMB, GZMK, VCAM1, ICAM1, and CXCL8 analysed by bulk RNA-seq in young and aged monkey spinal cords. i, Scatter and violin plots showing the percentage of mitochondrial genes per cell in each sample of snRNA-seq. Chromium, 10x Genomics Chromium platform. STRT-seq, single-cell tagged reverse transcription sequencing. j, Mapping rate (line) and sequencing saturation (bar) for different samples in snRNA-seq data with the Chromium method for spinal cords. k-l, Box plot showing the UMI counts (k) and gene numbers (l) per cell for different snRNA-seq samples with the Chromium method for spinal cords. m, UMI counts per cell for different samples of STRT-seq data. The distribution of the UMI counts per cell (left) and the UMI counts for different samples (right) are shown. n, The gene numbers per cell for different samples of STRT-seq data. The distribution of the gene numbers per cell (left) and the gene numbers for different samples (right) are shown. Data are shown as the mean ± s.e.m. (c, d, f-g) or mean ± s.d. (h). P values were calculated using two-tailed unpaired t-tests (c, d, f-g) or Wilcoxon rank sum test (h). YF, young female; YM, young male; OF, old female; OM, old male. In box plots (k-n), the centre line indicates the median, the box edges indicate the interquartile range, and the whiskers extend from the quartiles to the minimum and maximum values. Three discontinuous cross-sectional slices per monkey (c, d, f, g). n = 8 monkeys per group (c, d, f-h).
Extended Data Fig. 4 snRNA-seq analysis of young and aged monkey spinal cords.
a, t-SNE plot showing the cell distribution of integrated datasets coloured by different snRNA-seq strategies. b, t-SNE plots showing the distribution of young and aged cells. c, Expression levels of marker genes of different cell types in Fig. 2c. d, Dot plot showing the expression of marker genes of different immune cells in the monkey spinal cord. OL, oligodendrocytes. e, Pie plot showing the proportions of OL and microglia in total cells. Boxplot showing the number of detected genes per cell in OL, microglia, and microglia/OL doublets. f, Scaled expression levels of the top 30 marker genes of different cell types (left) and enriched pathways of these marker genes (right). g, Density plot showing the distribution of aging DEGs across different cell types. Dot plot showing the top upregulated and downregulated DEGs in multiple cell types. h, Enriched pathways of age-related DEGs across different cell types of monkey spinal cords. The colour key from white to pink (blue) indicates the absolute value of the DE score from low to high. A positive or negative DE score indicates that upregulated (pink) or downregulated (blue) DEGs comprise more than half of the enriched genes in the corresponding pathways, respectively. DE, differential expression. APP, antigen processing and presentation. i, Heatmaps showing the pathway enrichment analysis for upregulated and downregulated aging-related DEGs in different cell types of male and female monkey spinal cords. j, Violin plots showing the gene set scores of senescence-related genes in young and aged monkey MNs. k, Violin plots showing gene set scores of ALS related pathways in young and aged monkey MNs. P values were calculated using the Wilcoxon rank sum tests. In box plots (e, j, k), the centre line indicates the median, the box edges indicate the interquartile range, and the whiskers extend from the quartiles to the minimum and maximum values.
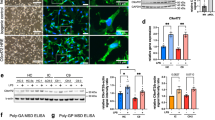
Extended Data Fig. 5 Microglia diversity analysis reveals the CHIT1-positive microglia in the monkey spinal cord.
a, Dot plots showing the marker genes (ordered by adjusted P value) for the three microglia states. b, Bar plot showing the cell proportions of microglia 1, microglia 2, and microglia 3 in total microglia. c, Cell proportions of microglia 1 and microglia 2 in total microglia from young and aged groups. The centre line indicates the median, the box edges indicate the interquartile range, and the whiskers extend from the quartiles to the minimum and maximum values. d, Bar plot showing the GO term analysis results of the top 50 marker genes for microglia 2 and microglia 3 in monkey spinal cords. e, Immunofluorescence staining for CHIT1 and CD68 in young and aged monkey spinal cords. Representative images (left) and the percentage of CD68+ or CD68− cells in total spinal CHIT1+ cells in the spinal cord (right) are shown. Scale bars, 50 μm. f, Immunofluorescence staining for CHIT1 and P2RY12 in the spinal WM and vGM regions of cervical (top), thoracic (middle) and lumbar (bottom) spinal cords in young and aged monkeys. Schematic diagram of spinal cord cephalocaudal axis segmentation (left), representative images (medium), and the percentage of spinal CHIT1+ cells in all spinal P2RY12+ microglia of cervical, thoracic and lumbar spinal cords in young and aged monkeys in the WM or vGM region (right) are shown. Scale bars, 25 μm. Data (c, e-f) are shown as the mean ± s.e.m. P values were calculated using a two-tailed unpaired t-test (f) or the Wilcoxon rank sum test (c). Three discontinuous cross-sectional slices per monkey (e, f). n = 8 monkeys per group (c, f). n = 16 monkeys (e).
Extended Data Fig. 6 Microglia diversity analysis demonstrates the absence of CHIT1-positive microglia in mouse spinal cord and primate brain.
a, Immunofluorescence staining for CHIT1, NeuN and IBA1 in GM and WM regions from young and aged monkey frontal lobes. n = 8 monkeys per group. Similar results were found in three discontinuous slices of coronal sections per monkey. Each slice contained intact grey matter structures and a portion of the white matter, within an area of 15 mm2. Scale bars, 100 μm. GM, grey matter. WM, white matter. b, Immunofluorescence staining for CHIT1 and IBA1 in the spinal cords from 2-, 13-, and 29-month-old C57BL/6 mice. n = 8 mice per group. Similar results were found in three discontinuous cross-sectional slices per mouse. Scale bars, 50 μm. c, Single-nucleus transcriptomic atlas of young and aged mouse spinal cords coloured by cell type (top) and age group (bottom). Ttn+, Ttn-positive cells. d, Dot plot showing the expression of marker genes of different cell types in mouse spinal cord. e, t-SNE plots showing the distribution of microglia in the mouse spinal cords. Upper, predicted microglia 3 is highlighted in red. Bottom, the points are coloured by age. f, Expression levels of the indicated genes. Top, expression levels of Chit1, Gpnmb, and Lgals3 in total microglia of mouse spinal cords. Bottom, expression levels of these three genes in both young and aged microglia of mouse spinal cords. g, Venn diagram showing the overlapped genes between the top 50 marker genes of microglia 3 in monkey spinal cord and predicted microglia 3 in mouse spinal cord. h, Heatmap showing the enriched pathways of marker genes for microglia 3 in monkey and mouse spinal cords. i, t-SNE plots showing the clusters and expression levels of P2RY12 and CHIT1 in the indicated datasets of myeloid cells, coloured by cluster (left) and expression level (right). j, Venn diagram showing the shared genes between core transcriptomic signatures of aging and neurodegenerative microglia and marker genes of microglia 3 of monkey spinal cord. k, ELISA analysis of CHIT1 concentration in young and aged human CSF and serum. Schematic diagram (top) and quantification for CHIT1 concentration in human CSF and serum (bottom) are shown. P values were calculated using two-tailed unpaired t-tests. Three biological replicates per individual. CSF, n = 3 individuals per group; serum, n = 40 individuals per group.
Extended Data Fig. 7 TGFBR expression in the spinal cord.
a, SA-β-Gal staining analysis and immunohistochemical staining of CHIT1 in the spinal vGM region from aged monkeys. Representative images (left) and the percentage of SA-β-Gal+ MNs in total MNs surrounded by CHIT1+ microglia with a distance of 100 μm in the aged spinal vGM region (right) are shown. Scale bars, 20 μm. b, Bar plot showing the percentage of CHIT1+ cells in WM or vGM in total CHIT1+ cells in spinal cords as quantification of Fig. 3d. c, Bar plot showing the percentage of CHIT1+ cells around the MNs in WM or vGM in total CHIT1+ cells in aged spinal cords. d, Bar plot showing the percentage of CHIT1+ cells adjacent to or distant from the MNs in total CHIT1+ cells in aged spinal cords. e, Representative images of Golgi staining of young and aged monkey spinal cords. The arrows indicate MNs (cells larger than 30 μm in cell soma diameter were considered as MNs in spinal vGM). Scale bars, 500 μm. f, Golgi staining of young and aged monkey spinal cords. Representative images (left) and quantification of spine number per 10 μm apical dendrites of MNs (right) are shown. More than 80 apical dendrites of MNs from three discontinuous cross-sectional slices per monkey were detected. Scale bars, 5 μm. g, Immunofluorescence staining of VAChT and ChAT in the spinal vGM region of young and aged monkeys. Representative images (left) and quantification of the number of puncta per MN (right) are shown. The arrows indicate the puncta. Scale bars, 10 μm. h, Immunofluorescence staining for VAChT and ChAT in the spinal vGM region from young and aged monkeys. VH, ventral horn. LH, lateral horn. Scale bars, 100 μm. The fields in the white rectangle are magnified and shown in Extended Data Fig. 7g. i, Schematic diagram of CHIT1+ microglia wrapping the MNs. j, Violin plots showing gene set scores of TGFBR signalling (left) and neuron apoptosis (right) in young and aged monkey MNs. The centre line indicates the median, the box edges indicate the interquartile range, and the whiskers extend from the quartiles to the minimum and maximum values. k, Representative images of immunofluorescence staining of TGFBR1 or TGFBR2 and ChAT in the monkey spinal vGM region. Scale bars, 25 μm. l, Representative images of immunofluorescence staining of TGFBR1/TGFBR2 and GFAP/IBA1 in the monkey spinal vGM region. Scale bars, 25 μm. m, Representative images of immunofluorescence staining of TGFBR1/TGFBR2 and GFAP/IBA1/ChAT in mouse spinal vGM region. Scale bars, 25 μm. n, Bar plots showing the mean TGFBR1 and TGFBR2 intensity per MN or per non-MN cells (right) in aged monkeys. All MNs and three hundreds non-MN cells (in the vGM region) from three discontinuous cross-sectional slices per monkey were detected. o, Representative images of immunofluorescence staining of TGFBR1 and ChAT or TGFBR2 and ChAT in the spinal WM of monkeys. Scale bars, 20 μm. Data (b-d, f-g, n) are shown as the mean ± s.e.m. P values were calculated using two-tailed unpaired t-tests (f-g, n) or the Wilcoxon rank sum test (j). a.u., arbitrary unit. Three discontinuous cross-sectional slices per monkey and n = 8 monkeys per group (a, c, d, f-h, k, l-o). n = 16 monkeys (b).
Extended Data Fig. 8 Intra-cerebrospinal fluid injection of CHIT1 induces accelerated MN senescence in mouse spinal cords.
a, Schematic diagram showing the time course of intra-cerebrospinal fluid injection of CHIT1 (1 ng per mouse) or Vehicle (equal volume of PBS (5 μL)) in 2-month-old C57BL/6 mice. CHIT1 was administered into the lateral ventricles every other day for 10 days. b, Immunofluorescence staining of p-SAMD2 and ChAT in spinal cords from vehicle- or CHIT1-treated C57BL/6 mice. Representative images (left) and the mean intensity of p-SMAD2 fluorescence per (ChAT+) MN (right) are shown. The arrows indicate the MNs. Scale bars, 50 μm. c, SA-β-Gal staining of spinal vGM sections from vehicle- or CHIT1-treated C57BL/6 mice. Representative images (left) and the percentage of SA-β-Gal-positive area in the total spinal vGM area or in the non-vGM area (right) are shown. Scale bars, 250 μm. The arrows indicate the MNs. Scale bars, 250 μm (lower magnification) and 25 μm (higher magnification). d, Double staining for SA-β-Gal and ChAT in the spinal cords of vehicle- or CHIT1-treated C57BL/6 mice. Representative images are shown on the left. The percentage of SA-β-Gal+ MNs in total MNs in the spinal vGM of vehicle- or CHIT1-treated C57BL/6 mice and the percentage of SA-β-Gal+ MN or SA-β-Gal+ non-MNs in total SA-β-Gal+ cells in spinal vGM of CHIT1-treated mice are shown on the right. Scale bars, 100 μm. e, Immunofluorescence staining of ChAT in spinal cords from vehicle- or CHIT1-treated C57BL/6 mice. Representative images (left) and the number of ChAT+ MNs in the spinal cord (right) are shown. The dashed lines indicate the boundary of vGM. The arrows indicate the MNs. Scale bars, 100 μm. f, Immunofluorescence staining of lamin B2 and ChAT in spinal cords from vehicle- or CHIT1-treated C57BL/6 mice. Representative images (left) and the mean lamin B2 intensity per (ChAT+) MN (right) are shown. Scale bars, 10 μm. The arrows indicate the MNs. g, Immunofluorescence staining of γ-H2A.X and ChAT in spinal cords from vehicle- or CHIT1-treated C57BL/6 mice. Representative images (left) and the percentage of γ-H2A.X+ MNs in the total MNs in spinal cord (right) are shown. The arrow indicates the γ-H2A.X+ MN. Scale bars, 30 μm. h, Representative evoked field currents from C57BL/6 mice distal limbs (left), and the statistics of amplitude of evoked currents (right) are shown. i, Co-staining of BTX and NF in NMJs in skeletal muscle from vehicle- or CHIT1-treated C57BL/6 mice. Representative images (left) and the percentage of nerve terminal area per 0.2 cm2 or the percentage of axon area per 0.2 cm2 (right) are shown. Scale bars, 100 μm. j, Representative images of Golgi staining of vehicle- or CHIT1-treated C57BL/6 mouse spinal cords. Scale bars, 500 μm. k, Representative images of Golgi staining showing MN morphology in young and aged monkey spinal cords. Zoomed-in from (j). Scale bars, 50 μm. l, Sholl analysis of Golgi staining showing basal and apical dendrites crossing through a series of concentric circles centred at the MN soma and spaced at 15 μm intervals of spinal cords from vehicle- or CHIT1-treated C57BL/6 mice. m, Golgi staining of spinal cords from vehicle- or CHIT1-treated C57BL/6 mice. Representative images (left) and quantification of spine numbers per 10 μm apical dendrites of neurons (right) are shown. More than 80 apical dendrites of MNs from three discontinuous cross-sectional slices per mouse were detected. Scale bars, 5 μm. n, Representative images of the open field test of vehicle- or CHIT1-treated C57BL/6 mice are shown on the left. Analysis of total distance travelled and mean speed of vehicle- or CHIT1-treated C57BL/6 mice throughout a ten-minute period are shown on the right. o, Bar plot showing the distance travelled in the treadmill test by vehicle- or CHIT1-treated C57BL/6 mice. p, Grip strength test (left) and grid test (right) of the four limbs of C57BL/6 mice treated with vehicle or CHIT1. Data (b-i, l-p) are shown as the mean ± s.e.m. P values were calculated using two-tailed unpaired t-tests. Three discontinuous cross-sectional slices per mouse (b-g, i, l). n = 8 C57BL/6 mice per group (a-g, i, l, n-p). n = 4 (h) or 7 (m) mice in Vehicle group and n = 5 (h) and 8 (m) mice in CHIT1 group.
Extended Data Fig. 9 Intra-cerebrospinal fluid injection of CHIT1 induces accelerated MN senescence in the spinal cords in nude mice.
a, Schematic diagram showing the time course of intra-cerebrospinal fluid injection of the vehicle or CHIT1 in 2-month-old nude mice. Every other day injection per mouse. The dose of CHIT1 was the same as that used for the C57BL/6 mice. b, Immunofluorescence staining of p-SAMD2 and ChAT in spinal cords from vehicle- or CHIT1-treated nude mice. Representative images (left) and the mean intensity of p-SMAD2 fluorescence per (ChAT+) MN (right) are shown. The arrows indicate the MNs. Scale bars, 50 μm. c, Immunofluorescence staining of ChAT in spinal cords from vehicle- or CHIT1-treated nude mice. Representative images (left) and the number of ChAT+ MNs in the spinal vGM (right) are shown. The dashed lines show the boundary of the vGM region. Scale bars, 100 μm. d, SA-β-Gal staining of the spinal vGM sections from vehicle- or CHIT1-treated nude mice. Representative images (left) and the percentage of SA-β-Gal-positive areas in total spinal vGM area (right) are shown. Scale bars, 100 μm. e-f, Co-staining for SA-β-Gal and ChAT in the spinal cords of vehicle- or CHIT1-treated nude mice. Representative images are shown in (e). The percentage of SA-β-Gal+ MNs in total MNs in vehicle- or CHIT1-treated nude mice and the percentage of SA-β-Gal+ MNs or SA-β-Gal+ non-MN cells in total SA-β-Gal+ cells in CHIT1-treated spinal vGM are shown in (f). Scale bars, 100 μm. g, Immunofluorescence staining of lamin B2 and ChAT in spinal cords from vehicle- or CHIT1-treated nude mice. Representative images (left) and the mean lamin B2 intensity per (ChAT+) MN (right) are shown. The arrows indicate the MNs. Scale bars, 20 μm. h, Grip strength test of the four limbs of nude mice treated with vehicle or CHIT1. Data (b-h) are shown as the mean ± s.e.m. P values were calculated using two-tailed unpaired t-tests. Three discontinuous cross-sectional slices per mouse (b-g) and n = 9 nude mice per group (a-h).
Extended Data Fig. 10 Intra-cerebrospinal fluid injection of CHIT1 induces accelerated MN senescence in monkey spinal cords.
a, ELISA analysis of CHIT1 concentration before vehicle- and CHIT1-treated monkey CSF at baseline. b, Imagiological diagnosis to confirm the implantation position of the Ommaya reservoir into the monkey ventricle by using 3D-reconstructed CT image. The arrows indicate the Ommaya reservoir. c, SA-β-Gal staining of the spinal vGM sections from vehicle- or CHIT1-treated monkey spinal cords. Representative images (left) and the percentage of SA-β-Gal-positive areas in the total vGM area (right) are shown. The arrows indicate SA-β-Gal-positive areas. The dashed lines indicate the boundary of vGM. Scale bars, 100 μm. d, The percentage of SA-β-Gal+ MNs or SA-β-Gal+ non-MN cells in total SA-β-Gal+ cells in the spinal vGM of CHIT1-treated monkeys. e, Immunofluorescence staining of 4-HNE in spinal cords from vehicle- or CHIT1-treated monkeys. Representative images (left) and the mean 4-HNE intensity per MN (right) are shown. The arrows indicate the MNs. Scale bars, 50 μm. f, Immunohistochemical staining of ChAT (left) or γ-H2A.X (right) in the adjacent sections from vehicle- or CHIT1-treated monkey spinal cords. Representative images of the vGM region (left) and the percentage of γ-H2A.X+ MNs in total MNs (right) are shown. Scale bars, 100 μm. Scale bars (zoomed in), 20 μm. The arrow indicates the γ-H2A.X+ MN. g, Representative images of MN morphology in vehicle- or CHIT1-treated monkey spinal cords. Scale bars, 100 μm. h, Sholl analysis of basal and apical dendrites crossing through a series of concentric circles centred at the MN soma and spaced at 15 μm intervals of spinal cords from vehicle- or CHIT1-treated monkeys. All MNs from three discontinuous cross-sectional slices per monkey were detected. i, Golgi staining of vehicle- or CHIT1-treated monkey spinal cords. Representative images (left) and quantification of spine numbers per 10 μm apical dendrites of neurons (right) are shown. More than 80 apical dendrites of MNs from three discontinuous cross-sectional slices per monkey were detected. Scale bars, 5 μm. j, Immunofluorescence staining of VAChT and ChAT in spinal cords from vehicle- or CHIT1-treated monkeys. Representative images (left) and the puncta number per MN (right) are shown. Scale bars, 50 μm. k, Co-staining of BTX and NF in the NMJ of skeletal muscle from vehicle- or CHIT1-treated monkeys. Representative images (left) and the percentage of nerve terminal area per 0.2 cm2 or the percentage of axon area per 0.2 cm2 (right) are shown. Scale bars, 50 μm. l, Representative evoked field currents in the distal limbs from vehicle- and CHIT1-treated monkeys (left), and the statistics of the amplitude of evoked currents (right) are shown. m, Line graph showing the cumulative change in movement distance in four hours of vehicle- or CHIT1-treated monkeys at baseline. The eight young monkeys were randomly divided into two groups, vehicle- and CHIT1-treated. The statistics data (Extended Data Fig. 10m–p) come from the eight young monkeys (Fig. 1i–k and Extended Data Fig. 2j). n, Bar plot showing movement distance in four hours of vehicle- or CHIT1-treated monkeys at baseline. o, Bar plot showing active time over a four-hour period of vehicle- or CHIT1-treated monkeys at baseline. p, Bar plot showing frequency of food grasping (in number of times) over a five-hour period of vehicle- or CHIT1-treated monkeys at baseline. Data (a, c, e-f, h-p) are shown as the mean ± s.e.m. P values were calculated using two-tailed unpaired t-tests. Three discontinuous cross-sectional slices per monkey (c-f, h-k) and n = 4 monkeys per group (a, c-f, h-k, m-p). n = 3 monkeys per group (l).
Extended Data Fig. 11 Senescence phenotypes of hMNs.
a, Immunofluorescence staining of ChAT (motor neuron marker) and MAP2 (pan-neuron marker) in hMNs differentiated from human embryonic stem cells (hESCs). Representative images (upper) and proportions of MAP2+ neurons in total cells, and ChAT+ hMNs in total MAP2+ neurons (bottom) are shown. Scale bars, 100 μm. b, Flow diagram for hMN culture. c, Co-staining of SA-β-Gal, 53BP1, lamin B2, H3K9me3 and MAP2/TUJ1 in hMNs at D0, D7, D14, and D21. Representative images (top) and the quantitative data (bottom) for SA-β-Gal+ hMNs, 53BP1+ hMNs, mean lamin B2 intensity and mean H3K9me3 intensity in hMNs are shown. Scale bars, 30 μm. d, Immunofluorescence staining of P21 and MAP2 in hMNs at D0 and D21. Representative images (left) and the percentage of P21+ hMNs in total hMNs (right) are shown. The arrows indicate the P21+ hMNs. Scale bars, 20 μm. e, Immunofluorescence staining of γ-H2A.X and MAP2 in hMNs at D0 and D21. Representative images (left) and the percentage of γ-H2A.X+ hMNs in total hMNs (right) are shown. The arrows indicate the γ-H2A.X+ hMNs. Scale bars, 20 μm. f, Western blot analysis of PSD95 in hMNs at D0 and D21. Representative images (left) and quantification of PSD95 protein levels (right) are shown. Loading control, GAPDH. g, Principal component analysis showing transcriptomic differences among hMNs collected at D0, D7, D14, and D21. h, Loading scores of the first and the second principal components of all genes when comparing hMNs. i-j, Scaled expression values and enriched pathways of genes upregulated (i) and downregulated (j) during hMN culture. Left, grey lines indicate the scale expression of genes upregulated or downregulated, while the red or blue line represents the fitting model of all grey lines. Right, enriched pathways of the upregulated or downregulated genes. k, Immunofluorescence staining of γ-H2A.X and MAP2 in hMNs cultured with medium containing young CSF, aged CSF, and aged monkey CSF after immunodepletion with IgG or anti-CHIT1 antibody. Representative images (upper) and quantification of the proportions of γH2A.X+ hMNs in total hMNs (lower) are shown. The arrows indicate the γ-H2A.X+ hMNs. Scale bars, 20 μm (all panels). l, Western blotting of CHIT1 in the same volume of aged CSF after immunodepletion with IgG (IgG-dep) or anti-CHIT1 antibody (anti-CHIT1-dep). m, Western blot analysis of CHIT1 in human microglia transduced with lenti-LUC or lenti-CHIT1. Loading control, GAPDH. n, Bar plot showing the CHIT1 protein secreted by microglia transduced with lenti-LUC or lenti-CHIT1. o, Western blotting of CHIT1 in the CM of CHIT1-Microglia (CHIT1-CM) immunodepleted with IgG or anti-CHIT1 antibody. p, Immunostaining of γ-H2A.X, lamin B2, or H3K9me3 and MAP2 in hMNs cultured with medium containing LUC-CM, CHIT1-OE CM, and CHIT1-OE CM after IgG-dep or anti-CHIT1-dep. Representative images (upper) and quantifications of the proportion of γH2A.X+ hMNs in total hMNs, as well as the mean intensity of lamin B2 or H3K9me3 fluorescence per hMN (lower) are shown. The arrows indicate the γ-H2A.X+ hMNs. Scale bars, 20 μm (all panels). Data (c-f, k, n, p) are shown as the mean ± s.e.m. The one-way ANOVA followed by Tukey’s post hoc test (c) and the P values were calculated using two-tailed unpaired t-tests (d-f, k, n, p). Three hundred hMNs per biological replicate (a, c-e, k, p) and n = 3 biological replicates per group (a, c-g, k-p).
Extended Data Fig. 12 CHIT1 induces hMN senescence through the TGFBR-SMAD cascade.
a, Immunofluorescence staining of H3K9me3 and MAP2 in hMNs treated with vehicle, CHIT1, or CHIT1 and ascorbic acid. Representative images (left) and quantifications of mean H3K9me3 intensity per hMN (right) are shown. Scale bars, 30 μm. b, Immunofluorescence staining of P21 and MAP2 in vehicle- or CHIT1-treated hMNs. Representative images (left) and the percentage of P21+ hMNs in total hMNs (right) are shown. Scale bars, 20 μm. c, Immunofluorescence staining of γ-H2A.X and MAP2 in vehicle- or CHIT1-treated hMNs. Representative images (left) and the percentage of γ-H2A.X+ hMNs in total hMNs (right) are shown. Scale bars, 20 μm. d, Left, images of patched hMNs; middle, the firing pattern of action potentials elicited by a 500 ms current pulse of 300 pA; right, the phase plot of spikes. Scale bars, 100 ms, 20 mV. e, Firing rates of action potentials from cultured hMNs treated with either vehicle or CHIT1. f, Bar plots, from left to right, showing the threshold of action potential generation from vehicle- or CHIT1-treated hMNs, half-width of evoked action potentials from vehicle- or CHIT1-treated hMNs, resting membrane potentials of the cell soma from vehicle- or CHIT1-treated hMNs, input resistance of cell membranes from vehicle- or CHIT1-treated hMNs. g, Left, the amplitude of outward current evoked by a voltage step from −80 mV to 60 mV. Right, the amplitude of the outward current response to a voltage stimulus of 0 mV (indicated by the arrowhead on the left). h, Western blot analysis of p-SMAD2 in vehicle- and TGFBR agonist (SRI-011381)-treated hMNs. Representative images (left) and quantification of p-SMAD2 levels in hMNs (right) are shown. Loading control, GAPDH. i, Co-staining for SA-β-Gal and TUJ1 in vehicle- and TGFBR agonist-treated hMNs. Representative images (left) and the percentage of SA-β-Gal+ hMNs in total hMNs (right) are shown. Scale bars, 20 μm. j, Immunofluorescence staining for lamin B2 or H3K9me3 and MAP2 in vehicle- and TGFBR agonist-treated hMNs. Representative images (left) and the mean lamin B2 intensity or mean H3K9me3 intensity per hMN (right) are shown. Scale bars, 20 μm. k, Western blot analysis of p-SMAD2 and SMAD2/3 in hMNs treated with vehicle, CHIT1, CHIT1 and TGFBR inhibitor (Galunisertib). Representative images (top) and quantifications of p-SMAD2 and SMAD2/3 protein levels (bottom) are shown. Loading control, GAPDH. l, Co-staining for SA-β-Gal and TUJ1, lamin B2 or H3K9me3 and MAP2 in vehicle, CHIT1-, CHIT1- and TGFBR inhibitor-treated hMNs. Representative images (left) and quantification of the percentage of SA-β-Gal+ hMNs in total hMNs and mean intensity of lamin B2 or H3K9me3 fluorescence per hMN (right) are shown. Scale bars, 20 μm. Data (a-c, e, f, g-l) are shown as the mean ± s.e.m. The one-way ANOVA followed by Tukey’s post hoc test (a, k, l), and P values were calculated using two-tailed unpaired t-tests (b, c, e-j). Three hundred hMNs per biological replicate (a, b, c, i, j, l) and n = 3 biological replicates per group (a, b, c, h-l). n = 29 (e, f) or 30 (g) cells in the vehicle group and n = 30 (e, f) or 33 (g) cells in the CHIT1 group.
Extended Data Fig. 13 Knockdown of SMAD2 alleviates CHIT1-induced hMN senescence and a schematic illustration for the findings of this study.
a, Western blot analysis of p-SMAD2 and SMAD2/3 in si-NC, si-SMAD2-1#, and si-SMAD2-2# transfected hMNs. Representative images (left) and quantifications of p-SMAD2 and SMAD2/3 protein levels (right) are shown. Loading control, GAPDH. b, Western blot analysis of p-SMAD2 in si-NC-, si-NC and CHIT1-, as well as CHIT1 and si-SMAD2-2#-treated hMNs. Representative images (left) and quantification of p-SMAD2 (right) are shown. Loading control, GAPDH. c, Co-staining for SA-β-Gal and TUJ1, lamin B2 and MAP2, or H3K9me3 and MAP2 in hMNs treated with si-NC, si-NC and CHIT1 as well as CHIT1 and si-SMAD2-2#. Scale bars, 20 μm. d, Quantifications of the percentage of SA-β-Gal+ hMNs, mean lamin B2 intensity, and mean H3K9me3 intensity in hMNs. e, Venn diagram showing the overlap between CHIT1-DEGs (CHIT1 vs. Vehicle) and ascorbic acid-DEGs (CHIT1 vs. Vehicle) in hMNs. The heatmap and bar plots showing the expression of overlapped DEGs and the corresponding enriched pathways were shown at the bottom. f, Detection of lipofuscin autofluorescence and ChAT immunostaining in the spinal vGM region of aged control (Vehicle) and ascorbic acid-treated monkeys. Representative images (left) and the mean intensity of lipofuscin fluorescence per (ChAT+) MN (right) in the spinal vGM region are shown. All MNs from three discontinuous cross-sectional slices per monkey were detected. Scale bars, 50 μm. g, A schematic illustration depicting the phenotypic and transcriptomic characteristics of NHP spinal cord aging. The first single-cell resolution survey of primate spinal cord aging reveals previously unreported pro-inflammatory microglia, which accumulate selectively around senescent MNs and highly expresses CHIT1, serving as both a potential biomarker and a pathogenic driver underlying hMN senescence. AIMoN-CPM, ageing-induced MN toxic CHIT1-positive microglia. Data (a, b, d, and f) are shown as the mean ± s.e.m. The one-way ANOVA followed by Tukey’s post hoc test (a, b, d), and P values were calculated using two-tailed unpaired t-tests (f). n = 3 biological replicates per group (a, b). Three hundred hMNs per biological replicate and n = 3 biological replicates per group (d). n = 5 monkeys per group (f).
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Fig. 1 (the uncropped blots) and the full legends for Supplementary Tables 1–11.
Supplementary Tables
Supplementary Tables 1–11.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, S., Li, J., Wang, S. et al. CHIT1-positive microglia drive motor neuron ageing in the primate spinal cord. Nature 624, 611–620 (2023). https://doi.org/10.1038/s41586-023-06783-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06783-1
This article is cited by
-
Compromised biogenesis of DHEA-S is implicated in primate adrenal aging
Nature Aging (2024)
-
CHIT1-positive microglia act as culprits for spinal motor neuron aging
Science China Life Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.