Abstract
Plants synthesize numerous alkaloids that mimic animal neurotransmitters1. The diversity of alkaloid structures is achieved through the generation and tailoring of unique carbon scaffolds2,3, yet many neuroactive alkaloids belong to a scaffold class for which no biosynthetic route or enzyme catalyst is known. By studying highly coordinated, tissue-specific gene expression in plants that produce neuroactive Lycopodium alkaloids4, we identified an unexpected enzyme class for alkaloid biosynthesis: neofunctionalized α-carbonic anhydrases (CAHs). We show that three CAH-like (CAL) proteins are required in the biosynthetic route to a key precursor of the Lycopodium alkaloids by catalysing a stereospecific Mannich-like condensation and subsequent bicyclic scaffold generation. Also, we describe a series of scaffold tailoring steps that generate the optimized acetylcholinesterase inhibition activity of huperzine A5. Our findings suggest a broader involvement of CAH-like enzymes in specialized metabolism and demonstrate how successive scaffold tailoring can drive potency against a neurological protein target.
Similar content being viewed by others
Main
The plant kingdom produces many compounds that affect cognition in animals6. These molecules probably act to protect against herbivory and also make plants a rich source of therapeutics for treating neurological diseases1,7. Many of these neuroactive compounds are alkaloids—nitrogen-containing compounds derived predominantly from amino acids—which act like neurotransmitter mimics to affect animal nervous systems1. Neuroactive alkaloids modulate the function of many different proteins involved in neuronal signalling, thereby causing alterations in behaviour and cognition. These bioactivities have long been recognized, as alkaloid-rich plants have served as important botanical medicines for thousands of years and many neuroactive alkaloids, such as the US Food and Drug Administration (FDA)-approved drugs morphine (analgesic), galantamine (dementia treatment) and atropine (muscarinic acetylcholine receptor antagonist), are still used in the clinic8.
The diversity in neuroactive alkaloid structures in plants is generated through complex biosynthetic mechanisms that convert primary building blocks (for example, amino acids) into a variety of scaffolds which can be tailored to produce specific, bioactive end-products. Alkaloid scaffolds are typically generated by an enzymatic transformation that condenses two substrates to yield a polycyclic structure2. However, unlike other major classes of plant natural products (for example, terpenoids and polyketides), there is no single chemical theme or enzyme class that is implicated in alkaloid scaffold generation. For example, although several alkaloid families are generated through Pictet–Spengler condensations, the enzymes which catalyse these reactions belong to unrelated protein families that have convergently evolved this activity9. Furthermore, many classes of plant alkaloids are derived through chemical transformations for which there is no known biosynthetic precedent. This is exemplified in the lysine-derived quinolizidine and Lycopodium alkaloids, which consist of hundreds of bioactive compounds10 and whose scaffolds are thought to be constructed through reactions for which no enzyme catalyst has yet been observed in nature4,11. This challenge to readily predict enzymes that build alkaloid scaffolds confounds the rapid elucidation of biosynthetic pathways and suggests that there are enzyme classes in plant metabolism yet to be identified.
Our interest in alkaloid scaffold biogenesis led us to focus on the Lycopodium alkaloids. These molecules are produced by plants in the Lycopodiaceae family (clubmosses)4 and consist of more than 400 structurally diverse, polycyclic alkaloids that have been studied as toxins and potential medicines12,13. Perhaps the most well-known member of this alkaloid class is huperzine A (HupA, 17)5, an acetylcholine mimic that reversibly inhibits acetylcholinesterase (AChE), an important enzyme at the neural synapse. This pharmaceutical activity has led to interest in the use of 17 as a potential treatment for the symptoms of dementia14 and elucidating its biosynthesis offers the possibility for the engineered production of this molecule, which has historically been non-sustainably sourced from wild Huperzia plants15. More broadly, the complexity and diversity of structures in the Lycopodium alkaloids has intrigued chemists for more than a century16 and these compounds continue to be targets for chemical synthesis strategies and isolation of unique structures17. However, although significant progress has been made in their total syntheses18,19, the mechanisms that plants use to synthesize the many, diverse Lycopodium alkaloid scaffolds have remained largely unknown and suggest the involvement of previously undescribed enzyme classes.
Discovery of scaffold-generating enzymes
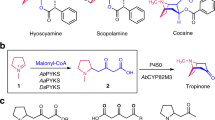
Previous isotope tracer studies (Supplementary Fig. 1) have demonstrated that the Lycopodium alkaloid scaffolds originate from two units each of a lysine-derived heterocycle (1-piperideine, 1) and a polyketide substrate derived from malonyl-CoA (3-oxoglutaric acid, 2, or its thioester analogue)4. These experiments enabled the recent identification of a biosynthetic route to 4-(2-piperidyl)acetoacetic acid (4PAA, 3) and pelletierine (4), the likely building blocks for all Lycopodium alkaloids (Fig. 1a), in several clubmoss species20,21,22,23. In our previous work, we demonstrated that three enzymes from the HupA-producing clubmoss Phlegmariurus tetrastichus (lysine decarboxylase, PtLDC; copper amine oxidase, PtCAO; and piperidyl ketide synthase, PtPIKS) are sufficient to convert the primary metabolites l-lysine and malonyl-CoA into 3, which can spontaneously decarboxylate to yield 4 (Fig. 1a)23. Although radio-isotope labelling studies with 4 have demonstrated this compound to be incorporated into downstream alkaloids, it was determined that this 8-carbon precursor is only incorporated into one half of 16-carbon Lycopodium alkaloid scaffolds24, 25 (Fig. 1a and Supplementary Fig. 1). By contrast, l-lysine, cadaverine, 1 and 2, which are presumed precursors to 4, were all shown to be incorporated into both halves of this scaffold24, 26,27,28,29,30 (Supplementary Fig. 1). These data suggest that a phlegmarane-type scaffold (Fig. 1a) is formed through the pseudodimerization of a 4-like molecule and a compound from which it is irreversibly derived, which has been proposed to be 3 or an oxidized derivative24,25.
a, An unknown series of chemical transformations are necessary to convert early precursors into the diverse Lycopodium alkaloid scaffolds found in club mosses. Note that the scaffold types shown in red/blue here are not compounds observed in nature but are visual representations of the main structural classes in the Lycopodium alkaloids. Shown in boxes are representative Lycopodium alkaloids, including the AChE inhibitor, HupA (17). b, Overview of transcriptomic-guided workflow for identifying new biosynthetic enzyme candidates. c.p.m., counts per million. The image of Phlegmariurus tetrastichus is adapted with permission from ref. 23 (Nett et al.), National Academy of Sciences.
Although a condensation between 3 and 4 is plausible (Supplementary Fig. 1), it was unclear what type of enzyme could catalyse this type of reaction. Moreover, it was not clear if 3 and/or 4 needed to be further tailored before coupling. Because of this, we chose to rely heavily on the high level of biosynthetic gene co-expression that we had previously observed in our transcriptome of P. tetrastichus23. To leverage our previous results, we performed hierarchical clustering with these data to generate co-expressed clusters of transcripts (Fig. 1b). This analysis revealed a single co-expressed cluster of 131 transcripts (cluster131) that contained all the previously identified biosynthetic genes (PtLDC-1, PtLDC-2, PtCAO-1, PtCAO-2, PtPIKS-1, PtPIKS-2, Pt2OGD-1, Pt2OGD-2 and Pt2OGD-3)23. This cluster was highly enriched with transcripts encoding for metabolic enzymes from several protein families commonly involved in natural product biosynthesis—for example, cytochromes P450 (CYPs), Fe(II)/2-oxoglutarate-dependent dioxygenases (2OGDs), methyltransferases, acyltransferases and dehydrogenase/reductase enzymes—suggesting that it may contain the requisite biosynthetic machinery for Lycopodium alkaloid scaffold biosynthesis.
It had previously been proposed that a 4-derived diene (8) could potentially serve as one of the cosubstrates for scaffold formation24. We considered that the formation of this compound would require two key events: an oxidation of 4 to form the imine and the reduction and elimination of the ketone oxygen. A related sequence of transformations had been reported in the context of morphine biosynthesis31, suggesting its plausibility in Lycopodium alkaloid biosynthesis. To test candidate enzymes for this proposed route, we used Agrobacterium-mediated DNA delivery in Nicotiana benthamiana as a transient gene expression platform. This allowed for production of 3 and 4 as substrates and the combinatorial testing of selected gene candidates (Methods). We were unable to identify an oxidase that could act directly on 4, so we instead gave priority to dehydrogenase/reductase family enzymes in cluster131 that could potentially catalyse the ketone reduction. Only one short-chain dehydrogenase/reductase (SDR) family gene was found in this cluster (PtSDR-1) and this had a close homologue (PtSDR-2; 88.6% amino acid identity) which could be found in a slightly expanded co-expression cluster (273 transcripts; cluster273). When added to the transient expression experiments in N. benthamiana (containing PtLDC, PtCAO and PtPIKS), both SDR homologues led to a decrease of 4 and the detection of two mass ions through liquid chromatography–mass spectrometry (LC–MS) that correspond to reduction of the ketone to the alcohol ([M + H]+ = m/z 144.1383) (Fig. 2 and Extended Data Fig. 1a,b). Comparison to a standard of 1-(piperidin-2-yl)propan-2-ol (that is, reduced pelletierine) stereoisomers (5) confirmed these two compound peaks to be diastereomers of 5. PtSDR-1 and PtSDR-2 seemed to form different ratios of 5 stereoisomers (Extended Data Fig. 1a). It had previously been noted that PtPIKS produces racemic 4 (and therefore 3, as 4 can be derived through spontaneous decarboxylation of 3)22, which suggested that these SDR enzymes may preferentially act on different enantiomers of 4 as substrate. Chiral LC–MS analysis confirmed the production of racemic 4 by PtPIKS and further demonstrated that PtSDR-1 mainly consumed (S)-4 to produce (2S, 4S)-5 (otherwise known as (+)-sedridine) but also apparently acted on (R)-4 to produce a small amount of (2S, 4R)-5 (otherwise known as (+)-allosedridine) (Extended Data Fig. 1c,d). PtSDR-2 consumed both enantiomers of 4 to produce an equimolar amount of (2S, 4S)-5 and (2S, 4R)-5 (Extended Data Fig. 1c,d) and seemed to be more active in our system. Taken together, these results demonstrate that these SDR enzyme homologues each catalyse the ketone reduction of 4 with conserved stereoselectivity to yield an alcohol in the (S) orientation but also that they have different enantioselectivity, with PtSDR-1 preferably reducing (S)-4, whereas PtSDR-2 seems to act equally well on both enantiomers of 4.
Shown are the extracted ion chromatograms (EICs) pertaining to the relevant m/z value for each proposed intermediate (m/z values shown under each compound) upon the transient co-expression of candidate biosynthetic genes from P. tetrastichus (blue boxes) in N. benthamiana. In general, compounds were observed as the [M + H]+ ion. For 9, the in-source fragment m/z 164.1434 ([M-C5H9N + H]+) is the principal detected ion and thus serves as a diagnostic for this compound. All compounds were detected through LC–MS using a HILIC column, with the exception of 6 diastereomers, which were observed by using a C18 column. Note that y axes for each set of chromatograms are on different scales but scales are constant within an EIC plot. Black arrows indicate observed depletion of substrates on addition of CAL-1 and CAL-2. The natures of compounds 4–6 were confirmed through comparison to synthesized or commercially available standards. The structure of 8 is proposed on the basis of MS2 and UV analysis (Extended Data Fig. 2). The structure of 9 is proposed on the basis of MS2, UV analysis and partial NMR of purified 9 (Extended Data Fig. 3 and Supplementary Figs. 10 and 11), as well as MS2 and full NMR of an oxidized byproduct (9′, Extended Data Fig. 3 and Supplementary Figs. 12–18).
Given the precedent for O-acylations in generating leaving groups for elimination in natural product biosynthesis31,32, we next screened BAHD acyltransferase family enzymes for activity, as six unique gene sequences from this family could be found in cluster131. Adding one acyltransferase (PtACT-1) to the transient co-expression system led to consumption of both (2S, 4S)-5 and (2S, 4R)-5 and production of a new compound ([M + H]+ = m/z 186.1489) consistent with the addition of an O-acetyl group (Fig. 2 and Extended Data Fig. 1e, f). Comparison to a synthesized standard and chiral LC–MS analysis confirmed this to be a mixture of two O-acetylated diastereomers, (2S, 4S)-6 and (2S, 4R)-6, which shows that PtACT-1 can catalyse O-acetylation regardless of the stereochemistry of the piperidine-alkyl (C3–C4) bond (Extended Data Fig. 1g,h).
The production of (2S, 4S)-6 and (2S, 4R)-6 was consistent with our hypothesis for elimination-mediated formation of the proposed diene (8). Because formation of 8 would require oxidation of the O-acetylated substrate(s), we next screened CYP and 2OGD family enzymes found in cluster131. One CYP enzyme (PtCYP782C1) was found to consume both (2S, 4S)-6 and (2S, 4R)-6 in our transient expression system (Fig. 2 and Extended Data Fig. 2a). This coincided with the presence of two new compounds: one that corresponded to a single oxidation (desaturation) of the O-acetylated substrate (7, [M + H]+ = m/z 184.1332, retention time 1.86 min), as well as another which shared the same exact mass as 8 ([M + H]+ = m/z 124.1121, retention time 1.93 min). Both compounds were almost entirely lost if samples were incubated at room temperature for 1 h (Extended Data Fig. 2b), which is consistent with previous descriptions of the instability of 8 (ref. 33). Although this limited our ability to access authentic standards, tandem mass spectrometry (MS/MS or MS2) analysis supported the proposed structures of 7 and 8 and ultraviolet (UV) analysis of 8 corroborated the presence of the predicted α,β-unsaturated imine in this molecule34 (Extended Data Fig. 2c,d). Reactions of 6 (mixture of stereoisomers) with PtCYP782C1-enriched microsomes produced in yeast confirmed the activity of this enzyme (Extended Data Fig. 2e–g) and allowed us to access 8 as an in vitro-generated substrate for any downstream enzymatic studies. These results indicate a series of transformations in which the diastereomers of 6 are oxidized to produce 7, which then undergoes an allylic elimination to yield 8 (Extended Data Fig. 2h,i), although it is uncertain whether the elimination is spontaneous or enzyme-catalysed by PtCYP782C1.
Production of 8 in our transient expression system suggested that we had potentially accessed the relevant substrates for initial phlegmarane scaffold formation (Fig. 1a). However, it was difficult to select candidate enzymes given the lack of precedence for enzymes that could promote this type of chemistry; furthermore, it was uncertain exactly what ‘dimer’ substrate combination should be tested. As a more untargeted approach, we opted to test candidates from cluster131 in batch combinations by enzyme family regardless of their previous association with specialized metabolism. In this process, we observed that a batch of four α-carbonic anhydrase (CAH) family proteins produced a new mass signature ([M + H]+ = m/z 164.1434) when transiently expressed in N. benthamiana leaves with the rest of the established pathway (Fig. 2 and Extended Data Fig. 3a,b). The calculated molecular formula of this feature (C11H18N) was unanticipated given the expected 8-carbon substrates and this metabolite was only found using untargeted metabolomic analysis of our data35. However, further analysis of in-source MS adducts and fragments36 identified two co-eluting mass signatures that corresponded to the mass of a 16-carbon molecule ([M + H]+ = m/z 247.2169 and [M + 2H]2+ = m/z 124.1121), suggesting that we had potentially accessed a scaffold from the condensation of two 8-carbon, nitrogen-containing substrates (Extended Data Fig. 3c,d). With this in mind, the m/z 164 ion seemed to correspond to an ionization-induced loss of 1-piperideine during MS analysis (Extended Data Fig. 3d). We note that of the three MS adducts observed for this molecule, the m/z 164 ion was the most abundant and therefore this was used as a diagnostic ion for all following analyses. Subsequent MS2 fragmentation of both the parent ion (m/z 247) and the in-source fragment (m/z 164) suggested that this compound (designated as 9, although the structure was not immediately evident) possesses a bicyclic, phlegmarane-type scaffold (Extended Data Fig. 3e–h) and UV analysis supported the presence of an α,β-unsaturated imine34 (Extended Data Fig. 3i). Compound 9 could be detected in extracts from the biosynthetically active tissue of P. tetrastichus (Extended Data Fig. 3e,f), which gave us confidence that this compound was relevant to Lycopodium alkaloid metabolism. We subsequently found that two of the batch-tested CAH-like (CAL) proteins (named as PtCAL-1a and PtCAL-2a) were required to be transiently expressed with the rest of the upstream pathway for this scaffold formation to occur and that no apparent activity could be detected with either of the CALs on their own (Fig. 2 and Extended Data Fig. 3a). Also, we found that cluster131 contained homologues of PtCAL-1a (PtCAL-1b, 89.6% amino acid identity) and PtCAL-2a (PtCAL-2b, 70.1% amino acid identity) which exhibited the same activity (Extended Data Fig. 3b).
We considered that formation of 9 could result from a dimerization of 8. However, when 8 was provided as a substrate to PtCAL-1a and PtCAL-2a independently of the full reconstituted pathway in N. benthamiana (8 was generated by co-infiltrating 6 as a substrate for transiently expressed PtCYP782C1), formation of 9 was not observed (Extended Data Fig. 3j,k). Given this result, we predicted that the scaffold may be a pseudodimer that requires 8 and another upstream pathway intermediate as a cosubstrate. In support of this hypothesis, we could reconstitute production of 9 in N. benthamiana through the combination of PtCAL-1a/PtCAL-2a with a module for producing 8 (PtCYP782C1 and synthetic 6 as substrate) and a module for producing 3 and 4 (PtLDC, PtCAO and PtPIKS) (Extended Data Fig. 3j,k). Also, we observed consumption of both 8 and 3 that was concurrent with production of 9, which supports these compounds as the ‘pseudodimers’ that are condensed to form the scaffold molecule (Fig. 2 and Extended Data Fig. 3l). Because PtPIKS produces a racemic mixture of 3 (Extended Data Fig. 1c; note that the stereochemistry of 3 is inferred by the measurement of 4 enantiomers), it was plausible that either (R)-3 or (S)-3 could be incorporated into this scaffold, which would result in the formation of two 9 diastereomers. Indeed, under optimal LC–MS conditions, we could observe a second, nearly co-eluting peak with an identical MS2 fragmentation pattern to 9 (Extended Data Fig. 3m). However, this compound was only present at around 10% the amount of the main 9 diastereomer, which suggests that a single enantiomer of 3 is preferably used as the cosubstrate. Through chiral LC–MS, we determined that (S)-3 was partially consumed by PtCAL-1a/PtCAL-2a (28% decrease, P = 0.09) whereas (R)-3 was not (P = 0.66), which supports the specific condensation of (S)-3 with 8 to form 9 (Extended Data Fig. 3n). Overall, these results from heterologous pathway expression in N. benthamiana are consistent with previously proposed mechanisms that implicate 3 as the nucleophile to initiate scaffold formation with an electrophilic cosubstrate, which we have shown to be 8 (refs. 24,25).
We scaled up production of 9 in N. benthamiana for purification and structural determination of this compound. This proved to be difficult, as the compound seemed to degrade during purification and we were only able to obtain a moderately pure proton NMR. However, we were able to purify a putative oxidized product of this scaffold (9′ [M + H]+ = m/z 263.2118) that accumulated during purification (Extended Data Fig. 3o–q) and structural analysis of this molecule through NMR and MS2 confirmed that it contained the predicted phlegmarane scaffold (see Supplementary Information for NMR data of 9 and 9′). Considering the structure of 9′, MS2 fragmentation and UV analysis of 9 and the chemical logic of a condensation between (S)-3 and 8, we predict the structure of 9 as a bicyclic phlegmarane scaffold with a conjugated α/β-unsaturated imine (Figs. 2 and 3a). Notably, a similar α/β-unsaturated imine moiety has been used in the chemical synthesis of Lycopodium alkaloid scaffolds17, for which it was noted to be oxygen sensitive and the NMR structure of our oxidized byproduct (9′) is consistent with an oxidation of 9. Thus, we propose that PtCAL-1 and PtCAL-2 homologues act together to form 9 through the condensation of (S)-3 and 8 and that this serves as the key phlegmarane scaffold-forming reaction in Lycopodium alkaloid biosynthesis (Fig. 3a and Extended Data Fig. 3r).
a, Proposed mechanism for biosynthesis of 9 by PtCAL-1a/PtCAL-2a. b, Representative western blot of 6xHis-tagged CALs expressed alone or co-expressed with untagged gene constructs in N. benthamiana. A 6xHis-tagged LcLDC construct was included as an intracellular protein control. This experiment was performed more than three times with similar results routinely observed. c, EICs for production of 9 (m/z 164.1434) by apoplastic PtCAL-1a and PtCAL-2a (alone or co-expressed) with different substrate combinations. d, Time course of 9 production by apoplastic PtCAL-1a/PtCAL-2a compared to a GFP apoplast control. n = 3 reactions per condition. e, Assessment of which 3 enantiomer is used as a substrate in the formation of 9. Because 3 enantiomers could not be directly observed, chirality was inferred by measuring 4 enantiomers, which form through spontaneous decarboxylation of 3. Enantiomers were analysed as N-acetylated derivatives. Average ratios are above each bar. n = 3 reactions per condition. f, Proposed condensation of 1 and 2 catalysed by PtCAL-3 to produce (S)-3. g, Co-expression of PtCAL-3 with the rest of the pathway required to produce 9. n = 3 infiltrated leaves per condition. h, EICs for 3 (m/z 186.1125) production by PtCAL-3 apoplast protein with different substrates. i, Formation of 3 over time by apoplastic PtCAL-3 with 1 and 2 as substrates, as compared to a GFP apoplast control. n = 3 reactions per condition. j, Assessment of enantiospecific product formation by PtCAL-3 through chiral LC–MS analysis of 4 enantiomers (after N-acetylation). k, Ratio of (S)-4 and (R)-4 over time in the apoplastic PtCAL-3 reaction. For bar and line graphs, plotted values represent the mean, with error bars representing ±s.d. Statistical analyses were performed using a two-tailed Welch’s t-test assuming unequal variance.
The ability of CAH-like proteins to act directly in specialized metabolite biosynthesis represents a striking neofunctionalization in this enzyme family, as CAH enzymes canonically catalyse the interconversion of CO2 and bicarbonate as an aspect of numerous biological functions including pH control, CO2 concentrating/solubilization and lipid metabolism37. Also, we were surprised by the apparent cofunctionality of PtCAL-1a/PtCAL-2a because proteins from the CAH family usually function as monomers38,39. To better understand the unique functionality of PtCAL-1a/PtCAL-2a, we next worked to establish an in vitro reaction assay. Although we could obtain solubilized versions of these proteins through both heterologous expression in Escherichia coli and cell-free protein production with wheat germ extract, we were unable to recapitulate the previously observed enzyme activity for PtCAL-1/PtCAL-2 obtained from either system, which indicated that there may be factors in the context of living plant cells that are critical for activity (for example, post-translation modifications or subcellular localization). Both proteins possess a predicted N-terminal signalling peptide that indicates trafficking through the secretory pathway, which suggested that they may be localized to the apoplast (the extracellular compartment in plant leaves). To assess this, we produced His-tagged versions of these proteins in N. benthamiana and used western blotting of different protein fractions (apoplast and cellular) to evaluate PtCAL-1a and PtCAL-2a localization. This demonstrated that both proteins can be found in the apoplast in this heterologous system but also that this localization is affected by their co-expression (Fig. 3b). In particular, whereas PtCAL-1a exhibited apoplastic localization independently of co-expression with PtCAL-2a, very little PtCAL-2a protein could be detected in the apoplast when it was expressed alone and it instead seemed to be mainly in the intracellular fraction, which contains both cytosolic and organellar proteins (Fig. 3b). However, on co-expression of PtCAL-1a, apoplastic PtCAL-2a was readily detected and seemed to exhibit post-translational modifications of an unknown nature (Fig. 3b). These results indicate that PtCAL-1a may have a critical role in the proper post-translational modification and/or trafficking of PtCAL-2a, although further work will be necessary to determine the mechanism by which this occurs. Beyond providing details on localization, this information was critical for enzyme assay development because the pH of the apoplast is typically relatively low (about pH 5)40, which suggested that these proteins may have optimal function at a lower pH. Also, apoplast extracts can be readily isolated from N. benthamiana leaves expressing these proteins40, thereby providing a potential means to evaluate CAL protein outside the living plant system.
Using isolated apoplast extracts in vitro, we demonstrated activity (Extended Data Fig. 4a–d) for PtCAL-1a/PtCAL-2a when 3 and 8 were supplied as substrates enzymatically (through the action of purified PtPIKS +1 and malonyl-CoA and PtCYP782C1-enriched microsomes +6 and NADPH, respectively). Notably, the enzymatic activity of PtPIKS yields both 3 and 4 (through spontaneous decarboxylation of 3) and no production of 9 was observed for PtCAL-1a/PtCAL-2a when synthesized 4 was added together with 8 (Fig. 3c and Extended Data Fig. 4c), which further supports 3 as the cosubstrate. We confirmed that the free acid of 3 (as opposed to a thioester conjugate) acts as the cosubstrate, as 3 generated in situ (through spontaneous condensation of 1 and 2) could replace the PtPIKS enzyme reaction in this in vitro system (Fig. 3c,d and Extended Data Fig. 4b–d). As observed with in planta experiments, only the (S) enantiomer of 3 was consumed in the presence of PtCAL-1a/PtCAL-2a (Fig. 3e and Extended Data Fig. 4e, P = 0.0032), thereby supporting enantiospecific scaffold generation with this cosubstrate. Consistent with N. benthamiana experiments and western blot analysis, we only observed activity in apoplast extracts from leaves in which PtCAL-1a and PtCAL-2a were co-expressed; individual expression of each protein and subsequent mixing did not yield detectable product formation (Fig. 3c and Extended Data Fig. 4c), indicating that the co-occurrence of each protein in the plant is critical for their proper production and function.
Condensation to form 9 could initiate through the nucleophilic attack of (S)-3, a β-keto acid, with the electrophilic, α,β-unsaturated imine of 8. In theory, this may occur through two routes (Extended Data Fig. 4f): (1) decarboxylation of (S)-3 to generate the enolate of 4, which would then serve as the nucleophile for condensation with 8 (‘decarboxylation-first’ mechanism) or (2) formation of the (S)-3 enolate through tautomerization, followed by addition to 8, then decarboxylation (‘addition-first’ mechanism)41. Although a decarboxylation-first mechanism is reminiscent of several canonical strategies for C–C bond formation (for example, in fatty acid biosynthesis), an addition-first mechanism could also leverage CO2 release to drive the reaction equilibrium to completion and thus seemed to be a plausible alternative. To probe these possibilities, we designed experiments to test if the CAL proteins accelerated decarboxylation (and presumably enolate formation) in the absence of their respective electrophiles. When we supplied 3 as the substrate to PtCAL-1a/PtCAL-2a in the absence of 8, we did not observe accelerated decarboxylation of 3 (beyond the rate of spontaneous decarboxylation of this β-keto acid) (Extended Data Fig. 4g,h). This result and the fact that 4, which would be in equilibrium with an equivalent enol tautomer, does not serve as a cosubstrate with 8 (Fig. 3c and Extended Data Fig. 4c), suggests that the addition of (S)-3 to 8 probably precedes decarboxylation. This mechanism implies that the CAL proteins may enhance formation of the enolate tautomer of 3, which could serve as the requisite nucleophile (Extended Data Fig. 4f); however, our results do not rule out the possibility that binding of 8 is required for the decarboxylation of (S)-3 to occur. Although further work will be necessary to firmly establish this enzymatic mechanism, our data suggest an addition-first mechanism by which one half of the Lycopodium alkaloid scaffold (8) is combined with a cosubstrate (3) from which it is irreversibly derived (Fig. 3a). The use of 3 as the nucleophilic cosubstrate for scaffold formation is in direct agreement with past isotope labelling studies that demonstrated incorporation of 4 into only one half of Lycopodium alkaloids scaffolds24,25. In this mechanism, the ‘4-derived’ half is represented by 8, which we have shown is enzymatically synthesized from 4, whereas (S)-3 serves as the other half. Although we favour the role of (S)-3 as the initial nucleophile attacking the 8 electrophile in this reaction, we note that an alternative sequence of bond formation is also plausible. For example, formation of the enamine tautomer of 8 could allow for this molecule to serve as the initial nucleophile, wherein the enamine would attack the carbonyl of (S)-3 first, followed by decarboxylative condensation to generate the final phlegmarane scaffold.
Encouraged by the identification of these neofunctionalized CAH proteins, we considered that other transcriptionally coregulated CAL genes (Pearson’s r > 0.9 when compared to expression of other pathway genes) might also have a role in this biosynthetic pathway. On testing another four CAL candidates with our established biosynthetic pathway through transient expression in N. benthamiana, we found a distinct CAL gene (PtCAL-3) that caused about a threefold increase (P = 0.0005) in the abundance of 9 (Fig. 3g). Analysis of all pathway intermediates that accumulated in this experiment showed a shift in the abundance of 5 diastereomers to an enrichment of (2S, 4S)-5, suggesting that PtCAL-3 could be acting to influence the stereochemistry of precursor substrates (Extended Data Fig. 5a). Including PtCAL-3 with different combinations of pathway genes demonstrated that this enzyme is acting upstream of 4 formation, as we could observe a shift from racemic 4 to an enrichment of (S)-4 when PtCAL-3 was included (Extended Data Fig. 5b–d, P = 0.009). As with PtCAL-1a and PtCAL-2a, PtCAL-3 contains an N-terminal signalling peptide and was found to be localized to the apoplast (Fig. 3b) and we were able to establish functional in vitro assays using apoplast extract from N. benthamiana leaves expressing this gene. In these assays, we demonstrated that including PtCAL-3 apoplast with the PIKS reaction (using 1 and malonyl-CoA as substrates) led to an enrichment of (S)-4 to (R)-4 over time (Extended Data Fig. 5h) and we speculated that PtCAL-3 protein may be accelerating the rate of 3 and/or 4 formation in a stereoselective manner. To decouple the activity of PtCAL-3 from PtPIKS, we generated 3 as a substrate in situ through the spontaneous condensation of 1 and 2 (Extended Data Fig. 4a). When this reaction mixture was added to PtCAL-3 apoplast, we observed a drastically accelerated increase in the formation of 3 in comparison to a control apoplast extract (Fig. 3h, i and Extended Data Fig. 5i). Also, we determined that the (S) enantiomer of 3 (inferred through measurement of 4 enantiomers) was enriched over time (Fig. 3j,k and Extended Data Fig. 5j,k), which indicated that PtCAL-3 is catalysing a stereospecific condensation of 1 and 2. Similar to the proposed addition-first mechanism of PtCAL-1a/PtCAL-2a, PtCAL-3 did not accelerate the rate of decarboxylation of 2 (Extended Data Fig. 5l, m). Furthermore, acetoacetate, the product of the decarboxylation of 2, did not serve as a viable cosubstrate with 1 (Fig. 3h). Thus, our data suggest a mechanism in which PtCAL-3 catalyses a Mannich-like addition of a 2 enolate to the imine of 1 in a stereospecific manner, after which decarboxylation occurs to yield (S)-3 (Fig. 3f). This mechanism aligns well with the observed data from biosynthetic pathway reconstitution in N. benthamiana because PtPIKS produces 2 as a major product22,23 and thus the requisite substrates for PtCAL-3 (1 and 2) are present from the activity of earlier enzymes in the pathway (PtCAO and PtPIKS).
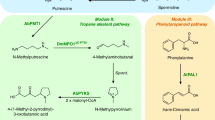
Beyond elucidation of the pathway for initial scaffold formation, our results on early Lycopodium alkaloid biosynthesis help rationalize the observed synthesis of racemic 3 and 4 by the PIKS enzyme22,23, which was relatively unusual given that most enzymes synthesize products in an optically pure form42. Specifically, we have shown that the subsequent enzymes in the biosynthesis of 8 (for example, PtSDR-2, PtACT-1 and PtCYP782C1) lack substrate stereoselectivity and thus both the (R) and (S) enantiomers of 4 can be converted into 8 (Fig. 4a). Although this is unusual for a metabolic pathway, we predict that the stereoselectivity of these particular enzymes may not have been strongly selected for during the evolution of 8 biosynthesis because the stereocentre of 4 (as well as 5 and 6) is eventually lost in the formation of 8. Ultimately, the activity of PtCAL-3 provides a bypass of these events to generate a specific enantiomer, (S)-3, for scaffold generation. This scenario would necessitate movement of 1 and 2 into the apoplast for PtCAL-3 because this protein seems to be secreted extracellularly. Because PtCAL-1a/PtCAL-2a condense (S)-3 with 8 to generate the core Lycopodium alkaloid scaffold, the specific production of (S)-3 by PtCAL-3 helps to explain the observed boost in the production of 9 when PtCAL-3 is present in N. benthamiana pathway reconstruction and in vitro enzyme assays (Fig. 3g and Extended Data Fig. 4d). Indeed, the addition of PtCAL-3 also leads to a significantly increased ratio (increased from 10:1 to 50:1, P = 0.01) of 9 over its minor diastereomer (Extended Data Fig. 5e,f). We predict that formation of the minor diastereomer is because of low-level use of (R)-3 as a substrate by PtCAL-1a/PtCAL-2a and that the increased proportion of (S)-3 from PtCAL-3 activity leads to further enrichment of 9 as the main product. The probable colocalization of these CAL proteins in the apoplast provides a mechanism by which (S)-3 can be directly used in scaffold formation without being fully consumed by the enzymatic steps that synthesize 8, which are localized to the cytosol (Fig. 4a). Together, these data reveal a pathway for how neofunctionalized CAL enzymes activate carboxylate substrates and catalyse stereoselective C–C bond formation in plant specialized metabolism.
a, Biosynthetic proposal for the early chemical transformations in Lycopodium alkaloid biosynthesis. Note that transport of intermediates across the membrane is speculative. b, Phylogenetic tree (MUSCLE alignment, neighbour-joining tree) of CAH family proteins from several kingdoms of life. Bootstrap values (100 replicates) are located at nodes. Also shown are the main active site residues for each aligned protein, with numbering corresponding to human carbonic anhydrase 2 (HsCA2, UniProt ID: P00918). Changes to the canonical/conserved sequence are highlighted in coloured boxes. Stars indicate proteins that have verified canonical CAH activity. An expanded alignment/phylogenetic tree can be found in Supplementary Fig. 2.
The identification of these three CALs demonstrates that proteins from the CAH family can participate directly in specialized metabolic pathways. The unexpected functions of the CALs suggest that their fundamental mechanisms of catalysis are probably distinct from archetypical CAHs. It has been well-established that canonical CAHs use an extremely highly conserved histidine triad to coordinate a Zn2+ cofactor, which acts as a Lewis acid for generating the reactive hydroxide ion that hydrates CO2 (refs. 43,44). Thus, it is notable that in homologues of both PtCAL-1 and PtCAL-2, this histidine triad has been mutated (Fig. 4b, Extended Data Fig. 6 and Supplementary Fig. 2). In the case of PtCAL-1, two of the three histidines are mutated, whereas all three are mutated in PtCAL-2. Previous analysis of analogous mutations in CAHs have determined that perturbation of this triad leads to a loss in Zn2+-binding and CAH activity45,46 and thus the mutations observed in PtCAL-1 and PtCAL-2 would seem to indicate a different mechanism of catalysis. Although PtCAL-3 retains this histidine triad, several other highly conserved active site residues involved in substrate binding have been altered (Fig. 4b and Supplementary Fig. 2), presumably to accommodate the increase in substrate size relative to CO2/bicarbonate. For each CAL, the addition of a Zn-chelating reagent44 to the apoplastic protein did not lead to any discernable loss in their biosynthetic activity, nor was there any effect from the supplementation of Zn to these reactions (Extended Data Figs. 4i and 5n). This is in contrast to the effect of Zn chelators on canonical CAH enzymes, which typically show near complete loss of activity after such treatments47. Although this suggests that the CALs no longer use Zn as a cofactor, more comprehensive examination of these proteins will be needed to understand their cofactor requirements as well as the fundamental mechanisms of their catalysis. Structural modelling48 of the three CALs demonstrates that they exhibit the conserved tertiary structure found in the CAH family37 (Extended Data Fig. 6). With that considered, we expect that the observed alterations in highly conserved active site residues in these CALs will provide a prominent starting point for future mechanistic studies.
Beyond understanding the detailed catalytic mechanisms of the CALs, further work will be necessary to establish the reason(s) for PtCAL-1/PtCAL-2 codependence. Although computational modelling48 predicts PtCAL-1a and PtCAL-2a to interact with a moderate amount of confidence (Supplementary Fig. 3), de novo prediction of protein heterodimers remains challenging without experimental validation. Thus, it will be necessary in future work to rigorously assess potential interaction between these two proteins, as well as how this interaction may affect function. For example, although we have shown that the co-expression of PtCAL-1 critically affects the localization and post-translational modification of PtCAL-2, it is not yet clear how PtCAL-1 may cause this change and more questions remain as to how these proteins may be cooperating to carry out phlegmarane scaffold formation. Thus, these CALs will provide an exciting model not only for investigating the catalytic mechanisms of a neofunctionalized subclass of enzymes but also for understanding the nuanced roles for transport and protein cooperativity in specialized metabolism.
Enzymatic tailoring for the production of neuroactive HupA
Although we were not immediately successful in finding enzymes that could process 9, we next sought to investigate further downstream reactions in Lycopodium alkaloid metabolism. In our previous study of HupA (17) biosynthesis, we identified three 2OGDs (Pt2OGD-1, Pt2OGD-2 and Pt2OGD-3) which function in the downstream tailoring reactions required to produce 17 from proposed precursors23. However, we were initially unable to identify an enzyme that could act on these substrates to form the 8,15-double bond (see Fig. 1a for numbering) that is present in 17 and many other Lycopodium alkaloids, suggesting that we had not been testing the correct substrate(s). The simplest Lycopodium alkaloid with the same ‘lycodane’ scaffold (Fig. 1a) as 17 is flabellidine (10)49, which contains an N-acetyl group on the A-ring nitrogen and could plausibly be derived from 9 (Fig. 5). Milligram quantities of this molecule had previously been purified50, which allowed us to test this as a substrate in N. benthamiana leaves expressing our oxidase gene candidates from cluster131 (CYPs and 2OGDs). Through this approach, we identified a pair of 2OGD enzymes which acted sequentially to convert 10 into downstream, oxidized products (Supplementary Results give a detailed description of these enzymes). The first of these enzymes (Pt2OGD-4) oxidized 10 to a molecule with an exact mass that is consistent with the installation of a carbonyl (proposed structure 11, [M + H]+ = m/z 303.2067) (Extended Data Fig. 7), whereas the second enzyme (Pt2OGD-5) consumed 11 and produced a desaturated compound (proposed structure 13, [M + H]+ = m/z 301.1911) (Extended Data Fig. 8). Although authentic standards were not available for these compounds, we suspected that Pt2OGD-4 was catalysing formation of the A-ring carbonyl, whereas Pt2OGD-5 was installing the 8,15-double bond.
New enzymes or new reactions for previously described enzymes, are coloured purple. Any Lycopodium alkaloids with common names have been verified with authentic standards. Shown below Lycopodium alkaloids are their IC50 values for the inhibition of AChE, if previously tested. Citations for these values can be found in the Methods. Note that the stereochemistry of the methyl group in 9 is predicted on the basis of the typical stereochemistry observed in isolated Lycopodium alkaloids53.
If our predictions for the oxidations catalysed by Pt2OGD-4 and Pt2OGD-5 were correct, then the only remaining oxidation would be A-ring desaturation, which we have shown to be catalysed by Pt2OGD-3 (ref. 23). However, Pt2OGD-3 did not consume 13 and thus we hypothesized that N-deacetylation must precede this desaturation. Accordingly, we found an α/β hydrolase family enzyme (PtABH-1) in cluster131 that consumed 13 to produce the N-deacetylated compound lycophlegmarinine D (14)51, which verified the positioning of the carbonyl and double bond installed by Pt2OGD-4 and Pt2OGD-5, respectively (Extended Data Fig. 9). Addition of Pt2OGD-3 to the transiently co-expressed combination of Pt2OGD-4, Pt2OGD-5 and PtABH-1 led to the consumption of 14 and the formation of huperzine B (15) (Extended Data Fig. 10a and Supplementary Fig. 4). Subsequent addition of Pt2OGD-1 and Pt2OGD-2 allowed for the production of huperzine C (16) and, ultimately, 17 (Extended Data Fig. 10a and Supplementary Fig. 5), thus establishing a biosynthetic route for the complete, stepwise biosynthesis of 17 from 10. Although 17 has generated the most interest as a potential pharmaceutical14, hundreds of Lycopodium alkaloids have been isolated and structurally characterized13, including many congeners of 17 pathway intermediates which differ in their degree of unsaturation. Indeed, by mixing and matching enzymes from this downstream biosynthetic module, we were able to reconstitute the biosynthesis of 15 different Lycopodium alkaloids from 10 as an initial substrate. This included nine previously isolated and characterized compounds which were verified with authentic standards, as well as six previously unreported alkaloids (Fig. 5, Extended Data Fig. 10a–d and Supplementary Fig. 5; Supplementary Results gives more details of these experiments). This demonstrates that the enzymes we identified contribute to a metabolic network of Lycopodium alkaloids in the endogenous plants, thereby explaining much of the structural diversity found among this class of alkaloids.
The biological functions for most Lycopodium alkaloids in the native plants have not been determined but the ability of many of these compounds to inhibit AChE, a critical enzyme in animal neuronal signalling, suggests that they may act to deter herbivory through this mechanism. In support of this, AChE is a common target of insecticides7 and 17 has been shown to exhibit antifeedant activity on several insect species52, suggesting a possible AChE inhibition mechanism for 17 in the defence of the plant against insect herbivory. It is notable that 17 exhibits the most potent AChE inhibitory activity of any Lycopodium alkaloid measured thus far and that this inhibition activity decreases with each previous intermediate in the pathway (Fig. 5). This seems to represent a metabolic structure–activity relationship among the Lycopodium alkaloids, wherein each of the enzymatic transformations en route to 17 enhances AChE inhibitory activity. Although we cannot be sure that the biological function of 17 is to inhibit animal AChE enzymes, the relationship between Lycopodium alkaloid biosynthesis and AChE inhibitory activity suggests that this metabolic pathway has evolved successive biosynthetic steps that increase the potency of these alkaloids step-by-step to achieve the production of an ‘optimized’ AChE inhibitor. However, we note that alternative explanations for the evolution of 17 biosynthesis are plausible, particularly given the complex, metabolic network of Lycopodium alkaloids that exists in extant plants. For example, it is possible that 17 was a minor component of the Lycopodium alkaloid cocktail present in a shared common ancestor and that the AChE activity of 17 was selected for, thereby refining and enhancing the biosynthetic production of this molecule. Regardless of the specific mechanism, the Lycopodium alkaloids could prove to be a powerful system for understanding the evolution of specialized metabolism in early diverging plants.
In support of our proposed biosynthetic pathway, all main biosynthetic intermediates from 4 to 9 (Supplementary Fig. 6) and 10 to 17 (Supplementary Fig. 7) could be detected in extracts from tissues in P. tetrastichus in which 17 biosynthesis actively occurs. Transformation of the phlegmarane scaffold of 9 into the tetracyclic lycodane scaffold found in downstream alkaloids would putatively only require a double-bond isomerization and enamine–imine condensation (Fig. 5). Final N-acetylation of this scaffold on the A-ring would then yield 10, thereby connecting upstream biosynthesis to the downstream transformations required to produce 17. The identification of 10 as a precursor to 17 sheds critical light on the tentative chemical logical of this final tetracyclic scaffold formation. In particular, the addition of the N-acetyl group to the A-ring probably serves as a protecting group that ‘locks’ the tetracyclic lycodane scaffold in place, which would otherwise be in equilibrium with the enamine/imine (Fig. 5). In agreement with this premise, the N-acetyl group is only lost following formation of the A-ring lactam by Pt2OGD-4, which would also serve to deactivate the basicity of the nitrogen and protect the stability of this tetracyclic ring structure. Although we do not know the nature of the enzyme(s) required to convert 9 into the theoretical enamine/imine intermediate, we can be confident that an acetyltransferase family enzyme is required for the final step to yield 10.
Our efforts in identifying new enzymes in 17 biosynthesis (Fig. 5) provide fundamental insight into the previously cryptic reactions used to build and tailor the scaffold structures of neuroactive Lycopodium alkaloids and greatly expand our broader understanding of the enzymatic capabilities present in the plant kingdom. Most notably, our identification of several, neofunctionalized CAH family enzymes suggests that proteins from this family may have more widespread roles throughout plant metabolism than previously realized. Ultimately, our results place CAL proteins among a relatively short list of enzymes known in plants for the biosynthesis of specialized metabolite scaffolds2,3.
Methods
Chemicals and reagents
All common chemicals and reagents were obtained from commercial vendors. A mixture of 1-(piperidin-2-yl)propan-2-ol stereoisomers (5) was obtained commercially (MilliporeSigma). Authentic standards of (2S,4S)-5 (otherwise known as (+)-sedridine) and (2R,4S)-5 (otherwise known as (−)-allosedridine) were provided by P. Evans (University College Dublin). An authentic standard of lycophlegmarinine D (14) isolated from Phlegmariurus phlegmaria51 was provided by K. Pan (China Pharmaceutical University) and 8,15-dihydrohuperzine (21) was provided by R. Sarpong (University of California, Berkeley)17. The following Lycopodium alkaloids were previously isolated from Lycopodium platyrhizoma50: flabellidine (10), des-N-methyl-α-obscurine (18), des-N-methyl-β-obscurine (19), casuarinine H (20) and lycoplatyrine B (24). Confirmatory NMR spectra for 10 and 20 can be found in the Supplementary Information; those of 18 and 19 were previously reported23. The following Lycopodium alkaloids were purchased from commercial vendors: huperzine B (15, MilliporeSigma), huperzine C (16; two independent sources: Shanghai Tauto Biotech and Toronto Research Chemicals) and HupA (17, ApexBio Technology).
Transcriptomic and co-expression analysis
Transcriptomic data of P. tetrastichus were previously generated using PacBio IsoSeq for establishing a high-quality reference transcriptome of full-length sequences and Illumina HiSeq 4000 for quantification of gene expression across many tissue types and biological samples23. Protein sequences encoded by each transcript were annotated with the best-hit Pfam term54 using HMMER (http://hmmer.org/). We performed differential expression analysis between samples from new growth leaves (biosynthetically active for HupA production) and mature shoot tissue (inactive for HupA production) using edgeR (ref. 55). This analysis yielded 2,227 unique transcripts that had significantly higher expression in the new growth leaves. These transcripts were then included in hierarchical clustering analysis using Cluster 3.0 (ref. 56). For this, expression counts (trimmed mean of M-values (TMM)-normalized, c.p.m.) for each transcript were normalized to the median expression value for that transcript and these values were then log2-transformed. Transcripts were then hierarchically clustered using the Pearson correlation (centred) metric with average linkage and visualized in TreeView software (https://jtreeview.sourceforge.net/). Relevant clusters were identified on the basis of the presence of previously characterized genes from Lycopodium alkaloid biosynthesis (PtLDC-1, PtLDC-2, PtCAO-1, PtCAO-2, PtPIKS-1, PtPIKS-2, Pt2OGD-1, Pt2OGD-2 and Pt2OGD-3). This allowed for the identification of a minimally sized cluster of 131 transcripts that contained all previously characterized transcripts. Specific clusters of transcripts (cluster131 and cluster273) referenced are given in the Supplementary Information.
Agrobacterium-mediated transient expression
Candidate genes were cloned using complementary DNA from P. tetrastichus new growth leaves, much as previously described23. Following PCR amplification with primers containing appropriate overhangs, PCR products were gel purified and inserted into previously digested (AgeI/XhoI) pEAQ-HT plasmid (KanR) using isothermal DNA assembly. Assembled plasmid reactions were transformed into E. coli NEB 10-beta cells (New England Biolabs) and plated on selective LB agar plates (50 µg ml−1 of kanamycin) for overnight growth at 37 °C. Colonies were screened using PCR and the sequences of PCR products were confirmed using Sanger sequencing. Positive transformants were then used to inoculate 4 ml of liquid LB cultures, which were then shaken overnight at 37 °C. Plasmids were subsequently purified through miniprep and inserts were again sequence verified using Sanger sequencing. Plasmids containing genes of interest were transformed into Agrobacterium tumefaciens GV3101 (GentR) using the freeze-thaw method, plated onto selective LB agar plates (50 µg ml−1 of kanamycin and 30 µg ml−1 of gentamycin) and grown for 2 days at 30 °C. Positive transformants were verified through colony PCR and these were then inoculated into 2 ml of liquid LB cultures, which were shaken for 2 days at 30 °C. Colony PCR was again used to verify the presence of the plasmid construct in the liquid cultures, after which 25% glycerol stocks were prepared and stored at −80 °C for future use.
Screening of candidate genes through Agrobacterium-mediated transformation in N. benthamiana was performed much as previously described23,57. Agrobacterium strains harbouring plasmid constructs of interest were first thickly streaked from glycerol stocks onto LB agar plates (50 µg ml−1 of kanamycin and 30 µg ml−1 of gentamycin) and grown for 2 days at 30 °C. This lawn of cell growth was then removed using a sterile pipette tip, resuspended in 0.5 ml of LB and then pelleted through centrifugation at 8,000g for 5 min. Cells were then resuspended in 0.5 ml of Agrobacterium induction media (10 mM MES, 10 mM MgCl2, 150 µM acetosyringone, pH 5.6) and allowed to incubate at room temperature for at least 1 h. The concentrations of cell resuspensions were measured by taking their optical density OD600 and combinations of strains of interest were then combined at a final OD600 of 0.2–0.3 for each strain. A needleless syringe was then used to infiltrate these strain mixtures into the abaxial side of N. benthamiana leaves from 4–5-week-old plants, which were germinated and grown exactly as previously described23,57. For a typical experiment, three leaves from three different plants were used for each strain mixture to minimize any batch effects or biological variation among plants. Following infiltration, plants were grown as usual for 3–5 days, after which leaves were excised for subsequent metabolite extraction. For substrate co-infiltration experiments, plants were grown for 3 days after Agrobacterium infiltration, after which 100 µl of substrate (25 µM in water) was infiltrated into the infected portion of the leaf using a needleless syringe. The area infiltrated with substrate was marked and after one more day of plant growth, this area was excised for subsequent metabolite analysis.
Metabolite extraction
Following transient gene expression, Agrobacterium-infected leaf tissue was excised, placed in a preweighed 2 ml Safe-Lock tube (Eppendorf) and immediately snap frozen in liquid nitrogen. Typically, only one-quarter of a leaf was excised for analysis. When substrate was co-infiltrated, the entire marked area of substrate infiltration was excised and snap frozen. Snap-frozen samples were either stored at −80 °C or immediately lyophilized to dryness. Following lyophilizing, samples were kept on ice or at 4 °C during all stages of processing. After removal from the lyophilizer, samples were weighed to collect dry masses. A 5 mm diameter steel bead was then added to each sample tube and plant tissue was homogenized to a powder by shaking at 25 Hz for 2 min on a ball mill homogenizer (Retsch MM 400). Steel beads were removed with tweezers and homogenized tissue was extracted with an appropriate volume of solvent. For routine extraction, 80% methanol in water was added at an amount of 20 µl of solvent per milligram of dry leaf weight and, after mixing, samples were incubated on ice for at least 20 min. During the course of our experiments, we noted that certain intermediates (for example, 3, 7 and 8) would be depleted over time, either because of decomposition or reactivity with other metabolites from N. benthamiana. We found that extracting samples with ice-cold water + 0.1% (v/v) formic acid would improve the stability of these compounds without any major losses in alkaloid yield. As such, most of the LC–MS chromatograms that are shown for early pathway intermediates were derived from experiments in which water + 0.1% formic acid was used as the extraction solvent.
After incubation, samples were briefly vortexed and cell debris was pelleted through centrifugation at 10,000g and 4 °C for 5 min. After centrifugation, samples were prepared differently on the basis of the type of chromatographic analysis that was to be used (for example, C18 versus hydrophilic interaction chromatography (HILIC)). Samples related to the analysis of the early biosynthetic pathway (that is, any of the products generated by PtLDC-1/2, PtCAO-1/2, PtPIKS-1/2, PtSDR-1/2, PtACT-1, PtCYP782C1, PtCAL-1/PtCAL-2 and PtCAL-3) were diluted tenfold in ice-cold acetonitrile (ACN) to better match the starting solvent conditions for HILIC analysis. Samples related to the analysis of downstream intermediates (that is, any intermediates downstream of 10) were diluted 1:1 with water + 0.1% formic acid. All samples were then filtered through Multiscreen Solvinert filter plates (MilliporeSigma, Hydrophilic PTFE, 0.45 µm pore size) and subsequently transferred into LC–MS vials, which were stored at −20 or −80 °C until analysis.
Preparation of metabolites for chiral analysis
Many of the early intermediates could only be observed by HILIC analysis, which made it difficult to resolve enantiomers with standard chiral chromatography. Protection of the secondary amines of 4 and its pathway derivatives through N-acetylation allowed us to readily separate enantiomers (Extended Data Fig. 1c). The N-acetylation of standards, plant extracts and enzyme reactions was performed as follows. A 10 µl aliquot of sample was diluted into 90 µl of ACN (for standards, 10 µl of a 10 mM stock solution in methanol was used) and 200 µl of acetic anhydride was then added. Samples were then heated at 60 °C for 30 min, although we noted that heating was not strictly necessary for N-acetylation to readily occur. After this incubation, samples were moved onto ice for at least 5 min, after which 300 µl of methanol was added to quench the reaction. Quenched samples were then filtered and transferred into LC–MS vials, as described above. Standards were subsequently diluted to a concentration of 10–20 µM in 80% methanol before analysis.
LC–MS analysis
Samples were routinely analysed on two different LC–MS instrument setups: (1) an Agilent 1260 high-performance liquid chromatography (HPLC) instrument paired with an Agilent 6520 accurate-mass quadrupole time-of-flight (Q-TOF) mass spectrometer (6520 LC–MS) or (2) an Agilent 1290 Infinity II UHPLC paired with a coupled Agilent 6546 Q-TOF mass spectrometer (6546 LC–MS). For both instruments, all samples were analysed using electrospray ionization (ESI) in positive ionization mode. Each instrument also had an in-line diode array detector (DAD) for routine analysis of UV active compounds (Agilent 1100 DAD for 6520 LC–MS; Agilent 1290 Infinity II DAD for 6546 LC–MS). UV data were typically collected at wavelengths of 210, 230, 254 and 280 nm (4 nm bandwidth for each) with reference to 360 nm (100 nm bandwidth). Reversed-phase (C18) analysis was predominantly performed on the 6546 LC–MS using a ZORBAX RRHD Eclipse Plus C18 column (Agilent, 1.8 μm, 2.1 × 50 mm) with water + 0.1% formic acid and ACN + 0.1% formic acid as mobile phases. HILIC analysis was predominantly performed on the 6520 LC–MS using a Poroshell 120 HILIC-Z column (Agilent, 2.7 μm, 2.1 × 100 mm) with water and 9:1 ACN:water, each with 0.1% formic acid and 10 mM ammonium formate, as mobile phases. Chiral chromatography was performed on the 6520 LC–MS using a CHIRALPAK IC-3 column (Daicel, 3 μm, 4.6 × 100 mm) with water + 0.1% formic acid and ACN + 0.1% formic acid as mobile phases. Specific LC–MS method parameters can be found in the Supplementary Methods. In general, early pathways intermediates (compounds 3 through 9) were observed with HILIC analysis, whereas downstream intermediates (compounds 10 to 25) were observed with C18 analysis. We note that 6 in particular could be observed using either C18 or HILIC analysis. However, although diastereomers of 6 could be resolved with C18 analysis, these seemed to co-elute as a single peak in HILIC analysis. When applicable, mass ions pertaining to individual metabolites were fragmented using targeted MS2. This was normally performed with several collision energies (10, 20 and 40 V) but most of the presented data were collected with a collision energy of 20 V.
LC–MS data were routinely visualized and analysed using MassHunter Qualitative Analysis software. Extracted ion chromatograms shown in each figure were typically generated by extracting for the exact m/z for the target ion of interest with a 20 ppm mass tolerance window. Quantification of relative ion abundance was performed using the automated ‘Agile2’ method in MassHunter Quantitative Analysis software. For untargeted analysis, data files were converted into mzML format and XCMS software35 was used to identify any differentially produced mass ions between different gene expression conditions/reactions. This output was typically filtered to remove low-abundance ions (less than 1 × 105 ion abundance) and any ions that were not clearly differential between treatments (P > 0.2). XCMS analysis was typically followed with CAMERA software36 analysis to identify potential in-source ion adducts of detected metabolites. UV spectra for 8 and 9 were produced by using the Extract Spectrum function on the corresponding compound peak in MassHunter Qualitative Analysis.
Apoplast protein isolation
The three CAL proteins identified in this study have predicted N-terminal signal peptides, which were identified using the TargetP-2.0 server (https://services.healthtech.dtu.dk/service.php?TargetP-2.0)58. Preliminary confocal microscopy of C-terminal, GFP-tagged proteins did not support their main localization to be the endoplasmic reticulum or Golgi and initial analysis of images suggested that they may be localized to the apoplast. To assess this possibility, CAL genes with or without C-terminal 6xHis tags were transiently expressed in N. benthamiana, as described above. Each CAL gene was expressed individually; also, PtCAL-1a and PtCAL-2a were transiently co-expressed in the same leaf because we had found them to cofunction in Lycopodium alkaloid biosynthesis. At 4 days after Agrobacterium infiltration, apoplast protein extracts were isolated using the infiltration–centrifugation method, much as previously described40. Two leaves per reaction were excised from the plant and submerged in ice-cold apoplast extraction buffer (100 mM MES, 300 mM NaCl, pH 5.5) in an open-capped 50 ml Falcon tube and these tubes were placed in a plastic vacuum chamber attached to a Welch Model 2025 vacuum pump. The chamber was brought down to full vacuum and after 2 min at this pressure, the vacuum was slowly released to allow for buffer to infiltrate the leaf apoplastic space. Buffer-infiltrated leaves were carefully removed from the Falcon tubes, blotted dry with paper towels and were then rolled into Parafilm and placed in a plungerless 5 ml plastic syringe. The syringe was placed in a 15 ml Falcon tube and this was then centrifuged at 1,000g and 4 °C for 10 min to collect apoplast extract. The resulting extract was centrifuged at 10,000g and 4 °C for 15 min to pellet any larger cellular debris and the supernatant was concentrated using an Amicon Ultra-4 Centrifugal Filter Unit (10 kDA MWCO, MilliporeSigma UFC501024). Protein concentrations were measured using the BIO-RAD Protein Assay or Bradford assay (Abcam 119216) and adjusted with apoplast extraction buffer to a final concentration between 0.5 and 1.5 mg ml−1. Aliquots of the extracts were snap frozen in liquid nitrogen and stored at −80 °C.
Western blot analysis of plant extracts
To determine localization of CAL proteins in our N. benthamiana transient expression system, we performed western blot analysis of epitope tagged versions of each protein. Each CAL gene was PCR amplified from previously generated plasmid constructs using primers with overhangs for subsequent isothermal assembly into pEAQ-HT plasmid digested at the AgeI/XmaI restriction sites, which creates constructs with a C-terminal 6xHis tag. The reverse primer in this cloning strategy omitted the native stop codon of the CAL coding sequences to ensure that the final coding sequence included the C-terminal tag. These constructs were sequence verified, transformed into A. tumefaciens GV3101 and these strains were then used to transiently express these genes in N. benthamiana, as described above.
For the analysis of different protein fractions, apoplast extracts were prepared exactly as described above. Once apoplast extracts were obtained, the remaining leaf tissue was flash-frozen in liquid nitrogen and lyophilized to dryness. Lyophilized leaf tissue was pulverized to a powder with 5 mm stainless steel beads in a ball mill homogenizer (Retsch MM400) at 25 Hz for 2 min. Protein from homogenized samples was then extracted with ice-cold phosphate-buffered saline (PBS) supplemented with Halt protease and phosphatase inhibitor cocktail (Thermo Scientific PI78443) using 20 µl of buffer per mg dry leaf mass. This was incubated on ice for 20 min with periodic, gentle inversion, after which samples were centrifuged at 18,210g for 10 min at 4 °C to remove insoluble plant material. The remaining supernatant was kept and represented the ‘internal’ cell fraction, which would presumably contain cytosolic and microsomal proteins. Protein concentration was determined by Bradford assay (Abcam 119216) and extracts were stored at −80 °C until future use.
Samples for immunoblots were prepared by adding 4× NuPAGE LDS sample buffer (Fisher Scientific AAJ61894AC) to a final concentration of 1× sample buffer with 2.5% β-mercaptoethanol and samples were then heated for 20 min at 70 °C. Total protein for apoplast (2.5 µg) and PBS extracts (5 μg) was separated on NuPAGE gels and then transferred onto a PVDF membrane (BIO-RAD 1704272) using a Trans-Blot semidry transfer system (BIO-RAD). Blots were blocked in EveryBlot blocking buffer (BIO-RAD 12010020) for more than 5 min at room temperature and incubated with mouse anti-His (Genscript A00186) at 0.1 µg ml−1 in EveryBlot buffer for 1 h at room temperature or overnight at 4 °C. After washing three times with PBST (PBS + 0.1% Tween), blots were incubated with horse antimouse IgG, HRP-linked antibody (Cell Signaling Technology 7076) at 1:3,000 dilution. Blots were then washed five times with PBST and imaged with an iBright FL1500 Imaging System (Thermo Fisher Scientific).
Heterologous expression of CYP782C1 in yeast, microsomal protein preparation and in vitro enzyme assays
Expression of PtCYP782C1 in Saccharomyces cerevisiae (yeast) was performed as previously described57,59. Briefly, the coding sequence of PtCYP782C1 was PCR amplified and annealed into the pYeDP60 plasmid. This plasmid construct was transformed into S. cerevisiae WAT11 (ade2) and positive transformants were selected on synthetic drop-out medium plates lacking adenine (6.7 g l−1 of yeast nitrogen base without amino acids, 20 g l−1 of glucose, 2 g l−1 of drop-out mix minus adenine, 20 g l−1 of agar) through growth at 30 °C for 2 days. Presence of the plasmid constructs was confirmed by colony PCR. A single, positive colony was used to inoculate a starter 4 ml of culture of liquid drop-out medium, which was grown at 28 °C and 250 r.p.m. Following 2 days of growth, 2 ml of the starter culture was used to inoculate 500 ml of YPGE medium (10 g l−1 of Bacto yeast extract, 10 g l−1 of Bacto peptone, 5 g l−1 of glucose and 3% (v/v) ethanol). This culture was grown at 28 °C and 250 r.p.m until reaching a cell density of 5 × 107 cells ml−1, which was estimated through OD600 measurements. After reaching this density, expression was induced by adding 50 ml of a sterile galactose solution (200 g l−1) to achieve a concentration of approximately 10% (v/v). The culture was then grown at 28 °C and 250 r.p.m. for another 16 h to achieve a cell density of approximately 5 × 108 cells ml−1, after which this culture was immediately used for microsomal protein isolation, which was performed exactly as previously described59. Microsomal protein was stored in TEG buffer (50 mM Tris-HCl, 1 mM EDTA, 20% (v/v) glycerol, pH 7.4), aliquoted into 1.5 ml microfuge tubes, snap frozen in liquid nitrogen and stored at −80 °C.
Enzyme reactions with PtCYP782C1-enriched microsomal protein were performed in potassium phosphate buffer (50 mM potassium phosphate, 100 mM sodium chloride, pH 7.8) and typically contained 4 µg of microsomal protein (final concentration of 0.02 µg µl−1), 500 µM NADPH and 50 µM of 6 substrate in a total reaction volume of 200 µl. Control reactions omitted NADPH or used microsomal protein that was heated at 95 °C for at least 10 min. Following addition of all components, reactions were incubated at room temperature for a minimum of 10 min. At specific time points, 20 µl aliquots of the reaction were added to 180 µl of ACN + 0.1% formic acid to quench the reaction. Quenched reactions were then filtered and transferred into LC–MS vials, as previously described. Products of PtCYP782C1 activity on 6 were assessed through LC–MS using HILIC analysis.
In vitro enzyme reactions with apoplastic CAL protein
Reactions with CAL-enriched apoplast were routinely performed in low-pH potassium phosphate buffer (50 mM potassium phosphate, 100 mM NaCl, pH 5.9) at a volume of 20 µl. For PtCAL-1a/PtCAL-2a, these reactions contained approximately 1.4 µg of apoplast protein for each CAL (final concentration of 0.07 µg µl−1). Control reactions used apoplast from leaves expressing only one CAL or with apoplast generated from GFP-expression N. benthamiana leaves. The requisite substrates for this reaction were generated through the activities of in vitro PtPIKS-1 and PtCYP782C1 enzyme reactions. We found that the PtCYP782C1 microsomal reaction did not work well at the lower pH (pH 5–6) at which the CAL enzymes seemed to be most active (Extended Data Figs. 2g and 5k). Therefore, before the CAL reactions, we ran a separate PtCYP782C1 microsomal protein assay in high pH buffer (50 mM potassium phosphate, 100 mM sodium chloride, pH 7.8), much as described above, for a minimum of 2 h to generate sufficient 8 as a substrate. To maximize the amount of 8 produced, substrate-generating PtCYP782C1 reactions (100 µl) contained 1.5 mM of substrate (6), 10 µg of PtCYP782C1 microsomes (final concentration of 0.1 µg µl−1) and 4 mM NADPH. After these incubations, a 2 µl aliquot of the PtCYP782C1 reaction (now containing 8) was added to the PtCAL-1a/PtCAL-2a apoplast enzyme assay setup (20 µl of total reaction volume). To generate 3 and 4 as potential substrates, 1 µg of previously purified PtPIKS-1 enzyme23 and 150 µM 1 and 300 µM malonyl-CoA were added directly to the CAL reaction mixtures. After thorough mixing, reactions were incubated at room temperature. An alternative route for producing 3 and 4 independently of thioester intermediates was achieved by mixing stocks of 1 (10 mM in water) and 2 (10 mM in water; always prepared fresh to minimize compound decomposition) in equal proportion, followed by incubation at room temp for 1–2 h, as these two substrates can non-enzymatically condense to yield 3 (which can spontaneously decarboxylate to produce 4). A 2 µl aliquot of this mixture was then added as a component of the PtCAL-1a/PtCAL-2a enzyme reaction (20 µl total reaction volume) in addition to the PtCYP782C1 microsomal reaction mixture. After predesignated incubation times, reactions were quenched by diluting tenfold into ACN with 0.1% formic acid.
For PtCAL-3 activity assays, 5 µg of PtCAL-3 apoplast (final concentration 0.1 µg µl−1 of apoplast protein), was diluted into in low-pH potassium phosphate buffer (50 mM potassium phosphate, 100 mM NaCl, pH 5.9) at a volume of 50 µl just as with PtCAL-1a/PtCAL-2a. To generate 3 and 4 as potential substrates in vitro, 1 µg of previously purified PtPIKS-1 enzyme23 was added to this reaction (final concentration 0.02 µg µl−1) with 150 µM 1 and 300 µM malonyl-CoA added as substrates. In follow-up experiments, the PIKS reaction was omitted and 1 and 2 were added as direct substrates to a final concentration of 500 µM each. When 4 was tested as a substrate, it was added at a concentration of 150 µM. All reactions were incubated at room temperature for predesignated amounts of time, after which aliquots were quenched through fivefold dilution in ice-cold ACN. For all CAL apoplast enzyme reactions, product formation was predominantly assessed through LC–MS using HILIC analysis. To assess the formation of specific enantiomers or consumption of specific enantiomeric substrates, quenched reactions were N-acetylated and analysed by chiral LC–MS, as described above.
To evaluate the potential decarboxylation of β-keto acid substrates by PtCAL-1/PtCAL-2 and PtCAL-3, only 3 or 2, respectively, were added as substrate. PtCAL-1/PtCAL-2 reactions were quenched by diluting aliquots tenfold into ACN with 0.1% formic acid and were subsequently analysed through HILIC LC–MS. PtCAL-3 reactions were quenched by mixing aliquots with an equal volume of water with 0.2% formic acid and were then analysed through C18 LC–MS. Decarboxylation was assessed by comparing the relative ion abundances of each substrate to that of their decarboxylated product; for 3, this pertained to 4 and for 2, this pertained to acetoacetic acid (AcAc). For all reactions, GFP apoplast with relevant substrates was analysed as a negative control. This was critical for relative quantification of decarboxylation, as this can happen readily to both 3 and 2 at room temperature.
For evaluation of Zn2+ as a cofactor for PtCAL-1/PtCAL-2 and PtCAL-3 catalytic activity, 0.3 ml of apoplast extract containing the CAL proteins was incubated with 13 ml of physiological pH potassium phosphate buffer (50 mM potassium phosphate, 100 mM NaCl, pH 7.5) containing 10 mM of the Zn2+ chelating reagent 2,6-pyridinedicarboxylic acid (PDCA)44 at 4 °C for 4 h with gentle rocking. PDCA was then diluted out by a factor of 108 through buffer exchange (50 mM potassium phosphate, 100 mM NaCl, pH 5.9) using Amicon Ultra-4 Centrifugal Filter Units (10 kDA MWCO, MilliporeSigma UFC501024). To control for possible loss of activity during this treatment and purification time, separate CAL apoplast extracts were treated and prepared as above but without PDCA. Protein concentrations were measured using the BIO-RAD Protein Assay or Bradford assay (Abcam 119216) and adjusted with potassium phophate buffer to a final concentration between 0.5 and 1.5 mg ml−1. Aliquots of the extracts were then snap frozen in liquid nitrogen and stored at −80 °C. Standard in vitro reactions for PtCAL-1/PtCAL-2 and PtCAL-3 were then run as described above to evaluate any effects on product formation. For Zn2+ supplementation, a final concentration of 1 mM ZnCl2 was added to reaction mixtures44.
Synthesis of 6 stereoisomers
To synthesize 6 stereoisomers, 150 mg of previously synthesized pelletierine (4, oil, 1 mmol)23 was added to 1 ml of methanol in a glass vial with a magnetic stir bar. This mixture was stirred on ice and 0.095 g (2.5 equiv.) of NaBH4 was added slowly. This reaction was allowed to incubate on ice for 2 h. The reaction was quenched through the addition of 2 ml of distilled water followed by 2 ml of 2 M HCl. The pH of the reaction was increased to pH 10 with 6 M NaOH (about 0.3 ml) and this was then extracted with diethyl ether (5 × 5 ml). The organic fractions were pooled, dried with anhydrous sodium sulfate, clarified using a filter and evaporated to dryness using a rotary evaporator system. A portion of this residue, which would be mainly composed of 5 stereoisomers, was then O-acetylated following an established protocol60. To accomplish this, 50 mg (0.35 mmol) of the synthesized 5 stereoisomers was dissolved in 100 µl of 6 N HCl in a glass vial. Next, 100 µl of acetic acid was added and this mixture was cooled to about 0 °C in an ice bath. Once this mixture was chilled, 1 ml of acetyl chloride was slowly added dropwise. This reaction was then incubated in the ice bath for 1 h, with periodic, gentle mixing. After this incubation, a 1 µl aliquot of this reaction was diluted in 1 ml of water + 0.1% (v/v) formic acid and this was analysed through C18 LC–MS to confirm the formation of the same acetylated compounds that were produced by PtACT-1. The full reaction was diluted in 25 ml of ice-cold distilled water, then clarified through filter paper.
The putative 6 stereoisomers were then purified by using a Sep-Pak C18 12 cc, 2 g Vac Cartridge (Waters). To do so, this cartridge was pre-equilibrated with 3 column volumes (CVs) of ACN + 0.1% (v/v) formic acid, followed by equilibration with 4 CVs of water + 0.1% (v/v) formic acid. The reaction mixture was then loaded onto the cartridge and the solvent was allowed to flow through. The loaded cartridge was then washed with 3 CVs of water + 0.1% (v/v) formic acid and the products (visibly yellow on the cartridge) were eluted with 30% ACN in water (with 0.1% v/v formic acid). Small (about 0.5 ml) fractions of the eluent were collected and 1 µl of each were diluted in water + 0.1% (v/v) formic acid and analysed through C18 LC–MS to confirm the presence of putative 6 diastereomers. Relatively pure fractions were combined, diluted into 20 ml of water + 0.1% (v/v) formic acid and repurified over the same type of cartridge, much as described above. For this second round of purification, ACN in water (+0.1% v/v formic acid) was added as an eluent at incrementally increasing concentrations (1 CV each of 2%, 4%, 6%, 8%, 10%, 20% and 40% ACN). Collected fractions were screened through LC–MS and pure fractions were combined, frozen and lyophilized to dryness. The resulting purified compound (about 20 mg) consisted of a yellowish powder. For structural confirmation, this was dissolved in CDCl3 and we then performed 1H and 13C NMR analysis using a Varian Inova 500 MHz NMR spectrometer (Supplementary Figs. 8 and 9).
Synthesis of enantio-enriched (R)- and (S)-pelletierine (4)
Enantiomers of 4 were synthesized by following a previously established protocol61. To a 25 ml round bottom flask with a magnetic stir bar were added 1-piperideine (1, 81 mg, 0.97 mmol, 1 equiv.), acetone (3.26 ml, 44.46 mmol, 46 equiv.), DMSO (3.26 ml), water (0.41 ml) and either d- or l-proline (21.2 mg, 0.19 mmol, 0.2 equiv.). l-proline was used to achieve enantio-enriched (S)-4, whereas d-proline was used to produce enantio-enriched (R)-4 (ref. 61). The reaction mixtures were stirred for 1 h at room temperature, after which 10 ml of saturated sodium bicarbonate in water was added. This was then extracted twice with 50 ml of dichloromethane. These organic fractions were combined and then extracted with 50 ml of brine. Residual water was removed from the remaining organic extract through the addition of anhydrous magnesium sulfate, after which this extract was clarified through filter paper and dried on a rotary evaporator system. The remaining yellow/brown oil represented the 4 product. Successful reactions were confirmed by N-acetylating a fraction of the product and analysing through chiral LC–MS, as described above. This method resulted in approximately 70% enantiomeric excess for each specified enantiomer.
Scaled-up production of CAL-1a/CAL-2a enzymatic product
To achieve milligram quantities of the observed product of PtCAL-1a/PtCAL-2a (m/z 164, 9), the leaves of 109 N. benthamiana plants (410 g fresh weight) were vacuum infiltrated62 with a combination of Agrobacterium strains necessary for engineering the production of this compound (PtLDC-2, PtCAO-1, PtPIKS-1, PtSDR-2, PtACT-1, PtCYP782C1, PtCAL-1a, PtCAL-2a and PtCAL-3). To prepare sufficient quantities of Agrobacterium for this scale, Agrobacterium strains harbouring the necessary gene constructs were first streaked on selective LB agar plates (50 µg ml−1 of kanamycin and 30 µg ml−1of gentamycin) and grown for 2 days at 30 °C to achieve colonies. Single colonies were then used to inoculate 1 l of liquid LB cultures (50 µg ml−1 of kanamycin and 30 µg ml−1 of gentamycin), which were shaken overnight at 30 °C and 250 r.p.m. Bacteria were then pelleted through centrifugated at 5,000g for 10 min, after which they were resuspended in a minimal volume of Agrobacterium induction buffer. Bacterial densities were measured through OD600 and strains were mixed together into a 3 l volume of induction buffer such that each strain had a final calculated density of OD600 = 0.2. This solution was transferred into a plastic beaker and this was placed into a plastic, vacuum desiccator. Each N. benthamiana plant was placed upside-down into the Agrobacterium mixture, and the desiccator chamber was brought down to vacuum for 2 min using a Welch Model 2025 vacuum pump, which removed air from the leaves. Pressure was then slowly released, which results in Agrobacterium solution infiltrating the previous air space of the leaves. This process was repeated for all 109 N. benthamiana plants. Infiltrated plants were then grown as usual for 6 days, after which they were collected and stored at −80 °C until compound purification.
To extract metabolites, frozen plant samples were homogenized in a blender along with 1.5 l of 100% ethanol. This extract was incubated overnight at room temperature in a 4 l flask, after which plant material was removed through clarification over filter paper (this was repeated twice to remove particulates). This ethanol extract was then dried on a rotary evaporator with gentle heating from a water bath (around 30 °C), after which about 50 ml of water still remained. This was resuspended in 400 ml of 3% tartaric acid in water (w/v) and then extracted with 3× 200 ml ethyl acetate to remove hydrophobic compounds. The pH of the aqueous extract was then increased to pH 8–9 using sodium bicarbonate and this was then extracted with 3× 200 ml ethyl acetate. LC–MS screening of extracts demonstrated that almost none of the Lycopodium alkaloid-related intermediates were extracted from the aqueous phase at pH 8–9; instead, this fraction largely contained nicotine-related alkaloids that are native to N. benthamiana metabolism. The aqueous phase was then basified to pH 10–11 using 6 M NaOH and this was extracted with 3× 400 ml ethyl acetate. LC–MS screening confirmed that nearly all of the Lycopodium alkaloid intermediates, including our desired compound (proposed 9), could be found in this organic extract. These ethyl acetate fractions were combined, dried with anhydrous magnesium sulfate, clarified through filter paper and then evaporated to dryness using a rotary evaporator. The remaining residue (about 170 mg) consisted mainly of a yellow/brown oil. This was redissolved in 20 ml of ethyl acetate and this was filtered to remove any insoluble components and then evaporated to dryness. This residue was then resuspended in a minimal volume of 50:50 hexanes/ethyl acetate (about 5 ml) and was purified using a Biotage Selekt Flash Purification System with a Biotage Sfär KP-Amino D column (50 µm particle size, 5 g volume). Purification conditions consisted of an initial isocratic elution of 100% hexanes/0% ethyl acetate for 3 CVs, followed by a gradient from 100% hexanes/0% ethyl acetate to 0% hexanes/100% ethyl acetate over 10 CVs, with a final 5 CVs at 0% hexanes/100% ethyl acetate. All fractions were collected in 10 ml increments. Each fraction was then screened for 9 through LC–MS with HILIC conditions. This purification strategy allowed for partial purification of our compound. Fractions containing 9 were combined, evaporated to dryness and subjected to the same purification workflow several times (with smaller fraction sizes) to achieve pure 9. All other fractions, which contained other Lycopodium alkaloid-related compounds, were dried and saved at 4 °C for future use.
We found that our isolated compound (predicted 9) was relatively unstable; resuspension of this compound in either deuterated chloroform (CDCl3) or deuterated methanol (CD3OD) and analysis through 1H NMR demonstrated loss of indicative chemical shifts over time, although this did allow us to obtain a crude 1H NMR (CDCl3, 500 MHz) for this compound (Supplementary Figs. 10 and 11). Also, on drying of our sample from CDCl3, we noted a colour change from yellow/brown to red. Loss of our compound was confirmed through LC–MS analysis. However, we observed that during the course of purification, a compound pertaining to an oxidation of 9 (m/z 263.2118; equal to 9 + oxygen) accumulated to high concentrations. This compound (9′) showed a similar LC–MS retention time to 9 and had an MS2 fragmentation pattern that seemed to indicate a phlegmarane-like scaffold structure, which suggested that it may be an oxidized byproduct of 9 (Extended Data Fig. 3o–q). As such, we purified this compound using the same strategy outlined above (yield of about 3 mg) and determined a putative structure (proposed 9′) through NMR analysis. For 9′, deuterated ACN (CD3CN) was used as a solvent and spectra were collected on a Varian Inova 600 MHz NMR spectrometer at room temperature (Supplementary Figs. 12–18). Although we were not able to resolve the stereochemistry of the C16 methyl with our NMR analyses, nearly all isolated Lycopodium alkaloids, including those with the phlegmarane scaffold, exhibit R stereochemistry at this location53 and thus, we tentatively predict this same R stereochemistry for the C16 methyl of 9′ and thus 9.
Sequence analysis and structural modelling of CAL genes and proteins
All analyses of CAL genes and proteins were performed in Geneious (v.2019.2). To generate protein alignments of CAH family proteins, an assortment of protein sequences containing the CAH domain were downloaded from UniProt (https://www.uniprot.org/). Most of these downloaded proteins were selected from plant species (these were selected pseudorandomly to capture a breadth of phylogenetic diversity) and we included all CAHs from plants that, to our knowledge, have been biochemically verified to have canonical CAH activity. We also included sequences from animals, fungi, algae and bacteria, including several proteins that have been biochemically verified to have canonical activity. The human CA2 protein (otherwise known as CAII, hCA II; UniProt ID: P00918) was used as a reference for amino acid numbering in alignments, as this is probably the most rigorously studied CAH protein37. The downloaded CAH proteins and the CAH family proteins identified in our transcriptomic dataset (for a set of 80 proteins total) were aligned using the MUSCLE algorithm and phylogenetic trees were constructed using the neighbour-joining method (100 bootstraps) with the Jukes–Cantor genetic distance model. The trees shown in Fig. 4b and Supplementary Fig. 2 have been transformed to align all sequence names. Shown adjacent to each tree in these figures are the amino acid sequences that align to well-defined active site residues in human CA2. Any changes to these residues are indicated in the figure and are colour-coded by amino acid.
The structures of PtCAL-1a, PtCAL-2a and PtCAL-3 were modelled using AlphaFold2 through ColabFold (v.1.5.2)48. Each of these proteins is predicted to have an N-terminal signal peptide58, which would be cleaved during processing and trafficking of these proteins, so structural models were generated with the predicted signal peptide removed (21 amino acid truncation for PtCAL-1a, 23 amino acid truncation for PtCAL-2a, 32 amino acid truncation for PtCAL-3). The highest confidence models are shown in Extended Data Fig. 6. We also used AlphaFold-Multimer through ColabFold48 to explore possible protein–protein interactions between PtCAL-1a and PtCAL-2a, given that these proteins must be co-expressed in N. benthamiana leaves to obtain biochemically active protein extracts. These data provide modest but not definitive support for the formation of a protein heterocomplex and the predicted aligned error plots for the top five ranked heterodimers, as well as the structural model for the top-ranked prediction, are shown in Supplementary Fig. 3.
IC50 values for AChE inhibition by lycodane-type Lycopodium alkaloids
Previous work has determined the ability of various Lycopodium alkaloids to inhibit AChE. A selection of these results are compiled and listed in Fig. 5. References for each of the IC50 values for each of the compounds are cited as follows: lycophlegmarinine D (14)51, huperzine B (15)63, huperzine C (16)63, HupA (17)64, des-N-methyl-α-obscurine (18)65, des-N-methyl-β-obscurine (19)66, casuarinine H (20)67, 8,15-dihydrohuperzine A (21)68 and lycoplatyrine B (24)50. Compounds annotated as ‘low/not detected’ were not found to have AChE inhibition in the detectable range of each experiment in question (typically, IC50 values in these experiments were not measurable or were greater than 30 µM).
General statistical analysis
All statistical analyses in this manuscript represent measurements from distinct biological samples, not repeat measurements. No statistical methods were used to predetermine sample sizes, and in general, three replicates were used in each experiment, unless stated otherwise. For experiments involving transient gene expression in N. benthamiana, triplicates were spread across three different plants to minimize any biological batch effects inherent to individual plants. All bar graphs shown in the manuscript represent the mean and error bars represent standard deviation from the mean. Essentially all experimental results reported in this manuscript were confirmed through at least two independent experiments and, in most cases, in more than three independent experiments. Blinding was not used during data collection and analysis, and randomization was not used in experimental design.
General software use and graph generation
Routine data compilation was performed in Microsoft Excel 2016. General analysis of LC–MS data was performed with Agilent MassHunter Qualitative Analysis 10.0. Chromatograms and mass spectra were plotted using IGOR Pro 6.0. Bar graphs and line graphs were plotted using GraphPad Prism 9 and this software was also used for routine statistical analysis. We performed hierarchical clustering analysis using Cluster 3.0 (ref. 56). R (v.4.2.2) was used for bar graph generation, visualization of hierarchical clustering data and for performing XCMS analysis69. Geneious Prime (v.2019.2.3) was used for bioinformatic analyses of nucleic acid and protein sequences. This software was also used for several sequence alignments (MUSCLE algorithm) and phylogenetic tree generation (Jukes–Cantor genetic distance model, neighbour-joining tree build method). The TargetP-2.0 server (https://services.healthtech.dtu.dk/service.php?TargetP-2.0)58 was used for predicting signal peptides and protein localization. MNova (v.1.6) was used for visualization and processing of NMR data. ChemDraw Professional (v.21.0.0.28) was used for chemical structure visualization and analysis. Structural modelling was performed using AlphaFold-Multimer through ColabFold (v.1.5.2) and protein models were visualized in PyMol (v.2.5.4).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data in this manuscript are available on request. The raw RNA-seq data analysed in this manuscript have previously been deposited to the NCBI Sequence Read Archive (BioProject PRJNA731132)23. Gene sequences for enzymes characterized in this study are deposited in the National Center for Biotechnology (NCBI) GenBank under the following accessions: Pt2OGD-4 (OR538095), Pt2OGD-5 (OR538096), PtABH-1 (OR538097), PtACT-1 (OR538098), PtCAL-1a (OR538099), PtCAL-1b (OR538100), PtCAL-2a (OR538101), PtCAL-2b (OR538102), PtCAL-3 (OR538103), PtCYP782C1 (four homologues; OR538104, OR538105, OR538106, OR538107), PtSDR-1 (OR538108) and PtSDR-2 (OR538109). The UniProt database (https://www.uniprot.org/) was used for identifying and obtaining CAH family sequences that were used in phylogenetic analyses. The human CA2 protein structure (2vva) was acquired from PDB (https://www.rcsb.org/). Any materials generated in this manuscript will be made available, as possible. Source data are provided with this paper.
References
Wink, M. in Modern Alkaloids: Structure, Isolation, Synthesis and Biology (eds Fattorusso, E. & Taglialatela‐Scafati, O.) 1–24 (Wiley, 2007).
Lichman, B. R. The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep. 38, 103–129 (2021).
Anarat-Cappillino, G. & Sattely, E. S. The chemical logic of plant natural product biosynthesis. Curr. Opin. Plant Biol. 19, 51–58 (2014).
Ma, X. & Gang, D. R. The Lycopodium alkaloids. Nat. Prod. Rep. 21, 752–772 (2004).
Liu, J.-S. et al. The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can. J. Chem. 64, 837–839 (1986).
Pluskal, T. & Weng, J. K. Natural product modulators of human sensations and mood: molecular mechanisms and therapeutic potential. Chem. Soc. Rev. 47, 1592–1637 (2018).
Houghton, P. J., Ren, Y. & Howes, M. J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 23, 181–199 (2006).
Gutiérrez-Grijalva, E. P., López-Martínez, L. X., Contreras-Angulo, L. A., Elizalde-Romero, C. A. & Heredia, J. B. in Plant-Derived Bioactives: Chemistry and Mode of Action (ed. Swamy, M. K.) 85–117 (Springer, 2020).
Roddan, R., Ward, J. M., Keep, N. H. & Hailes, H. C. Pictet–Spenglerases in alkaloid biosynthesis: future applications in biocatalysis. Curr. Opin. Chem. Biol. 55, 69–76 (2020).
Bunsupa, S., Yamazaki, M. & Saito, K. Lysine-derived alkaloids: overview and update on biosynthesis and medicinal applications with emphasis on quinolizidine alkaloids. Mini-Rev. Med. Chem. 17, 1002–1012 (2017).
Mancinotti, D., Frick, K. M. & Geu-Flores, F. Biosynthesis of quinolizidine alkaloids in lupins: mechanistic considerations and prospects for pathway elucidation. Nat. Prod. Rep. 39, 1423–1437 (2022).
Xu, M., Heidmarsson, S., de Boer, H. J., Kool, A. & Olafsdottir, E. S. Ethnopharmacology of the club moss subfamily Huperzioideae (Lycopodiaceae, Lycopodiophyta): a phylogenetic and chemosystematic perspective. J. Ethnopharmacol. 245, 112130 (2019).
Wang, B., Guan, C. & Fu, Q. The traditional uses, secondary metabolites and pharmacology of Lycopodium species. Phytochem. Rev. 21, 1–79 (2022).
Ma, X., Tan, C., Zhu, D., Gang, D. R. & Xiao, P. Huperzine A from Huperzia species—an ethnopharmacological review. J. Ethnopharmacol. 113, 15–34 (2007).
Ma, X., Tan, C., Zhu, D. & Gang, D. R. Is there a better source of huperzine A than Huperzia serrata? Huperzine A content of Huperziaceae species in China. J. Agric. Food Chem. 53, 1393–1398 (2005).
Bödeker, K. Lycopodin, das erste Alkaloïd der Gefässkryptogamen. Justus Liebigs Ann. Chem. 208, 363–367 (1881).
Haley, H. M. S. et al. Bioinspired diversification approach toward the total synthesis of lycodine-type alkaloids. J. Am. Chem. Soc. 143, 4732–4740 (2021).
Kitajima, M. & Takayama, H. Lycopodium alkaloids: isolation and asymmetric synthesis. Top. Curr. Chem. 309, 1–31 (2012).
Siengalewicz, P., Mulzer, J. & Rinner, U. in Alkaloids: Chemistry and Biology (ed. Knolker, H.-J.) 1–151 (Elsevier, 2013).
Bunsupa, S. et al. Molecular evolution and functional characterization of a bifunctional decarboxylase involved in Lycopodium alkaloid biosynthesis. Plant Physiol. 171, 2432–2444 (2016).
Xu, B., Lei, L., Zhu, X., Zhou, Y. & Xiao, Y. Identification and characterization of l-lysine decarboxylase from Huperzia serrata and its role in the metabolic pathway of Lycopodium alkaloid. Phytochemistry 136, 23–30 (2017).
Wang, J. et al. Deciphering the biosynthetic mechanism of pelletierine in Lycopodium alkaloid biosynthesis. Org. Lett. 22, 9725–8729 (2020).
Nett, R. S., Dho, Y., Low, Y.-Y. & Sattely, E. S. A metabolic regulon reveals early and late acting enzymes in neuroactive Lycopodium alkaloid biosynthesis. Proc. Natl Acad. Sci. USA 118, e2102949118 (2021).
Castillo, M., Gupta, R. N., Ho, Y. K., MacLean, D. B. & Spenser, I. D. Biosynthesis of lycopodine. Incorporation of 1-piperideine and of pelletierine. Can. J. Bot. 48, 2911–2918 (1970).
Braekman, J.-C., Gupta, R. N., MacLean, D. B. & Spenser, I. D. Biosynthesis of lycopodine. Pelletierine as an obligatory intermediate. Can. J. Chem. 50, 2591–2602 (1972).
Gupta, R. N., Castillo, M., MacLean, D. B., Spenser, I. D. & Wrobel, J. T. Biosynthesis of lycopodine. J. Am. Chem. Soc. 90, 1360–1361 (1968).
Castillo, M., Gupta, R. N., MacLean, D. B. & Spenser, I. D. Biosynthesis of lycopodine from lysine and acetate. The pelletierine hypothesis. Can. J. Chem. 48, 1893–1903 (1970).
Marshall, W. D., Spenser, I. D., Nguyen, T. T. & MacLean, D. B. Biosynthesis of lycopodine. The question of the intermediacy of piperidine-2-acetic acid. Can. J. Chem. 53, 41–50 (1975).
Hemscheidt, T. & Spenser, I. D. Biosynthesis of lycopodine: incorporation of acetate via an intermediate with C2v symmetry. J. Am. Chem. Soc. 115, 3020–3021 (1993).
Hemscheidt, T. & Spenser, I. D. A classical paradigm of alkaloid biogenesis revisited: acetonedicarboxylic acid as a biosynthetic precursor of lycopodine. J. Am. Chem. Soc. 118, 1799–1800 (1996).
Lenz, R. & Zenk, M. H. Acetyl coenzyme A: salutaridinol-7-O-acetyltransferase from Papaver somniferum plant cell cultures. J. Biol. Chem. 270, 31091–31096 (1995).
Matsuda, Y., Wakimoto, T., Mori, T., Awakawa, T. & Abe, I. Complete biosynthetic pathway of anditomin: nature’s sophisticated synthetic route to a complex fungal meroterpenoid. J. Am. Chem. Soc. 136, 15326–15336 (2014).
Roberts, M. F., Cromwell, B. T. & Webster, D. E. The occurrence of 2-(2-propenyl)-Δ1-piperideine in the leaves of pomegranate (Punica granatum L.). Phytochemistry 6, 711–717 (1967).
Kosower, E. M. & Sorensen, T. S. Some unsaturated imines. J. Org. Chem. 28, 692–695 (1963).
Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R. & Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching and identification. Anal. Chem. 78, 779–787 (2006).
Kuhl, C., Tautenhahn, R., Böttcher, C., Larson, T. R. & Neumann, S. CAMERA: an integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 84, 283–289 (2012).
Supuran, C. T. Structure and function of carbonic anhydrases. Biochem. J 473, 2023–2032 (2016).
DiMario, R. J., Clayton, H., Mukherjee, A., Ludwig, M. & Moroney, J. V. Plant carbonic anhydrases: structures, locations, evolution and physiological roles. Mol. Plant 10, 30–46 (2017).
DiMario, R. J., Machingura, M. C., Waldrop, G. L. & Moroney, J. V. The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci. 268, 11–17 (2018).
O’Leary, B. M., Rico, A., McCraw, S., Fones, H. N. & Preston, G. M. The infiltration–centrifugation technique for extraction of apoplastic fluid from plant leaves using Phaseolus vulgaris as an example. J. Vis. Exp. 94, e52113 (2014).
Walsh, C. T. Biologically generated carbon dioxide: nature’s versatile chemical strategies for carboxy lyases. Nat. Prod. Rep. 37, 100–135 (2020).
Finefield, J. M., Sherman, D. H., Kreitman, M. & Williams, R. M. Enantiomeric natural products: occurrence and biogenesis. Angew. Chemie Int. Ed. 51, 4802–4836 (2012).
McCall, K. A., Huang, C. C. & Fierke, C. A. Function and mechanism of zinc metalloenzymes. J. Nutr. 130, 1437S–1446S (2000).
Kim, J. K. et al. Elucidating the role of metal ions in carbonic anhydrase catalysis. Nat. Commun. 11, 4557 (2020).
Picaud, S. S. et al. Crystal structure of human carbonic anhydrase-related protein VIII reveals the basis for catalytic silencing. Proteins Struct. Funct. Bioinformatics 76, 507–511 (2009).
Nishimori, I. et al. Restoring catalytic activity to the human carbonic anhydrase (CA) related proteins VIII, X and XI affords isoforms with high catalytic efficiency and susceptibility to anion inhibition. Bioorg. Med. Chem. Lett. 23, 256–260 (2013).
Hunt, J. B., Rhee, M. J. & Storm, C. B. A rapid and convenient preparation of apocarbonic anhydrase. Anal. Biochem. 79, 614–617 (1977).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Alam, S. N., Adams, A. H. & MacLean, D. B. Lycopodium alkaloids. XV. Structure and mass spectra of some minor alkaloids of L. flabelliforme. Can. J. Chem. 42, 2456–2466 (1964).
Yeap, J. S. Y. et al. Lycopodium alkaloids: lycoplatyrine A, an unusual lycodine-piperidine adduct from Lycopodium platyrhizoma and the absolute configurations of lycoplanine D and lycogladine H. J. Nat. Prod. 82, 324–329 (2019).
Jiang, J.-M. et al. Lycophlegmarinines A–F, new Lycopodium alkaloids from Phlegmariurus phlegmaria. Tetrahedron 114, 132782 (2022).
Ainge, G. D., Lorimer, S. D., Gerard, P. J. & Ruf, L. D. Insecticidal activity of huperzine A from the New Zealand clubmoss, Lycopodium varium. J. Agric. Food Chem. 50, 491–494 (2002).
Bosch, C., Bradshaw, B. & Bonjoch, J. Decahydroquinoline ring 13C NMR spectroscopic patterns for the stereochemical elucidation of phlegmarine-type Lycopodium alkaloids: synthesis of (−)-serralongamine A and structural reassignment and synthesis of (−)-huperzine K and (−)-huperzine. J. Nat. Prod. 82, 1576–1586 (2019).
Finn, R. D. et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44, D279–D285 (2016).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
de Hoon, M. J. L., Imoto, S., Nolan, J. & Miyano, S. Open source clustering software. Bioinformatics 20, 1453–1454 (2004).
Nett, R. S., Lau, W. & Sattely, E. S. Discovery and engineering of colchicine alkaloid biosynthesis. Nature 584, 148–153 (2020).
Armenteros, J. J. A. et al. Detecting sequence signals in targeting peptides using deep learning. Life Sci. Alliance 2, e201900429 (2019).
Nett, R. S. & Sattely, E. S. Total biosynthesis of the tubulin-binding alkaloid colchicine. J. Am. Chem. Soc. 143, 19454–19465 (2021).
Wilchek, M. & Patchornik, A. The synthesis of O-acetylhydroxy-α-amino acids. J. Org. Chem. 29, 1629–1630 (1964).
Zaidan, R. K. & Evans, P. Strategies for the asymmetric construction of pelletierine and its use in the synthesis of sedridine, myrtine and lasubine. European J. Org. Chem. 2019, 5354–5367 (2019).
Stephenson, M. J., Reed, J., Brouwer, B. & Osbourn, A. Transient expression in Nicotiana benthamiana leaves for triterpene production at a preparative scale. J. Vis. Exp. 138, e58169 (2018).
Zhang, D. B., Chen, J. J., Song, Q. Y., Zhang, L. & Gao, K. Lycodine-type alkaloids from Lycopodiastrum casuarinoides and their acetylcholinesterase inhibitory activity. Molecules 19, 9999–10010 (2014).
Wang, Y. E., Yue, D. X. & Tang, X. C. Anti-cholinesterase activity of huperzine A. Acta Pharmacol. Sin. 7, 110–113 (1986).
Li, B. et al. New alkaloids from Lycopodium japonicum. Chem. Pharm. Bull. 60, 1448–1452 (2012).
Feng, Z. et al. Lycodine-type alkaloids from Lycopodiastrum casuarinoides and their acetylcholinesterase inhibitory activity. Fitoterapia 139, 104378 (2019).
Tang, Y. et al. Casuarinines A–J, lycodine-type alkaloids from Lycopodiastrum casuarinoides. J. Nat. Prod. 76, 1475–1484 (2013).
Thorroad, S. et al. Three new Lycopodium alkaloids from Huperzia carinata and Huperzia squarrosa. Tetrahedron 70, 8017–8022 (2014).
Tautenhahn, R., Bottcher, C. & Neumann, S. Highly sensitive feature detection for high resolution LC/MS. BMC Bioinf. 9, 504 (2008).
Acknowledgements
We thank F. Schroeder (Cornell University) and J. Liu (Stanford University) for assistance and useful discussion related to NMR analysis. We also thank D. Nelson (University of Tennessee) for providing cytochrome P450 nomenclature. Thank you to K. Pan (China Pharmaceutical University), R. Sarpong (University of California, Berkeley) and P. Evans (University College Dublin) for providing us with authentic standards. We acknowledge G. Lomonossoff for providing us with the pEAQ-HT plasmid. The research in this manuscript was supported by NIH R01 GM121527 to E.S.S. and NIH R35 GM150908 to R.S.N. Also, R.S.N. was supported as a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation. We dedicate this paper to the memory of our late mentor and friend C. T. Walsh.
Author information
Authors and Affiliations
Contributions
R.S.N. and E.S.S. led the project and conceived experimental procedures. R.S.N. performed transcriptomic analysis, cloned candidate genes, characterized enzyme function through transient expression in N. benthamiana and in vitro enzyme assays, synthesized chemical substrates, isolated chemical intermediates, carried out structural analysis of small molecules and performed bioinformatic analyses. Y.D. performed in vitro biochemical assays characterizing CAL proteins. C.T. analysed heterologous production of CAL proteins through western blots. D.P. assisted with in vitro enzyme characterization of CALs and analysis of their heterologous production. J.M.G. analysed heterologous production and biochemical properties of CAL proteins. Y.Y.L. isolated and structurally verified Lycopodium alkaloid standards. R.S.N. and E.S.S. wrote the manuscript. All authors contributed to data analysis and presentation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jing-Ke Weng, Benjamin Lichman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Functional characterization of PtSDR-1, PtSDR-2 and PtACT-1.
a) Transient expression of PtSDR-1 and PtSDR-2 together with a biosynthetic module for 4 production (PtLDC, PtCAO, and PtPIKS) in N. benthamiana. Shown are LC–MS extracted ion chromatograms (EICs) for 4 ([M + H]+ = m/z 142.1226) and products of PtSDR-1 and PtSDR-2 (A, B, C and D) that each pertain to a single reduction ([M + H]+ = m/z 144.1383), which are shown to represent stereoisomers of 5 via comparison to authentic standards. b) MS2 spectra (m/z 144.1383, 20 V) for the new compounds produced by PtSDR-1 (A and B) and PtSDR-2 (C and D) in comparison to the co-eluting stereoisomers of 5 standard. c) Chiral LC–MS analysis of the biosynthetic products produced in N. benthamiana. Samples were N-acetylated to allow for retention and separation on a chiral column. Note that hydroxy groups were also acetylated under our derivatization conditions. The left panel shows biosynthetic N-acetyl (NAc)-4 enantiomers ([M + H]+ = m/z 184.1332) in comparison to synthesized standards, while the right panel shows biosynthetic NAc, O-acetyl (OAc)-5 stereoisomers ([M + H]+ = m/z 228.1594) in comparison to authentic standards. d) Quantification of 4 enantiomers (as N-acetylated derivatives) that are consumed by PtSDR-1 and PtSDR-2 and 5 diastereomers that are produced in the N. benthamiana transient expression system. n = 3 for each gene combination. Each bar graph shows the mean +/− standard deviation. e) Transient expression of PtACT-1 with a biosynthetic module for production of 5 diastereomers (PtLDC, PtCAO, PtPIKS and PtSDR-1 or PtSDR-2) in N. benthamiana. Shown are LC–MS EICs for 5 diastereomers ([M + H]+ = m/z 144.1383) and products of PtACT-1 (E, F, G and H) that each pertain to the addition of an acetyl group ([M + H]+ = m/z 186.1489), which are shown to represent diastereomers of 6 via comparison to a synthesized standard (which consists of multiple stereoisomers). f) MS2 spectra (m/z 186.1489, 20 V) for the new compounds produced by PtACT-1 (E and F for experiments with PtSDR-1; G and H for experiments with PtSDR-2) in comparison to the co-eluting stereoisomers of 6 standard. g) Chiral LC–MS analysis of the biosynthetic products produced by PtACT-1 in N. benthamiana. Samples were N-acetylated to allow for retention and separation on a chiral column. Note that hydroxy groups were also acetylated under our derivatization conditions, so products of PtSDR-1/PtSDR-2 would also gain an O-acetyl moiety. Shown are biosynthetic NAc-6 diastereomers ([M + H]+ = m/z 228.1594) in comparison to authentic standards. h) Biosynthetic proposal for the activities of PtSDR-1, PtSDR-2 and PtACT-1 to yield 6 diastereomers. spon., spontaneous.
Extended Data Fig. 2 Functional characterization of PtCYP782C1.
a) Transient expression of PtCYP782C1 with a biosynthetic module for the production of 6 diastereomers (PtLDC, PtCAO, PtPIKS, PtSDR-1 or PtSDR-2 and PtACT-1) in N. benthamiana. Shown are LC–MS extracted ion chromatograms (EICs) for 6 diastereomers ([M + H]+ = m/z 186.1489) and a product from PtCYP782C1 activity that represents both an oxidation and elimination of the O-acetyl group ([M + H]+ = m/z 124.1121). Note that 6 is detected here with C18 analysis, while m/z 124.1121 is observed with HILIC analysis. b) The two new mass features (putative 7, left panel, [M + H]+ = m/z 184.1332; putative 8, right panel, [M + H]+ = m/z 124.1121) generated by PtCYP782C1 activity were detected in leaf extract that was prepared under cold conditions, but were mostly lost upon incubation at room temperature. c) MS2 spectra of the two new compounds produced by PtCYP782C1 (m/z 184.1332, 20 V and m/z 124.1121, 20 V), along with predicted ion fragment structures. d) UV analysis of 8 produced via the activity of yeast microsomes enriched with PtCYP782C1, with 6 and NADPH as substrates. Shown in the top panel are the DAD (λ = 254 nm) and extracted ion (m/z 124.1121) chromatograms from LC-DAD-MS analysis. The bottom panel shows the background-extracted UV spectrum of 8 from LC-DAD analysis. Note that retention time differences between this panel and panel b are due to different columns and LC methods. e) In vitro assays with yeast microsomes containing PtCYP782C1 protein. Shown are HILIC LC–MS chromatograms representing 6, as well as the two mass features (7, m/z 184.1332 and 8, m/z 124.1121) previously identified as putative products of PtCYP782C1. We note that diastereomers of 6 are not resolved during HILIC analysis (as they are in C18 analysis) and thus only one co-eluting peak is observed here. f) Formation of 7 and 8 over the course of an in vitro reaction with PtCYP782C1-enriched yeast microsomes. Product abundance is calculated as the integration of the peak generated in the EIC for each mass ion. g) Relative activity of PtCYP782C1 microsomes at varying pH, as determined via production of both 7 and 8. Note that the scales differ between the left and right axes. h) Biosynthetic proposal for the activity of PtCYP782C1 on 6 diastereomers to produce 7 and 8. i) Possible catalytic mechanisms for the conversion of 6 into 8.
Extended Data Fig. 3 Functional characterization of PtCAL-1 and PtCAL-2 in Nicotiana benthamiana.
a) Transient expression of PtCAL-1a and PtCAL-2a with a biosynthetic module for producing 8 (PtLDC, PtCAO, PtPIKS, PtSDR-2, PtACT-1 and PtCYP782C1). Shown is an LC–MS extracted ion chromatogram (EIC) for the major ion ([M + H]+ = m/z 164.1434) associated with the activity of PtCAL-1a/PtCAL-2a when they are both co-expressed in the transient expression system. b) Quantification of new product (m/z 164.1434) abundance through the activity of PtCAL-1 and PtCAL-2 homologues in different combinations. Each bar graph shows the mean +/− standard deviation. n = 3 infiltrated leaves for each condition. c) Multiple mass ions were found to co-elute with m/z 164.1434, suggesting that this ion could be an artefact of in-source fragmentation. d) MS1 profile of the new compound generated by PtCAL-1/PtCAL-2. Note the presence of presumed parent mass ions ([M + H]+ = m/z 247.2169, [M + 2H]2+ = m/z 124.1121), which suggest that m/z 164.1434 results from an in-source loss of 1-piperideine from the proposed product 9 during ionization in the mass spectrometer. e) LC–MS EICs (m/z 164.1434, m/z 124.1121 and m/z 247.2169) comparing the biosynthetic product of PtCAL-1/PtCAL-2 (9, proposed) to a co-eluting compound in the new growth leaf tissue of Phlegmariurus tetrastichus. f) MS2 spectra (m/z 164.1434, 40 V) comparing the biosynthetic product (9) to the compound identified in P. tetrastichus extract. g) Proposed structures for major ion fragments shown in panel f. h) MS2 spectrum (m/z 247.2169, 10 V) of the parent ion for the new compound (9) with predicted structures of fragments. i) UV analysis of 9 produced via biosynthetic reconstitution in N. benthamiana. Shown in the top panel are the DAD (λ = 280 nm) and extracted ion (m/z 164.1434) chromatograms from LC-DAD-MS analysis. The bottom panel shows the background-extracted UV spectrum of 9 from LC-DAD analysis. Note that retention time differences between this panel and panels a, c and e are due to different columns and LC methods. j) Co-infiltration of 6 (m/z 186.1489, left panel) as a substrate for transiently expressed PtCYP782C1, with PtCAL-1 and PtCAL-2a co-expressed, leads to production of 8 (m/z 124.1121, middle panel). However, as shown in panel k, this does not lead to production of 9. k) Deconvolution of the substrates required for PtCAL-1/PtCAL-2 activity. For this, PtCYP782C1, PtCAL-1 and PtCAL-2 were transiently expressed in N. benthamiana and 6 was co-infiltrated as substrate. With this established, different combinations of upstream genes were included in the transient co-expression system to provide putative cosubstrates necessary for the formation of 9 (m/z 164.1434). l) Production of 9 (m/z 164.1434) coincides with the depletion of 3 (m/z 186.1125) and 8 (m/z 124.1121). Relative product abundance was quantified via integration of peaks generated in EICs. Each bar graph shows the mean +/− standard deviation. n = 3 infiltrated leaves for each condition. m) Observations of major (9-A) and minor (9-B) diastereomers of 9 upon biosynthetic reconstitution. Shown here is an EIC LC–MS chromatogram of 9, as well as MS2 comparison between the two diastereomers. n) Chiral chromatography of N-acetylated precursors was performed to assess which enantiomer of 3 (measured here via consumption of 4) serves as the substrate for production of 9. Each bar graph shows the mean +/− standard deviation. n = 3 infiltrated leaves for each condition. Statistical comparisons were made using a two-tailed Welch’s t-test assuming unequal variance. o) HILIC LC–MS analysis of a new major compound ([M + H]+ = m/z 263.2118) purified while trying to isolate 9. This compound corresponds to the addition of an oxygen, suggesting this to be an oxidized product of 9. We also observed an in-source ion fragment that pertains to the addition of a water ([M + H]+ = m/z 281.2224). p) MS2 spectrum (m/z 263.2118, 20 V) of putative 9’ and proposed oxidation of 9 to produce 9’, which can undergo water addition during ionization. q) Predicted structures of major MS2 ion fragments. The corresponding NMR data for 9’ can be found in Supplementary Figs. 12–18. r) Biosynthetic proposal for the condensation of (S)-3 and 8 by PtCAL-1 and PtCAL-2 to produce the proposed phlegmarane scaffold of 9. Partial NMR data for the structural characterization of 9 can be found in Supplementary Figs. 10 and 11.
Extended Data Fig. 4 In vitro characterization of PtCAL-1 and PtCAL-2 from isolated apoplast extract.
a) Enzymatic and synthetic reactions used to produce substrates for assays with PtCAL-1a and PtCAL-2a apoplast extracts. b) Confirmation of PtCAL-1a/PtCAL-2a (co-expressed) apoplast activity when the PIKS reaction or 1 and 2 are provided as substrates along with the CYP782C1 reaction. Shown is a LC–MS extracted ion chromatogram (EIC) for 9 (m/z 164.1434). Note that production of 9 in this system was dramatically higher when 1 and 2 were used as substrates (to generate 3) with the CYP782C1 reaction. For all other panels in this figure, indication of +3 indicates that 1 and 2 were used to produce this substrate spontaneously in vitro. c) In vitro apoplast extract reactions with different combinations of apoplast extracts and substrates. The different conditions are listed and numbered to the left of this panel. Shown are the EICs for the substrates (3 and 8) as well as the product (9). Note that in these experiments, 3 is generated by the spontaneous condensation of 1 and 2. d) Time course of 9 production, as measured via ion abundance (m/z 164.1434) Shown are GFP apoplast extracts (control) or PtCAL-1a/PtCAL-2a (co-expressed) extracts with 3 and 8 generated as in vitro substrates. Additionally, the presence of PtCAL-3 in this reaction was assessed here. n = 3 individual reactions for each condition. Shown in the inset are P values for the statistical comparison between PtCAL-1a/PtCAL-2a +/− PtCAL-3. e) Chiral LC–MS EICs analysing the abundance of N-acetylated 4 enantiomers in the PtCAL-1a/PtCAL-2a +3, +8 reaction. Note the decrease in the abundance of NAc-(S)-4 in the presence of PtCAL-1a/PtCAL-2a (indicated with arrow). This is quantified in Fig 3e. f) Two possible mechanisms to initiate formation of the 9 scaffold. g) Analysis to determine if PtCAL-1a/PtCAL-2a accelerates decarboxylation of 3. Shown are the ratios of 4 to 3 ion abundances over two hours when 3 is included as a substrate alone with either GFP or PtCAL-1a/PtCAL-2a. n = 3 individual reactions for each condition. n.s. = not significant, P > 0.05. h) Eight-hour time point for assessing the potential decarboxylation of 3 by PtCAL-1a/PtCAL-2a apoplast. n = 3 individual reactions for each condition. i) Effect of a zinc (Zn) chelator (2,6-pyridinedicarboxylic acid, PDCA) and zinc supplementation of the enzyme activity of PtCAL-1a/PtCAL-2a, as measured by 9 ion abundance. n = 3 individual reactions for each condition. For all statistical analyses in this figure, a two-tailed Welch’s t-test assuming unequal variance was used. All bar graphs in this figure show the mean +/− standard deviation.
Extended Data Fig. 5 Functional characterization of PtCAL-3 in Nicotiana benthamiana and in vitro with isolated apoplast extract.
a) Transient expression of PtCAL−3 with the pathway to produce 9 (PtLDC, PtCAO, PtPIKS, PtSDR-2, PtACT-1, PtCYP782C1, PtCAL-1a and PtCAL-2a). Shown are LC–MS extracted ion chromatograms (EICs) for the 5 diastereomer (m/z 144.1383) intermediates that remain in this biosynthetic system. b) Chiral LC–MS analysis of N-acetylated products from a transient expression system that generates 4 (NAc-4 = m/z 184.1332) with or without co-expression of PtCAL-3. c) Effect on the ratio of (S)-4 to (R)-4 when PtCAL-3 is included with PtLDC, PtCAO and PtPIKS in N. benthamiana. n = 3 infiltrated leaves for each condition. d) Effect of PtCAL-3 on the total accumulation of 4 in N. benthamiana. For both panels c and d, each bar graph shows the mean +/− standard deviation. n = 3 infiltrated leaves for reach condition. The statistical comparison was made using a two-tailed Welch’s t-test assuming unequal variance. e) Effect of including PtCAL-3 on the ratio of 9 diastereomers (m/z 164.1434), as observed via LC–MS. f) Quantification of the ratio of 9 diastereomers when PtCAL-3 is absent or co-expressed with the rest of the pathway for 9 biosynthesis. Bar graphs show the mean +/− standard deviation, with the mean shown above each bar. n = 3 infiltrated leaves for each condition. The statistical comparison was made using a two-tailed Welch’s t-test assuming unequal variance. g) Biosynthetic proposal for the function of PtCAL-3 based upon its effect on pathway reconstitution in N. benthamiana. The specific production of (S)-3 by PtCAL-3 explains the enrichment of (S,S)-5 shown in panel a, the enrichment of (S)−4 shown in panels b and c, as well as the increase in the major 9 diastereomer (9-A) shown in panels e and f. We propose that the minor 9 diastereomer (9-B) is formed via the low incorporation of (R)-3 as a cosubstrate with 8. spon., spontaneous. h) In vitro assay with PtCAL-3-enriched apoplast and purified PtPIKS. Shown here are chiral LC–MS EICs for N-acetylated 4 enantiomers (m/z 184.1332). Apoplast from plants expressing GFP was used as a negative control. Reactions contained an enzymatic mixture for the production of 2, 3 and 4 (purified PtPIKS-1 +malonyl-CoA, +1), as defined in Extended Data Fig 4a. Note the enrichment of (S)−4 over time in the reactions that contain PtCAL-3. i) LC–MS analysis (HILIC) of in vitro PtCAL-3 apoplast reactions where 1 and 2 are used as substrates. Shown are EICs for 3 (m/z 186.1125) over time. Biosynthetic 3 (shown as a positive control) was generated via transient expression of PtLDC, PtCAO and PtPIKS in N. benthamiana, as usual. j) In vitro assay with PtCAL-3 apoplast where either racemic 4 or 1 and 2 (which can spontaneously condense to produce 3 and subsequently, 4) are included as substrates. Shown here are chiral LC–MS EICs for N-acetylated 4 enantiomers (m/z 184.1332). The ratio of enantiomers is listed next to the peaks for each reaction. k) Assessment of PtCAL-3 apoplast activity at different pH conditions. This was measured by determining the ratio of (S)-4 to (R)-4 (N-acetylated derivatives) via chiral LC–MS at the end point of each reaction. l) Two possible mechanisms for the PtCAL-3-catalysed condensation of 1 and 2 to produce (S)-3. m) Analysis to determine if PtCAL-3 accelerates decarboxylation of 2. Shown are the ion abundance ratios of 2 ([M+Na]+ = m/z 169.0107) to acetoacetic acid ([M+Na]+ = m/z 125.0209) over four hours when 2 is included as a substrate alone with either GFP or PtCAL-3. n = 3 individual reactions for each condition. n.s. = not significant, P > 0.05. n) Effect of a zinc (Zn) chelator (2,6-pyridinedicarboxylic acid, PDCA) and zinc supplementation on the enzyme activity of PtCAL-3, as measured by 3 ion abundance. n = 3 individual reactions for each condition. Boiled PtCAL-3 was included as a negative control since spontaneous formation of 3 can occur when 1 and 2 are co-incubated. For all statistical analyses in this figure, a two-tailed Welch’s t-test assuming unequal variance was used. All bar graphs in this figure show the mean +/− standard deviation.
Extended Data Fig. 6 Structural modelling of CAL proteins.
a) Structures of PtCAL-1a, PtCAL-2a and PtCAL-3 were modelled using AlphaFold2 via ColabFold (v1.5.2)48. The predicted N-terminal signal peptide for each CAL protein was removed prior to structural prediction. Shown here are the highest-ranked models for each structure, which are coloured according to the predicted local distance difference test (pLDDT) confidence score for each residue. Note that the top left, disordered region of each protein corresponds to the N-terminal sequence immediately downstream from the predicted signal peptide. b) Comparison of overall structure and active site architecture of modelled P. tetrastichus CALs compared to human carbonic anhydrase 2 (CA2, PDB structure 2VVA). For clarity, the disordered N-terminal regions were removed from the CAL proteins in this panel. Residues are numbered based upon the full-length version of each protein.
Extended Data Fig. 7 Functional characterization of Pt2OGD-4.
a) Transient expression of Pt2OGD-4 in N. benthamiana with co-infiltration of 10 as substrate. Shown are LC–MS extracted ion chromatograms (EICs) for the 10 substrate ([M + H]+ = m/z 289.2274, left panel) and a product (*) of Pt2OGD-4 that corresponds to the addition of a carbonyl ([M + H]+ = m/z 303.2067, right panel). b) MS2 spectra of the new compound (m/z 303.2067, 20 V) in comparison to that of 18 (m/z 261.1961). Note the similarity in major ion fragments, which suggests that the new compound (proposed as 11) bears structural similarity to 18. c) Minor products (peaks A and B) pertaining to the addition of a hydroxyl ([M + H]+ = m/z 305.2224) are also generated by Pt2OGD-4 activity. d) MS2 spectra (m/z 305.2224, 20 V) for compounds “A” and “B” generated by Pt2OGD-4. e) Putative structures of the ion fragments shown in bold in panel d. f) Biosynthetic proposal for the conversion of 10 into 11 by Pt2OGD-4. Note that the right panel shows the same chemistry as the left panel, but in a different 3D orientation.
Extended Data Fig. 8 Functional characterization of Pt2OGD-5.
a) Transient expression of Pt2OGD-5 with Pt2OGD-4 in N. benthamiana with co-infiltration of 10 as substrate. Shown are LC–MS extracted ion chromatograms (EICs) for the product of Pt2OGD-4 (11, m/z 303.2067, left panel) and a product (A) of Pt2OGD-5 that corresponds to a desaturation ([M + H]+ = m/z 301.1911, right panel). b) MS2 spectra of the new compound “A” (m/z 301.1911, 20 V) in comparison to that of 14 (m/z 259.1805). Note the similarity in major ion fragments, which suggests that the new compound (proposed as 13) bears structural similarity to 14. c) Transient expression of Pt2OGD-5 alone in N. benthamiana with co-infiltration of 10 as substrate. Shown are LC–MS extracted ion chromatograms (EICs) for 10 as substrate (m/z 289.2274, left panel) and a product (B) of Pt2OGD-5 that corresponds to a desaturation ([M + H]+ = m/z 287.2118, right panel). d) MS2 spectra of the new compound “B” (m/z 287.2118, 20 V) in comparison to that of 10 (m/z 289.2274). Note that the major ion fragments in “B” are typically 2 m/z units less than those of 10, which supports that the new compound (proposed as 12) bears the same scaffold as 10, but with a desaturation. e) Comparison of 10 consumption by Pt2OGD-4 vs. Pt2OGD-5. Each of the bar graphs represents an independent experiment. Pairwise comparisons between Pt2OGD-4 and Pt2OGD-5 reactions were assessed using a two-tailed Welch’s t-test, assuming unequal variance. n = 3 infiltrated leaves for each condition. Each bar graph shows the mean +/− standard deviation. f) Biosynthetic proposal for the activity of Pt2OGD-5. While Pt2OGD-5 can desaturate 10 to produce 12 (putative), Pt2OGD-4 appears to have higher activity on 10, suggesting that Pt2OGD-4 activity prior to Pt2OGD-5 activity is the major metabolic route for producing 13 (putative).
Extended Data Fig. 9 Functional characterization of PtABH-1.
a) Transient expression of PtABH-1 with Pt2OGD-5 and Pt2OGD−4 in N. benthamiana with co-infiltration of 10 as substrate. Shown are LC–MS extracted ion chromatograms (EICs) for the product of Pt2OGD−4 and Pt2OGD-5 (13, m/z 301.1911, left panel) and a product (A) of PtABH-1 that corresponds to a loss of an acetyl group ([M + H]+ = m/z 259.1805, right panel), which is confirmed to be 14 via comparison to an authentic standard. b) MS2 spectra of the new compound “A” (m/z 259.1805, 20 V) in comparison to that of 14 (m/z 259.1805, 20 V). c) Transient expression of PtABH-1 with Pt2OGD-4 (Pt2OGD-5 omitted) in N. benthamiana with co-infiltration of 10 as substrate. Shown are LC–MS EICs for the product of Pt2OGD-4 (11, m/z 303.2067, left panel) and a new product (B) of PtABH-1 that corresponds to the loss of an acetyl group, ([M + H]+ = m/z 261.1961, right panel), which is confirmed to be 18 via comparison to an authentic standard. d) MS2 spectra of the new compound “B” (m/z 261.1961, 20 V) in comparison to that of 18 (m/z 261.1961, 20V). e) Biosynthetic proposal for the activity of PtABH-1, which can deacetylate either 11 or 13 to produce 18 or 14, respectively. Critically, the ability to access confirmed standards biosynthetically verifies the proposed location of the carbonyl installed by Pt2OGD-4 and the double bond installed by Pt2OGD-5.
Extended Data Fig. 10 Step-by-step biosynthesis of downstream Lycopodium alkaloids.
a) Generation of HupA (17). b) Generation of 8,15-dihydro congeners. c) Generation of 2,3-dihydro congeners. d) Generation of 2,3,8,15-tetrahydro congeners. For all panels, filled in boxes to the left indicate presence of biosynthetic genes in our N. benthamiana transient expression system. Flabellidine (10) was co-infiltrated as a substrate in all experiments. Shown below each compound is the mean ion abundance for the indicated mass ions (m/z) for each compound. In all panels, n = 6 infiltrated leaves for each experimental condition. Error bars represent +/− standard deviation. New enzymes, or new reactions for previously described enzymes, are coloured purple. Lycopodium alkaloids with common names have been verified with authentic standards. All other structures are proposed based upon MS2, biosynthetic logic and/or insight gained from downstream products or known enzyme activities. Additional details can be found in Extended Data Figs. 7–9, Supplemental Results and Supplementary Figs. 4 and 5.
Supplementary information
Supplementary Information
Supplementary Methods, Results, Figs. 1–23 and References.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nett, R.S., Dho, Y., Tsai, C. et al. Plant carbonic anhydrase-like enzymes in neuroactive alkaloid biosynthesis. Nature 624, 182–191 (2023). https://doi.org/10.1038/s41586-023-06716-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06716-y
This article is cited by
-
Mining and functional characterization of NADPH-cytochrome P450 reductases of the DNJ biosynthetic pathway in mulberry leaves
BMC Plant Biology (2024)
-
Metabolic engineering of the paclitaxel anticancer drug
Cell Research (2024)
-
Assembling neuroactive alkaloids
Nature Plants (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.