Abstract
Microglia are specialized brain-resident macrophages that arise from primitive macrophages colonizing the embryonic brain1. Microglia contribute to multiple aspects of brain development, but their precise roles in the early human brain remain poorly understood owing to limited access to relevant tissues2,3,4,5,6. The generation of brain organoids from human induced pluripotent stem cells recapitulates some key features of human embryonic brain development7,8,9,10. However, current approaches do not incorporate microglia or address their role in organoid maturation11,12,13,14,15,16,17,18,19,20,21. Here we generated microglia-sufficient brain organoids by coculturing brain organoids with primitive-like macrophages generated from the same human induced pluripotent stem cells (iMac)22. In organoid cocultures, iMac differentiated into cells with microglia-like phenotypes and functions (iMicro) and modulated neuronal progenitor cell (NPC) differentiation, limiting NPC proliferation and promoting axonogenesis. Mechanistically, iMicro contained high levels of PLIN2+ lipid droplets that exported cholesterol and its esters, which were taken up by NPCs in the organoids. We also detected PLIN2+ lipid droplet-loaded microglia in mouse and human embryonic brains. Overall, our approach substantially advances current human brain organoid approaches by incorporating microglial cells, as illustrated by the discovery of a key pathway of lipid-mediated crosstalk between microglia and NPCs that leads to improved neurogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw scRNA-seq and bulk-seq sequencing data of this study have been deposited in Gene Expression Ominbus with accession nos. GSE242894 and GSE241127 respectively. Proteomic data on purified cell populations have been deposited on JPOST (JPST001822) and Proteome Exchange (PXD042344). Neural rosette proteomic data are available via ProteomeXchange with identifier PXD044406. All lipidomic data have been deposited in Mendeley Data, which can be accessed at https://doi.org/10.17632/t573dphyc5-1.
Code availability
All codes and software used in this study are listed in the reporting summary and in the Methods section where appropriate. No new software or code was generated and used for data collection and analysis.
References
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Cunningham, C. L., Martinez-Cerdeno, V. & Noctor, S. C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33, 4216–4233 (2013).
Marin-Teva, J. L., Cuadros, M. A., Martin-Oliva, D. & Navascues, J. Microglia and neuronal cell death. Neuron Glia Biol. 7, 25–40 (2011).
Sierra, A. et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 7, 483–495 (2010).
Shigemoto-Mogami, Y., Hoshikawa, K., Goldman, J. E., Sekino, Y. & Sato, K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 34, 2231–2243 (2014).
Squarzoni, P. et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 8, 1271–1279 (2014).
Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340 (2014).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Pasca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016).
Quadrato, G. et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017).
Camp, J. G. et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl Acad. Sci. USA 112, 15672–15677 (2015).
Kelava, I. & Lancaster, M. A. Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev. Biol. 420, 199–209 (2016).
Qian, X., Song, H. & Ming, G. L. Brain organoids: advances, applications and challenges. Development 146, dev166074 (2019).
Lee, C. Z. W., Kozaki, T. & Ginhoux, F. Studying tissue macrophages in vitro: are iPSC-derived cells the answer? Nat. Rev. Immunol. 18, 716–725 (2018).
Monier, A. et al. Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J. Neuropathol. Exp. Neurol. 66, 372–382 (2007).
Bian, Z. et al. Deciphering human macrophage development at single-cell resolution. Nature 582, 571–576 (2020).
Kanton, S. et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574, 418–422 (2019).
Popova, G. et al. Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids. Cell Stem Cell 28, 2153–66 e6 (2021).
Xu, R. et al. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Reports 16, 1923–1937 (2021).
Schafer, S. T. et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 186, 2111–2126 e20 (2023).
Takata, K. et al. Induced-pluripotent-stem-cell-derived primitive macrophages provide a platform for modeling tissue-resident macrophage differentiation and function. Immunity 47, 183–198 e6 (2017).
Bohlen, C. J. et al. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94, 759–773 e8 (2017).
Gosselin, D. et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017).
Su, N. et al. Occurrence of transmembrane protein 119 in the retina is not restricted to the microglia: an immunohistochemical study. Transl. Vis. Sci. Technol. 8, 29 (2019).
Vankriekelsvenne, E. et al. Transmembrane protein 119 is neither a specific nor a reliable marker for microglia. Glia 70, 1170–1190 (2022).
Bennett, M. L. et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl Acad. Sci. USA 113, E1738–1746 (2016).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Lee, C. Y. & Landreth, G. E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. (Vienna) 117, 949–960 (2010).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Fagerlund, I. et al. Microglia-like cells promote neuronal functions in cerebral organoids. Cells. 11, 124 (2021).
Abud, E. M. et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94, 278–293 e9 (2017).
Ormel, P. R. et al. Microglia innately develop within cerebral organoids. Nat. Commun. 9, 4167 (2018).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Herbomel, P., Thisse, B. & Thisse, C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev. Biol. 238, 274–288 (2001).
Yuan, S. H. et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 6, e17540 (2011).
Portier, M. M., Escurat, M., Landon, F., Djabali, K. & Bousquet, O. Peripherin and neurofilaments: expression and role during neural development. C. R. Acad. Sci. III 316, 1124–1140 (1993).
Hawrylycz, M. J. et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399 (2012).
Favuzzi, E. et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 184, 5686 (2021).
Townshend, R. F. et al. Effect of cell spreading on rosette formation by human pluripotent stem cell-derived neural progenitor cells. Front. Cell. Dev. Biol. 8, 588941 (2020).
Bengoechea-Alonso, M. T. & Ericsson, J. SREBP in signal transduction: cholesterol metabolism and beyond. Curr. Opin. Cell Biol. 19, 215–222 (2007).
Kontush, A., Lhomme, M. & Chapman, M. J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 54, 2950–2963 (2013).
Chroni, A., Liu, T., Fitzgerald, M. L., Freeman, M. W. & Zannis, V. I. Cross-linking and lipid efflux properties of apoA-I mutants suggest direct association between apoA-I helices and ABCA1. Biochemistry 43, 2126–2139 (2004).
Chung, S. et al. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J. Biol. Chem. 285, 12197–12209 (2010).
Kypreos, K. E. ABCA1 promotes the de novo biogenesis of apolipoprotein CIII-containing HDL particles in vivo and modulates the severity of apolipoprotein CIII-induced hypertriglyceridemia. Biochemistry 47, 10491–10502 (2008).
Smith, J. D. et al. ABCA1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein A-I. J. Lipid Res. 45, 635–644 (2004).
Favari, E. et al. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler. Thromb. Vasc. Biol. 24, 2345–2350 (2004).
Jin, X. et al. ABCA1 contributes to macrophage deposition of extracellular cholesterol. J. Lipid Res. 56, 1720–1726 (2015).
Thion, M. S. et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172, 500–516 e16 (2018).
Loo, L. et al. Single-cell transcriptomic analysis of mouse neocortical development. Nat. Commun. 10, 134 (2019).
Cakir, B. et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175 (2019).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 51, 404–413 (2019).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet. 51, 414–430 (2019).
Ferris, H. A. et al. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc. Natl Acad. Sci. USA 114, 1189–1194. (2017).
Mauch, D. H. et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357 (2001).
Ioannou, M. S. et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 177, 1522–1535 e14 (2019).
Ito, J., Nagayasu, Y., Miura, Y., Yokoyama, S. & Michikawa, M. Astrocytes endogenous apoE generates HDL-like lipoproteins using previously synthesized cholesterol through interaction with ABCA1. Brain Res. 1570, 1–12 (2014).
Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055–1066 (2020).
Scott-Hewitt, N. et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 39, e105380 (2020).
Knobloch, M. The role of lipid metabolism for neural stem cell regulation. Brain Plast. 3, 61–71 (2017).
Driver, A. M., Kratz, L. E., Kelley, R. I. & Stottmann, R. W. Altered cholesterol biosynthesis causes precocious neurogenesis in the developing mouse forebrain. Neurobiol. Dis. 91, 69–82 (2016).
Knobloch, M. et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 493, 226–230 (2013).
Shi, Y., Kirwan, P. & Livesey, F. J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 7, 1836–1846 (2012).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Lopez-Hernandez, G. Y. et al. Electrophysiological properties of basal forebrain cholinergic neurons identified by genetic and optogenetic tagging. J. Neurochem. 142, 103–110 (2017).
Trombin, F., Gnatkovsky, V. & de Curtis, M. Changes in action potential features during focal seizure discharges in the entorhinal cortex of the in vitro isolated guinea pig brain. J. Neurophysiol. 106, 1411–1423 (2011).
Ho, V. et al. Expression analysis of rare cellular subsets: direct RT-PCR on limited cell numbers obtained by FACS or soft agar assays. Biotechniques 54, 208–212 (2013).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 e21 (2019).
Becht E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2019).
Mi, H. et al. PANTHER version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 49, D394–D403 (2021).
Bindea, G., Galon, J. & Mlecnik, B. CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics 29, 661–663 (2013).
Bindea, G. et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009).
Sieber-Ruckstuhl, N. S. et al. Changes in the canine plasma lipidome after short- and long-term excess glucocorticoid exposure. Sci. Rep. 9, 6015 (2019).
Henriet, E. et al. Argininosuccinate synthase 1 (ASS1): a marker of unclassified hepatocellular adenoma and high bleeding risk. Hepatology 66, 2016–2028 (2017).
Bouyssie, D. et al. Proline: an efficient and user-friendly software suite for large-scale proteomics. Bioinformatics 36, 3148–3155 (2020).
Acknowledgements
We thank all the members of the Ginhoux laboratory for helpful discussions. Flow cytometry and cell sorting, microscopy and RNA library preparation were carried out on SIgN platforms (supported by BMRC IAF311006 grant and BMRC transition funds no. H16/99/b0/011), Agency of Science, Technology and Research (A*STAR), Singapore. F.G. was supported by EMBO YIP, A*STAR UIBR award, Singapore National Research Foundation Senior Investigatorship (NRFI2017-02) and the Fondation Gustave Roussy. R.M.S. was supported by BMRC, A*STAR core funding and Singapore NRF SIS. M.A.P. was supported by the BC Children’s Health Research Institute Investigator Grant (IGAP) Award, and a Scholar Award from the Michael Smith Health Research BC. We acknowledge S. Garel for discussion at IBENS, Astou Tangara and the IBENS imaging facility (IMACHEM-IBiSA), member of the French National Research Infrastructure France-BioImaging (ANR-10-INBS-04), with support from the ‘Fédération pour la Recherche sur le Cerveau - Rotary International France’ (2011) and from the programme « Investissements d’Avenir » ANR-10-LABX-54 MEMOLIFE. N.O. is supported by the Fondation pour la Recherche Médicale (FRM, EQU202003010195). M.S.T. is supported by CNRS and the Fondation pour la Recherche sur le Cerveau (FRC). We thank L. Robinson, Insight Editing London, for editing the manuscript.
Author information
Authors and Affiliations
Contributions
D.S.P., T.K., M.A.P., O.N.F.C. and F.G. designed experiments and interpretation. D.S.P., J.Z., M.A.P. and F.G. designed the coculture protocol. D.S.P., T.K. and S.K.T. performed functional experiments. D.S.P., O.L., K.H.U., W.T.K., Z.Y.B, N.W.D. and W.J.W. performed iMac and organoid cultures. A.S., C.A.D. and S.A. provided advice and interpretation. D.S.P., A.B., L.T., Y.T. and L.G.N. performed imaging analysis. F.T., A.T., W.K.T., L.G. and M.R.W. performed lipidomic mass spectrometry and data analysis. N.O. and M.S.T. performed microscopy analysis on organoids. C.H.T., V.A., M.C.M., I.B. and B.M. characterized the lipoprotein particles. B.V. and R.T.K. provided the CTRL iPS cell line. M.M., L.G. and C.P. generated the iPS cells from paediatric patients and associated experiments. D.C., J.G. and V.M. provided fibroblasts from paediatric patients. J.K.Y.C. provided the human fetal brain tissues. S.D.T., J.-W.D. and F.S. characterized neural rosettes and performed proteomic analysis. W.W.P. and R.M.S. performed proteomic mass spectrometry analysis on purified cell subsets. A.K., X.M.Z., S.K., J.L., K.D., J.C., S.W.H., G.D. and S.R. performed analysis of RNA-seq data. D.S.P., I.L., Z.M.L., J.M.O. and S.L. performed flow cytometry analysis and sorting. X.Y.Y. and S.Y.J. ran patch-clamp analysis and electrophysiological characterization of brain organoids. D.S.P., O.N.F.C. and F.G. wrote the manuscript. All authors read and agreed with the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.S.P., J.Z., M.A.P. and F.G. are inventors in ‘Microglia-sufficient brain organoids’ Patent Application No: 10201902893S.
Peer review
Peer review information
Nature thanks Veronique Miron and the other, anonymous, reviewer(s) for their contributionto the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Phenotypic characterization of iMicro.
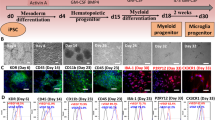
a, Full Schematic overview of the generation of microglia-sufficient brain organoids. iMac and brain organoid are obtained 26 days after initial iPSC culture and constitute day 0 iMac and organoids. Co-cultured organoids are analysed from 15 days onwards (day 15) of co-culture of day 0 iMac and organoids. iMac co-cultured with brain organoids are characterised as co-iMac initially and then iMicro. Unless otherwise stated, experiments throughout the study are performed using the KYOU1 iPSC line. b, Flow cytometry analysis of activation markers by iMac in various conditions: treated for 24 h with fresh iMac medium, fresh cerebral organoid medium, cerebral organoids conditioned medium, or fresh cerebral organoids medium supplemented with LPS (100 ng/ml) for 24 hrs (representative of n = 3). c, RT-qPCR data showing the expression levels of microglia-specific markers in iMac grown for 15 days in iMac medium, fresh cerebral organoid medium and cerebral organoid conditioned medium (n = 3 each). Statistical analysis, one-way ANOVA. Error bars, mean +/− s.e.m. d, Sectioning and immunofluorescence analysis of brain organoids co-cultured with (n = 2) or without (n = 2) iMac for 19 days for NESTIN, SOX2 and IBA1. Individual staining profile are shown below each merged staining. Scale bar, 100 µm. e, Immunofluorescence staining for SOX2, NESTIN and IBA1 of day 18 co-culture organoids (3 represented from n = 4). Boxed areas are shown on the right in higher magnification. Scale bar, 100 µm and 20 µm. f, Sectioning and immunofluorescence staining of brain organoids co-cultured with iMac for 18 days showing the positivity of IBA1+ iMac for Ki67. Quantification performed using the Imaris Imaging software. Scale bar, 100 µm (n = 4). g, Immunofluorescence staining for expression of P2RY12 by IBA-1+ co-iMac (day 18). Scale bar, 20 µm. Representative of 3 independent staining. h, EGFP-expressing iMac elongates its dendrites towards the neuronal injury induced by two-photon laser ablation. Scale bar, 20 µm. (Representative of multiple experiments with same observation). i, Live imaging showing EGFP-expressing iMac that actively moved on the organoid and contained Ab1-42 peptide-TAMRA suggesting their ability to survey the organoid and phagocytose the peptides. Scale bar, 50 µm. Boxed area highlighted below. Scale bar: 10 µm (n = 3). Quantification performed using the Imaris Imaging software.
Extended Data Fig. 2 Identity of cell populations from single-cell RNAseq data.
a, Expression of microglia-specific markers P2RY12, SALL1, CX3CR1 or TMEM119 in iMac alone (n = 3, day 0) and co-iMac co-cultured in 2 dimension (2D) with iPSC-derived cortical neurons supplemented with 50 ng/ml CSF-1 (n = 3, day 14). Data obtained from bulk RNA-seq. Statistical analysis, one-way ANOVA. ns, non-significant. Error bars, mean +/− s.e.m. b, Flow cytometry panel and gating strategy used to assess the expression levels of microglia-specific markers in co-iMac. c, Schematic overview of the single-cell RNA-seq experimental procedure. Number of cells analysed is shown for purified NPC & neuron (n = 3), co-NPC, & co-neuron (n = 3), iMac (n = 2) and co-cultured iMac (n = 3) obtained at day 18. The relative proportions of each cell type (and associated absolute counts) analysed in each condition are represented. d, Violin plot depicting the number of genes detected in each single cell analysis. e, Violin plot for nUMI. f, Violin plot depicting the percentage of mitochondrial genes. g, Violin plots showing the expression level of canonical markers used to define iMac, NPC, neurons and mesenchymal cells isolated from organoids and co-culture organoids. h, tSNE plots showing the expression level of canonical markers used to define NPC, neurons and mesenchymal cells. i, tSNE plots showing the expression levels of canonical markers used to define iMac (termed co-iMac/iMicro in co-cultured organoids). d-i, Data displayed correspond to data analysis from RNA-seq performed on cells obtained from day 18 organoid and co-culture organoids. h, i, tSNE plots are of the concatenated data ie. organoid alone and co-culture organoids.
Extended Data Fig. 3 Transcriptomic signature of iMicro.
a, Heatmap showing the expression levels of genes more- or less-highly expressed in iMac in co-culture organoids (co-iMac) at day 18. b, Violin plots comparing the expression levels of genes involved in neurogenesis and axon development between day 18 iMac and co-iMac. Statistical analysis, Mann-Whitney test. c, Heatmap showing the expression levels of regulons more- or less-highly expressed in day 18 iMac and co-iMac. a-c, Data displayed correspond to data analysis from RNA-seq performed on cells obtained at day 18.
Extended Data Fig. 4 Electrophysiological profile of co-culture brain organoids.
a, Plots representing the capacitance (Cm) of neuronal cells between organoids (n = 15) and co-culture organoids (n = 20) at day 15. b, Representative current traces of spontaneous postsynaptic currents (sPSC) obtained from whole cell patch clamp recording of day 15 brain organoids with or without iMac (n = 15, n = 20 respectively). c, d, Amplitude and Frequency of neurons from day 15 organoids co-cultured with (n = 15, n = 20 respectively) or without iMac. e, Representative traces of individual spontaneous postsynaptic current kinetic responses (sPSC) in day 15 organoid cultures with (n = 20) and without (n = 15) iMac. f, Rise and decay time (Tau) of sPSC responses of neurons in the presence (n = 15) or absence (n = 20) of iMac. a, c, d, f, Statistical analysis, Mann-Whitney U test. Error bars, mean +/− s.e.m.
Extended Data Fig. 5 Generation of co-culture organoids from various human iPSC clones.
a, Images of organoids cultured in the absence (organoid alone, n = 9) or presence (co-culture organoid, n = 9) of iMac for 18 days. Scale bar, 500 µm. b, Organoid size was measured over time in the absence and presence of iMac. Statistical analysis, 2-way ANOVA (n = 9 for each condition, KYOU-1 iPSC clone). c, Evolution of the size of organoids generated from the XCL-1 iPSC clone over time from day 0 to day 20 in the absence and presence of iMac. Statistical analysis, 2-way ANOVA (n = 3 for each condition, representative of at least 2 separate experiments). d, Size of brain organoids generated from iPSC clones HD33i and IMR90 devoid of innate microglia in the presence or absence of autologous iMac from day 0 to day 16, (n = 12 each condition for each iPSC line). Statistical analysis, 2-way ANOVA. Boxed, proportion (%) of “innate” microglia in each organoid at day 16 (n = 8, n = 12 respectively). e, Size of brain organoids generated from iPSC clones CTRL, CAU, pCi-ASI and XCL-1 from day 0 and up to day 15 (day 20 for XCL-1 iPSC clone) (n = 14, 17, 11 and 9 respectively). Boxed plots represent the proportion (%) of innate microglia in each organoid at day 16, and the change of sizes between organoids with and without innate microglia (number of each organoid displayed on the graph). Statistical analysis, 2-way ANOVA. f, Impact of the presence of M-CSF in the culture medium on the proportion of innate microglia in brain organoids generated from CTRL, CAU and pCi-ASI iPSC clones (n = 8, 6 and 8 respectively). Statistical analysis, Mann Whitney test. g, Impact of the presence (n = 3) and absence (n = 3) of M-CSF in the organoid culture medium on the size of organoids from CTRL and CAU iPSC clones grown for up to15 days. Statistical analysis, 2-way ANOVA. h, Evolution of the size of organoids generated from the iPSC of 4 distinct patients (pediatric brain cancer) over 15 days with and without iMac (n = 3 for each condition). Statistical analysis, 2-way ANOVA. Statistical analysis reported on the plot are between organoids and co-culture organoids for each day under the “ashed” bracket. i, Proportion of four different cell types identified in organoids cultured in the absence (organoid alone 1, 2 and 3) or presence of iMicro (co-culture 1, 2 and 3) for 18 days. Relative cell proportions were calculated from the single cell RNA-seq data. j, Bar graphs showing the relative proportion of each cell type in organoids alone (n = 3) and co-cultured organoids (n = 3). The relative proportions were calculated from the single-cell RNA-seq data. Statistical analysis, Mann Whitney test. k, 3D encasing of TUJ1 (TUBB3) signal by IBA1+ iMicro in co-culture organoid (day 16) Scale bar, 10 µm. Right panels represent four different angles. Scale bar, 5 µm. (one representation of multiple similar observations). l, Mass-spectrometry analysis of TUJ1 protein describing the presence of TUJ1 protein in day 18 iMac, iMicro, co-NPC and co-neuron. Statistical analysis, top plot: one-way ANOVA; bottom plot: Mann-Whitney test. Three independent samples (2 co-cultured organoids and 2 organoids) from the same experiment were sorted for proteomic analysis and each sample was run in triplicate. b-f, j, l. Error bars: mean +/− s.e.m.
Extended Data Fig. 6 Characteristics of NPC, co-NPC, neurons and co-neurons.
a, Heatmap and associated violin plots showing the expression level of genes highly expressed in NPC or co-NPC and related pathways. b, Heatmap and associated violin plots showing the expression level of genes highly expressed in neuron or co-neuron and related pathways. a, b, Right panels, violin plot representation of the levels of expressions of specific genes. c, UMAP plots showing the expression level of canonical markers used to define proliferating cells and characterizing NPC/co-NPC in concatenated clustering analysis of organoid and co-culture organoids. d, Proportion of four different cell types (excluding iMicro/iMac) in organoids cultured in the absence (organoid alone 1, 2 and 3) or presence of iMac (co-culture 1, 2 and 3) for 18 days. Relative proportions of each cell types were calculated from the single cell RNA-seq data. Statistical analysis, Mann Whitney test. a-d, Data displayed correspond to data analysis from RNA-seq performed on cells obtained from organoid and co-culture organoid at day 18. e, Z-projections of immunofluorescence staining of day 15 organoids and co-culture organoids for TUBB3 (TUJ1), Ki67 and Hescht. (n = 3 and n = 2 respectively). f, Immunohistochemistry of brain organoid alone (n = 6) and co-culture (n = 6) for CASPASE3. Quantification analysis using QuPath software. Boxed areas are displayed on the right and below each pictures respectively. Scale bar, 1250 µm, 275 µm and 150 µm. Statistical analysis, Mann-Whitney U test. Error bars, mean +/− s.e.m.
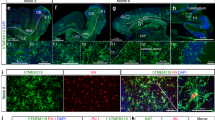
Extended Data Fig. 7 Description of neural rosettes in co-culture organoids.
a, Immunochemistry staining for SOX2 of neural rosettes from organoids (representative of 115 rosettes from 11 organoids) and co-culture organoids (representative of 40 rosettes from 8 organoids) at day 15. Scale bar, 100 µm. b, Description of the intrinsic features of neural rosettes from day 15 organoids and co-culture organoids. Number of rosettes per organoid section (n = 8 for each organoid culture). Representation of the internal and external rosette areas (µm2) (and associated internal and external density per rosette) for each measured rosette from organoids (n = 115) and co-culture organoids (n = 40). Statistical analysis, Mann-Whitney test. c. Z-stacks and associated projection showing the repartitions of IBA1+ iMicro, SOX2+ neural rosettes and NeuN+ neurons. Picture representative of 5 different co-culture organoids (day 15). White arrows, neural rosettes. Scale bar, 100 µm. d. Heatmap of DEPs from mass-spectrometry proteomic analysis of rosettes from day 15 organoids and co-culture organoids (n = 6 each, 40−50 rosettes per replicate). e, Expression of major proteins common between day 15 neural rosettes; and NPC, neuron, co-NPC and co-neurons (2 independent samples, 3 replicates each). Statistical analysis, Mann-Whitney test and one-way ANOVA respectively. a, e, Error bars, mean +/− s.e.m.
Extended Data Fig. 8 Pathway analysis from proteomic data, impact of conditioned medium on organoid growth.
a, b, c, Pathway network analysis highlighting the enrichment/loss of pathways using differentially expressed proteins (DEP) from proteomic data in iMac versus iMicro, NPC versus co-NPC and neuron versus co-neuron, respectively; and showing an increased neurogenesis upon the addition of iMicro. Enrichment is coded with a colour gradient. Prevalence of the pathway is reflected by its p-Value (only pathway with p-Value < 0.05 are represented). d, Images of organoids cultured in the absence (organoid day 0 and day 28) or presence (co-culture organoid) of iMac, or under supernatant transfer from iMac culture for 28 days. Scale bar, 500 µm. n = 6 for each condition. e, Change in the size of brain organoids grown in medium replaced with fresh organoid medium (n = 9) or fresh organoid conditioned medium (n = 9) every 3 days (grey arrows). Statistical analysis, 2-way ANOVA. f, Changes in organoid size generated from XCL-1 iPSC clone over-time in the absence and presence of iMac conditioned medium replaced every 2 days (grey arrows). Co-culture organoids were used as control. Statistical analysis, 2-way ANOVA. (n = 3 per conditions, representative of at least 2 independent experiments). e, f, Error bars, mean +/− s.d. regu. Regulation, pos. positive, neg. negative.
Extended Data Fig. 9 Composition of co-culture organoid conditioned medium.
a, Mass-spectrometry lipidomic analysis of culture medium (cM) from iMac, organoid and co-culture organoids at day 18. (n = 4 for cell samples, n = 1 for medium). Statistical analysis, one-way ANOVA. b, Western blot (W-B) analysis of iMac cell lysates and conditioned medium (cM) from iMac and organoid culture (n = 2 each). Quantitation of APOE is normalised to ponceau S signals representing 40 µg of protein loaded on the gel. Positive control: human HDL. Individual gels are shown in Supplementary Fig. 1a. c, Expression profile of APOE transcripts and APOE proteins in NPC, co-NPC, neurons and co-neurons (left and right panels respectively). Transcript, RNA-seq data from day 18 organoids. Protein, mass-spectrometry proteomic analysis on sorted cells from day 18 organoids (Three independent samples (3 co-cultured organoids and 3 organoids) sorted for proteomic analysis and each sample was run in triplicate). Statistical analysis, one-way ANOVA. d, e, Western blot (W-B) analysis for the expression of APOE from concentrated and HDL fraction (respectively) of day 18 organoids and co-culture organoids conditioned medium (cM) in the presence or absence of probucol. Human HDL and HDL negative medium are used as negative and positive controls respectively. HDL fractions isolated from density gradient centrifugation of cM and plasma (HDL ctrl). Quantification normalised with the protein amount loaded (40 µg). Gels are representative of 3 independent experiments. Statistical analysis, one-way ANOVA. Individual gels are shown in Supplementary Fig. 1c and d respectively. f, Expression profile of ABCA1 protein by flow cytometry analysis on iMac (day 18). Representative of n = 3. g, Mass-spectrometry lipidomic analysis of culture medium (cM) from iMac with or without treatment with probucol. n = 6 (iMac day 0), n = 3 (control medium, iMac day 18 and iMac day 18 + probucol). Statistical analysis, one-way ANOVA. h, Impact of probucol (added every 2 days, grey arrows) on the size of organoid and co-culture organoids from XCL-1 iPSC clone over time (up to 20 days). Untreated organoids were used as control. Statistical analysis, 2-way ANOVA. (Representative of 2 independent experiments with n = 4 each). a, c, d, e, g, Bar represents the mean. h, Error bars: mean +/− s.d). ctrl., control.
Extended Data Fig. 10 Identification of iMicro signature in datasets and during fetal development.
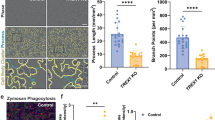
a, Heatmaps showing transcripts involved in cholesterol biosynthesis and regulation, cholesterol transport and lipid storage in human microglial cells at the embryonic development and adulthood stages. b, Immunofluorescence staining of the neocortex region of E14.5 brain for IBA1, PLIN2 and Hoechst. Analysis and quantification using the Imaris Imaging software. Picture representative of n = 4. c, d, Heatmaps showing transcripts involved in cholesterol biosynthesis and regulation, cholesterol transport and lipid storage in microarray data of E14.5 cerebral cortex (microglia and neuron; Loo et al., 2019) and in our organoid model (iMicro, co-NPC and co-neuron) respectively. e, Immunofluorescence staining of human foetal microglia (17, 20 and 21 weeks, n = 3 each) for Lipid-spot 610, PLIN2 and IBA1. Different magnifications are used on the samples. Scale bar, 50 µm. Yellow arrows characterize Lipid-spot 610+ PLIN2+ IBA1+ cells. Picture representative of 3 independent staining from one foetal brain at week 17, 20 and 21. Quantification using the Imaris Imaging software.
Supplementary information
Supplementary Information
This file contains more detailed legends for Figs. 1–4, Supplementary Figs. 1–3, Tables 1–2 and full descriptions for Supplementary Data Tables 1–11 (tables supplied separately).
Supplementary Tables
Supplementary Tables 1–11.
Supplementary Video 1
Morphology of iMac in brain organoids. 3D reconstitution of cryo-sectioned image or organoid cocultured for 15 days. Staining for IBA (green), TUJ1 (red) and DAPI (blue). Scale bar, 5 µm to 30 µm.
Supplementary Video. 2
Response of iMac to laser-induced neuronal ‘injury’. Left, 3D reconstitution of cryo-sectioned of EGFP+ iMac (green) extending protuberance to site of laser injury (orange) using a two-photons microscope. Scale bar, 50 µm. Images were taken immediately after the damage and for at least 2 h. Right, selected snapshots of the 3D video at time of laser injury and then 30 min and 2 h after laser injury. Scale bar, 20 µm.
Supplementary Video 3
iMac phagocytose amyloid beta peptides in brain organoid cocultures. Cryosection of EGFP+ iMac (green) cocultured with brain organoids for 15 days; and incubated with amyloid beta (1–42) peptides conjugated with Fluo-555 (red) for 48 h. Scale bar, 40 µm.
Supplementary Video 4
Organoid morphology in the presence of iMac. 3D reconstitution of cryosection of organoid alone (day 14) and cocultured organoids (day 18) stained for IBA1 (green), NESTIN (red) and SOX2 (blue). Scale bar, 300 µm. Far right, zoom of surface view of coculture organoids at day 18. Scale bar, 30 µm to 100 µm.
Supplementary Video 5
iMac prompt the remodelling of brain organoid size. 3D reconstitution of cryosection of organoid alone (day 14) and cocultured organoids (day 18) stained for IBA1 (green), TUJ1 (red) and SOX2 (blue). Scale bar, 300 µm.
Supplementary Video 6
co-NPC present a reduced proliferative activity in coculture organoids. 3D reconstitution of cryosection of organoids alone (day 15, n = 3) and coculture organoid (day 15, n = 3) stained for TUBB3 (green), ki67 (red), IBA1 (sky blue) and the nuclear counterstain Hoescht (dark blue). Scale bar, 100 µm.
Supplementary Video 7
iMicro and co-NPC are in close contact in coculture organoids. Top, 3D reconstitution of whole coculture organoid staining for TUBB3 (TUJ1, green) and IBA-1 (red) at day 22 (each view representative of n = 3) and depicting the proximity between IBA1+ iMac and TUBB3+ neuronal cells. Scale bar, 10 µm. Scale video, 5–25 µm. Bottom, respective Z-stack cross-section snapshots emphasizing on the interaction between Neuronal cells and IBA1+ iMicro. Scale bar, 10 µm.
Supplementary Video 8
iMicro engulf traces of TUJ1 neurons in coculture organoids. Top, 3D reconstitution of whole coculture organoid staining for TUBB3 (TUJ1, green) and IBA-1 (red) at day 22 (each view representative of n = 3) and depicting traces of TUBB3+ neuronal cells in IBA-1+ cells, thus suggesting that iMicro have engulfed TUBB3+ neuronal cells. Each view is representative of n = 3. Scale bar, 10 µm. Scale video, 0.2–10.0 µm. Bottom, respective Z-stack cross-section snapshot demonstrating the presence of TUJ1 signal within IBA1+ cells. Scale bars, 10 µm and 70 µm respectively.
Supplementary Video 9
iMac prompt a reduction of neural rosette in size and morphology in coculture organoids. 3D reconstitution of cryosection of coculture (day 15, n = 3) stained for NeuN (green), SOX2 (red), IBA1 (sky blue) and the nuclear counterstain Hoescht (dark blue). Scale bar, 100 µm.
Supplementary Video 10
iMicro contains lipid droplets. 3D reconstitution of cryosection of cocultured organoid (day 15) containing EGFP+ iMicro (green) stained for LipidSpot 610 (red). Scale bar, 70 µm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, D.S., Kozaki, T., Tiwari, S.K. et al. iPS-cell-derived microglia promote brain organoid maturation via cholesterol transfer. Nature 623, 397–405 (2023). https://doi.org/10.1038/s41586-023-06713-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06713-1
This article is cited by
-
In conversation with Ukpong Eyo
Nature Neuroscience (2024)
-
Humanized brain organoids-on-chip integrated with sensors for screening neuronal activity and neurotoxicity
Microchimica Acta (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.