Abstract
Monoamine neurotransmitters such as dopamine and serotonin control important brain pathways, including movement, sleep, reward and mood1. Dysfunction of monoaminergic circuits has been implicated in various neurodegenerative and neuropsychiatric disorders2. Vesicular monoamine transporters (VMATs) pack monoamines into vesicles for synaptic release and are essential to neurotransmission3,4,5. VMATs are also therapeutic drug targets for a number of different conditions6,7,8,9. Despite the importance of these transporters, the mechanisms of substrate transport and drug inhibition of VMATs have remained elusive. Here we report cryo-electron microscopy structures of the human vesicular monoamine transporter VMAT2 in complex with the antichorea drug tetrabenazine, the antihypertensive drug reserpine or the substrate serotonin. Remarkably, the two drugs use completely distinct inhibition mechanisms. Tetrabenazine binds VMAT2 in a lumen-facing conformation, locking the luminal gating lid in an occluded state to arrest the transport cycle. By contrast, reserpine binds in a cytoplasm-facing conformation, expanding the vestibule and blocking substrate access. Structural analyses of VMAT2 also reveal the conformational changes following transporter isomerization that drive substrate transport into the vesicle. These findings provide a structural framework for understanding the physiology and pharmacology of neurotransmitter packaging by synaptic vesicular transporters.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kandel, E. R., Koester, J. D., Mack, S. H. & Siegelbaum, S. A. Principles of Neural Science 6th edn (McGraw Hill Professional, 2021).
Ng, J., Papandreou, A., Heales, S. J. & Kurian, M. A. Monoamine neurotransmitter disorders—clinical advances and future perspectives. Nat. Rev. Neurol. 11, 567–584 (2015).
Eiden, L. E. & Weihe, E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N.Y. Acad. Sci. 1216, 86–98 (2011).
Schuldiner, S., Shirvan, A. & Linial, M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol. Rev. 75, 369–392 (1995).
Yaffe, D., Forrest, L. R. & Schuldiner, S. The ins and outs of vesicular monoamine transporters. J. Gen. Physiol. 150, 671–682 (2018).
Wimalasena, K. Vesicular monoamine transporters: structure–function, pharmacology, and medicinal chemistry. Med. Res. Rev. 31, 483–519 (2011).
Davis, M. C., Miller, B. J., Kalsi, J. K., Birkner, T. & Mathis, M. V. Efficient trial design—FDA approval of valbenazine for tardive dyskinesia. N. Engl. J. Med. 376, 2503–2506 (2017).
Huntington Study Group. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. J. Am. Med. Assoc. 316, 40–50 (2016).
Siddiqui, M., Bhatt, H., Judd, E. K., Oparil, S. & Calhoun, D. A. Reserpine substantially lowers blood pressure in patients with refractory hypertension: a proof-of-concept study. Am. J. Hypertens. 33, 741–747 (2020).
Takahashi, N. et al. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc. Natl Acad. Sci. USA 94, 9938–9943 (1997).
Wang, Y.-M. et al. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron 19, 1285–1296 (1997).
Fon, E. A. et al. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron 19, 1271–1283 (1997).
Saida, K. et al. Brain monoamine vesicular transport disease caused by homozygous SLC18A2 variants: a study in 42 affected individuals. Genet. Med. 25, 90–102 (2023).
Drew, D. & Boudker, O. Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem. 85, 543–572 (2016).
Drew, D., North, R. A., Nagarathinam, K. & Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 121, 5289–5335 (2021).
Yan, N. Structural biology of the major facilitator superfamily transporters. Annu. Rev. Biophys. 44, 257–283 (2015).
Parsons, S. M. Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 14, 2423–2434 (2000).
Erickson, J. D., Eiden, L. E. & Hoffman, B. J. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc. Natl Acad. Sci. USA 89, 10993–10997 (1992).
Liu, Y. et al. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell 70, 539–551 (1992).
Weihe, E., Schäfer, M. K., Erickson, J. D. & Eiden, L. E. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J. Mol. Neurosci. 5, 149–164 (1994).
Peter, D. et al. Differential expression of two vesicular monoamine transporters. J. Neurosci. 15, 6179–6188 (1995).
Erickson, J. D. & Eiden, L. E. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J. Neurochem. 61, 2314–2317 (1993).
Nikkhah, A. A brief review on the role of vesicular monoamine transporter2 inhibitors in hyperkinetic movement disorders. Iran. J. Child Neurol. 15, 29–33 (2021).
Chen, J. J., Ondo, W. G., Dashtipour, K. & Swope, D. M. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin. Ther. 34, 1487–1504 (2012).
Harriott, N. D., Williams, J. P., Smith, E. B., Bozigian, H. P. & Grigoriadis, D. E. VMAT2 inhibitors and the path to Ingrezza (valbenazine). Prog. Med. Chem. 57, 87–111 (2018).
Weir, M. R. Reserpine: a new consideration of an old drug for refractory hypertension. Am. J. Hypertens. 33, 708–710 (2020).
Fraser, H. S. Reserpine: a tragic victim of myths, marketing, and fashionable prescribing. Clin. Pharmacol. Ther. 60, 368–373 (1996).
Lobay, D. Rauwolfia in the treatment of hypertension. Integr. Med. 14, 40–46 (2015).
Scherman, D. & Henry, J. P. Reserpine binding to bovine chromaffin granule membranes. Characterization and comparison with dihydrotetrabenazine binding. Mol. Pharmacol. 25, 113–122 (1984).
Darchen, F., Scherman, D. & Henry, J. P. Reserpine binding to chromaffin granules suggests the existence of two conformations of the monoamine transporter. Biochemistry 28, 1692–1697 (1989).
Schuldiner, S., Liu, Y. & Edwards, R. H. Reserpine binding to a vesicular amine transporter expressed in Chinese hamster ovary fibroblasts. J. Biol. Chem. 268, 29–34 (1993).
Liu, Y. & Edwards, R. H. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu. Rev. Neurosci. 20, 125–156 (1997).
Peter, D., Jimenez, J., Liu, Y., Kim, J. & Edwards, R. H. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J. Biol. Chem. 269, 7231–7237 (1994).
Støve, S. I., Skjevik, Å. A., Teigen, K. & Martinez, A. Inhibition of VMAT2 by β2-adrenergic agonists, antagonists, and the atypical antipsychotic ziprasidone. Commun. Biol. 5, 1283 (2022).
Ugolev, Y., Segal, T., Yaffe, D., Gros, Y. & Schuldiner, S. Identification of conformationally sensitive residues essential for inhibition of vesicular monoamine transport by the noncompetitive inhibitor tetrabenazine. J. Biol. Chem. 288, 32160–32171 (2013).
Kanner, B. I., Fishkes, H., Maron, R., Sharon, I. & Schuldiner, S. Reserpine as a competitive and reversible inhibitor of the catecholamine transporter of bovine chromaffin granules. FEBS Lett. 100, 175–178 (1979).
Erickson, J. D., Schafer, M. K., Bonner, T. I., Eiden, L. E. & Weihe, E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc. Natl Acad. Sci. USA 93, 5166–5171 (1996).
Yaffe, D., Vergara-Jaque, A., Forrest, L. R. & Schuldiner, S. Emulating proton-induced conformational changes in the vesicular monoamine transporter VMAT2 by mutagenesis. Proc. Natl Acad. Sci. USA 113, E7390–E7398 (2016).
Steiner-Mordoch, S., Shirvan, A. & Schuldiner, S. Modification of the pH profile and tetrabenazine sensitivity of rat VMAT1 by replacement of aspartate 404 with glutamate. J. Biol. Chem. 271, 13048–13054 (1996).
Merickel, A., Kaback, H. R. & Edwards, R. H. Charged residues in transmembrane domains II and XI of a vesicular monoamine transporter form a charge pair that promotes high affinity substrate recognition. J. Biol. Chem. 272, 5403–5408 (1997).
Merickel, A., Rosandich, P., Peter, D. & Edwards, R. H. Identification of residues involved in substrate recognition by a vesicular monoamine transporter. J. Biol. Chem. 270, 25798–25804 (1995).
Yaffe, D., Radestock, S., Shuster, Y., Forrest, L. R. & Schuldiner, S. Identification of molecular hinge points mediating alternating access in the vesicular monoamine transporter VMAT2. Proc. Natl Acad. Sci. USA 110, E1332–E1341 (2013).
Yaffe, D. et al. Functionally important carboxyls in a bacterial homologue of the vesicular monoamine transporter (VMAT). J. Biol. Chem. 289, 34229–34240 (2014).
Chen, H. et al. Structural and functional insights into Spns2-mediated transport of sphingosine-1-phosphate. Cell 186, 2644–2655 (2023).
Binz, H. K. et al. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat. Biotechnol. 22, 575–582 (2004).
Hu, G. et al. New fluorescent substrate enables quantitative and high-throughput examination of vesicular monoamine transporter 2 (VMAT2). ACS Chem. Biol. 8, 1947–1954 (2013).
Weaver, J. A. & Deupree, J. D. Conditions required for reserpine binding to the catecholamine transporter on chromaffin granule ghosts. Eur. J. Pharmacol. 80, 437–438 (1982).
Stern-Bach, Y., Greenberg-Ofrath, N., Flechner, I. & Schuldiner, S. Identification and purification of a functional amine transporter from bovine chromaffin granules. J. Biol. Chem. 265, 3961–3966 (1990).
Rudnick, G., Steiner-Mordoch, S. S., Fishkes, H., Stern-Bach, Y. & Schuldiner, S. Energetics of reserpine binding and occlusion by the chromaffin granule biogenic amine transporter. Biochemistry 29, 603–608 (1990).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006).
Bolte, S. & Cordelières, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006).
Rana, M. S., Wang, X. & Banerjee, A. An improved strategy for fluorescent tagging of membrane proteins for overexpression and purification in mammalian cells. Biochemistry 57, 6741–6751 (2018).
Zheng, S. Q. et al. MotionCor2—anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D 74, 519–530 (2018).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Black, C. A. et al. Assessing vesicular monoamine transport and toxicity using fluorescent false neurotransmitters. Chem. Res. Toxicol. 34, 1256–1264 (2021).
Acknowledgements
We thank scientists in the Cryo-EM Center of St. Jude Children’s Research Hospital for their support in data collection. We thank scientists in the Cell and Tissue Imaging Center of St. Jude Children’s Research Hospital and P. Zheng, P. Du and S. Jian for their support in cellular imaging. We thank Y. Wang for cell culture. We thank the members of the Lee and Zhang laboratories and F. Liu for helpful discussions; Z. Luo for assistance in preparing cartoon diagrams; and I. Chen for editing the manuscript. We thank the Roussel laboratory and the Schuetz laboratory at St. Jude for sharing equipment for radioisotope experiments. We thank the National Center for Protein Science at Peking University for other technical support. This work was supported by the National Key Research and Development Program of China (2021YFA1302300 to Z.Z.), the National Natural Science Foundation of China (32171201 to Z.Z.), the Center for Life Science, School of Life Science (SLS) of Peking University (to Z.Z.), the SLS-Qidong innovation fund (to Z.Z.), the Li Ge-Zhao Ning Life Science Youth Research Foundation (to Z.Z.) and the State Key Laboratory of Membrane Biology of China (to Z.Z.) and by National Institutes of Health (R01GM143282 to C.-H.L.) and ALSAC (to C.-H.L.).
Author information
Authors and Affiliations
Contributions
S.P. performed and analysed radioligand binding/transport assays. S.P. expressed and purified protein samples. S.P. and Y.D. performed cryo-EM structural experiments. S.P., S.L. and Y.D. analysed structural data. S.L. and Y.D. performed fluorescent substrate transport assays. X.L., S.P., Y.D., S.L. and C.-L.C. performed colocalization experiments. C.L. performed initial construct characterization and biochemical experiments. Z.Z. and C.-H.L. conceived the research and supervised the project. Z.Z. and C.-H.L. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Shimon Schuldiner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Construct design and sequence alignment of VMAT2.
a, Time course of 3H-dopamine uptake in permeabilized VMAT2-transfected or untransfected cells. The inset shows the uptake of the first 10 min. Data are shown as mean ± s.d.; n = 3 biological replicates. b, Schematic of the VMAT2EM construct. c, Representative size exclusion chromatography profile and SDS-PAGE analysis of purified VMAT2EM in saposin nanodisc. For gel source data, see Supplementary Fig. 1. The trace and gel image are representative of 3 experimental replicates. d, Sequence alignment of the SLC18 family. Residues with functional roles are highlighted with different colors: green, residues that contribute to TBZ selectivity toward VMAT2; blue, residues forming the cytoplasmic gates; yellow, residues forming the luminal gates and the lid; red, acidic residues potentially involved in proton coupling.
Extended Data Fig. 2 Cryo-EM analyses of VMAT2 in complex with TBZ.
a, Summary of image processing procedures of VMAT2EM in complex with TBZ. All procedures were done with cryoSPARC, except for particle polishing which was done with RELION. b, Representative micrograph (left) (out of 26,389 similar micrographs) and 2D class averages (right). c, Fourier shell correlation (FSC) curves between two half maps. d, Angular distribution of particles for the final 3D reconstructions. e, Local resolution of the cryo-EM map. f, Cryo-EM densities of the transmembrane helices. Map contour level = 0.15–0.24 in ChimeraX.
Extended Data Fig. 3 Cryo-EM analyses of VMAT2 in complex with reserpine.
a, Summary of image processing procedures of VMAT2EM Y418S in complex with reserpine. All procedures were done with cryoSPARC, except for particle polishing which was done with RELION. b, Representative micrograph (left) (out of 25,947 similar micrographs) and 2D class averages (right). c, Fourier shell correlation (FSC) curves between two half maps. d, Angular distribution of particles for the final 3D reconstructions. e, Local resolution of the cryo-EM map. f, Cryo-EM densities of the transmembrane helices. Map contour level = 0.15–0.23 in ChimeraX.
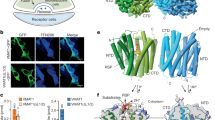
Extended Data Fig. 4 Topology and architecture of VMAT2.
a, Schematic of VMAT2 topology. VMAT2 adopts a canonical MFS fold with 12 TMs. The N-domain (TM1–6) and the C-domain (TMs 7–12) are connected by a cytosolic loop and a short amphipathic helix. Each domain is made up from 3-TM structurally inverted repeats, represented by triangles. b, Architecture of VMAT2. The TBZ-bound structure is shown. For clarity, TBZ is not shown. Left, viewed parallel to the membrane plane. Right, viewed from the luminal side of the vesicle.
Extended Data Fig. 5 Ligands-VMAT2 interaction diagrams.
a, Schematic of TBZ binding interactions. b, Schematic of reserpine binding interactions. c, Schematic of 5-HT binding interactions. Ligand interactions are analyzed using Schrödinger Maestro.
Extended Data Fig. 6 Mutations on the TBZ binding site affect TBZ sensitivity.
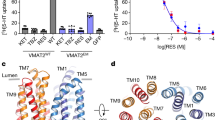
a, TBZ sensitivity of VMAT2 variants with alanine mutations in the binding pocket. For each construct, 100% uptake is defined as the average FFN206 signal in the absence of TBZ. Data are shown as mean ± s.d.; n = 3 biological replicates. The IC50 of VMAT2WT is 19 nM, in line with reported values for human and rat VMAT2 (18–37 nM)34,66. The IC50 values of F135A and Y433A are 514.5 and 230 nM, respectively. b, TBZ sensitivity of VMAT2 variants with VMAT1 substitutions in the binding pocket. For each construct, 100% uptake is defined as the average FFN206 signal in the absence of TBZ. Data are shown as mean ± s.d.; n = 3 biological replicates. The IC50 of L37F, I308V, Y433F, and L37F/I308V/Y433F is 82.4, 42.9, 26.1, and 1034 nM, respectively. c, FFN206 uptake of VMAT2 variants. 100% uptake is defined as the average FFN206 signal of VMAT2WT. Data are shown as mean ± s.d.; n = 3 biological replicates. d, Representative images of the FFN206 uptake assay. The FFN206 images have been brightened for enhanced visibility. The raw fluorescent intensities without adjustments are displayed in the lower right. e, TBZ sensitivity of VMAT2WT-transfected or untransfected cells in the FFN206 uptake assay. Data are shown as mean ± s.d.; n = 3 biological replicates.
Extended Data Fig. 7 Cryo-EM analyses of VMAT2 in complex with 5-HT.
a, Summary of image processing procedures of VMAT2EM Y418S in complex with 5-HT. All procedures were done with cryoSPARC, except for particle polishing and the final 3D classification which were done with RELION. b, Representative micrograph (left) (out of 19,023 similar micrographs) and 2D class averages (right). c, Fourier shell correlation (FSC) curves between two half maps. d, Angular distribution of particles for the final 3D reconstructions. e, Local resolution of the cryo-EM map. f, Cryo-EM densities of the transmembrane helices and 5-HT. Map contour level = 0.15–0.19 or 0.13 (TM12) in ChimeraX.
Extended Data Fig. 8 Conformational changes of the luminal gate from the cytoplasm-facing to lumen-facing occluded state.
Structural transition in the luminal gates of VMAT, viewed from the luminal side. The TM7 segment that undergoes secondary structural changes is highlighted in red.
Extended Data Fig. 9 Acidic residues in VMAT2 as potential proton sites.
a, Local interaction clusters of the potential proton sites in TBZ-bound, reserpine-bound, or 5-HT-bound state. b, 3H-dopamine uptake of VMAT2 variants with mutations in local interaction clusters of the potential proton sites. Uptake data of E312 mutants are plotted in Fig. 4e. 100% uptake is defined as the average 3H-dopamine signal of VMAT2WT. Data are shown as mean ± s.d.; n = 3 biological replicates. Average measurements in 10 µM cold reserpine were used for background correction.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pidathala, S., Liao, S., Dai, Y. et al. Mechanisms of neurotransmitter transport and drug inhibition in human VMAT2. Nature 623, 1086–1092 (2023). https://doi.org/10.1038/s41586-023-06727-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06727-9
This article is cited by
-
Packaging monoamine neurotransmitters
Cell Research (2024)
-
Transport and inhibition mechanism for VMAT2-mediated synaptic vesicle loading of monoamines
Cell Research (2024)
-
Structural insights into vesicular monoamine storage and drug interactions
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.