Abstract
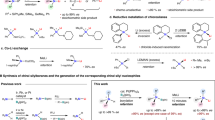
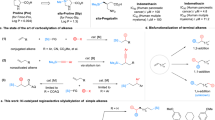
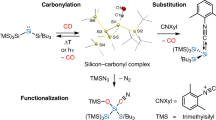
‘Organic silicon’ is not found in nature but modern chemistry is hard to imagine without silicon bound to carbon. Although silicon-containing commodity chemicals such as those emerging from the ‘direct process’1,2,3,4 look simple, it is not trivial to selectively prepare aryl-substituted and alkyl-substituted (functionalized) silicon compounds, known as silanes. Chlorosilanes such as Me4−nSiCln (n = 1–3) as well as SiCl4 (n = 4) are common starting points for the synthesis of silicon-containing molecules. Yet these methods often suffer from challenging separation problems5. Conversely, silanes with four alkyl groups are considered synthetic dead ends. Here we introduce an arenium-ion-catalysed halodealkylation that effectively converts Me4Si and related quaternary silanes into a diverse range of functionalized derivatives. The reaction uses an alkyl halide and an arene (co)solvent: the alkyl halide is the halide source that eventually engages in a Friedel–Crafts alkylation with the arene to regenerate the catalyst6, whereas the arenium ion acts as a strong Brønsted acid for the protodealkylation step7. The advantage of the top-down halodealkylation methodology over reported bottom-up procedures is demonstrated, for example, in the synthesis of a silicon drug precursor. Moreover, chemoselective chlorodemethylation of the rather inert Me3Si group attached to an alkyl chain followed by oxidative degradation is shown to be an entry into Tamao–Fleming-type alcohol formation8,9.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all other data supporting the findings of this study are available in the Article and its Supplementary Information and are also available from the corresponding author on request.
References
Zhang, P., Zhang, D., Dong, J., Chen, G. & Li, J. Direct synthesis of methylchlorosilanes: catalysts, mechanisms, reaction conditions, and reactor designs. Org. Process Res. Dev. 26, 2270–2280 (2022).
Zhang, Y., Li, J., Liu, H., Zhong, Z. & Su, F. Recent advances in Rochow-Müller process research: driving to molecular catalysis and to a more sustainable silicone industry. ChemCatChem 11, 2757–2779 (2019).
Seyferth, D. Dimethyldichlorosilane and the direct synthesis of methylchlorosilanes. The key to the silicones industry. Organometallics 20, 4978–4992 (2001).
Clarke, M. P. The direct synthesis of methylchlorosilanes. J. Organomet. Chem. 376, 165–222 (1989).
Arkles, B. in Handbook of Grignard Reagents (eds Silverman, G. S. & Rakita, P. E.) 667–675 (Dekker, 1996).
He, T., Klare, H. F. T. & Oestreich, M. Silylium-ion regeneration by protodesilylation enables Friedel–Crafts alkylation with less isomerization and no defunctionalization. ACS Catal. 11, 12186–12193 (2021).
Wu, Q. et al. Cleavage of unactivated Si–C(sp3) bonds with Reed’s carborane acids: formation of known and unknown silylium ions. Angew. Chem. Int. Ed. 57, 9176–9179 (2018).
Fleming, I., Henning, R. & Plaut, H. The phenyldimethylsilyl group as a masked form of the hydroxy group. J. Chem. Soc. Chem. Commun. 29–31 (1984).
Jones, G. R. & Landais, Y. The oxidation of the carbon-silicon bond. Tetrahedron 52, 7599–7662 (1996).
Benkeser, R. A. & Muench, W. C. New synthesis of internally substituted alkyl silanes. J. Am. Chem. Soc. 95, 285–286 (1973).
Watanabe, H., Aoki, M., Sakurai, N., Watanabe, K. & Nagai, Y. Selective synthesis of mono-alkyldichlorosilanes via the reaction of olefins with dichlorosilane catalyzed by group VIII metal phosphine complexes. J. Organomet. Chem. 160, C1–C7 (1978).
Out, G. J. J., Klok, H. A., Schwegler, L., Frey, H. & Möller, M. Hydrosilylation of 1-alkenes with dichlorosilane. Macromol. Chem. Phys. 196, 185–194 (1995).
Yuan, W., Smirnov, P. & Oestreich, M. Custom hydrosilane synthesis based on monosilane. Chem 4, 1443–1450 (2018).
Smirnov, P. & Oestreich, M. Merging platinum-catalyzed alkene hydrosilylation with SiH4 surrogates: salt-free preparation of trihydrosilanes. Organometallics 35, 2433–2434 (2016).
Fan, X. et al. Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y. Nat. Chem. 15, 666–676 (2023).
Chan, T. H. & Fleming, I. Electrophilic substitution of organosilicon compounds—applications to organic synthesis. Synthesis 761–786 (1979).
Matsuoka, K. et al. Chemoselective cleavage of Si–C(sp3) bonds in unactivated tetraalkylsilanes using iodine tris(trifluoroacetate). J. Am. Chem. Soc. 143, 103–108 (2021).
Roy, A. & Oestreich, M. At long last: the Me3Si group as a masked alcohol. Angew. Chem. Int. Ed. 60, 4408–4410 (2021).
Klare, H. F. T. & Oestreich, M. The power of the proton: from superacidic media to superelectrophile catalysis. J. Am. Chem. Soc. 143, 15490–15507 (2021).
Kipping, F. S. Organic derivatives of silicon. Part III. dl-Benzylmethylethylpropylsilicane and experiments on the resolution of its sulphonic derivative. J. Chem. Soc. Trans. 91, 717–747 (1907).
Sommer, L. H., Marans, N. S., Goldberg, G. M., Rockett, J. & Pioch, R. P. A new reaction in organosilicon chemistry. J. Am. Chem. Soc. 73, 882 (1951).
Sommer, L. H. et al. Aliphatic organo-functional siloxanes. J. Am. Chem. Soc. 75, 2932–2934 (1953).
Sommer, L. H., Barie, W. P. & Gould, J. R. Kinetics and mechanism of methyl–silicon cleavage by sulfuric acid. J. Am. Chem. Soc. 75, 3765–3767 (1953).
Shorr, L. M., Freiser, H. & Speier, J. L. Methyl–silicon cleavage of certain substituted carboxylic acids in sulfuric acid. Kinetics and mechanism. J. Am. Chem. Soc. 77, 547–551 (1955).
O’Brien, D. H. & Harbordt, C. M. Cleavage of alkylsilanes by strong acids. J. Organomet. Chem. 21, 321–328 (1970).
Olah, G. A., Husain, A., Gupta, B. G. B., Salem, G. F. & Narang, S. C. Synthetic methods and reactions 104. Silylations with in situ generated trimethylsilyl triflate reagent systems. J. Org. Chem. 46, 5212–5214 (1981).
Finke, U., Moretto, H.-H., Niederprüm, H. & Vorbrüggen, H. Silylester der Perfluoralkansulfonsauren sowie Verfahren zu deren Herstellung. German patent DE2803125A1 (1978).
Demuth, M. & Mikhail, G. A convenient in situ preparation of trimethylsilyl trifluoromethanesulfonate. Synthesis 827 (1982).
Reed, C. A. Myths about the proton. The nature of H+ in condensed media. Acc. Chem. Res. 46, 2567–2575 (2013).
Reed, C. A. H+, CH3+, and R3Si+ carborane reagents: when triflates fail. Acc. Chem. Res. 43, 121–128 (2010).
Russell, G. A. Catalysis by metal halides. III. The question of the existence of siliconium ions. J. Am. Chem. Soc. 81, 4831–4833 (1959).
Eaborn, C. Organosilicon compounds. Part I. The formation of alkyliodosilanes. J. Chem. Soc. 2755–2764 (1949).
Russell, G. A. Catalysis by metal halides. I. Mechanism of the disproportionation of ethyltrimethylsilane. J. Am. Chem. Soc. 81, 4815–4825 (1959).
Sakurai, H., Tominaga, K., Watanabe, T. & Kumada, M. Aluminium chloride-catalyzed reactions of organosilicon compounds II. Facile syntheses of alkylchlorosilanes, -germanes, and -stannanes. Tetrahedron Lett. 7, 5493–5497 (1966).
Haubold, W., Gemmler, A. & Kraatz, U. Tetramethylsilan – ein Methylierungsreagenz für Bortrihalogenide. Z. Naturforsch. 33b, 140–141 (1978).
Bordeau, M., Djamei, S. M., Calas, R. & Dunogues, J. Nouvelles utilisations du tétraméthylsilane comme agent de méthylation des chlorosilanes; valorisation du méthyltrichlorosilane. J. Organomet. Chem. 288, 131–138 (1985).
Heyduk, A. F., Labinger, J. A. & Bercaw, J. E. Catalytic alcoholysis of tetramethylsilane via Pt-mediated C–H bond activation. J. Am. Chem. Soc. 125, 6366–6367 (2003).
Alyev, I. Y., Rozhkov, I. N. & Knunyants, I. L. Anode chemistry of organosilanes. Oxidative replacement of alkyl group by fluoride ion. Tetrahedron Lett. 17, 2469–2470 (1976).
Schäfer, A. et al. A new synthesis of triarylsilylium ions and their application in dihydrogen activation. Angew. Chem. Int. Ed. 50, 12636–12638 (2011).
Labbow, R., Reiß, F., Schulz, A. & Villinger, A. Synthesis of the first persilylated ammonium ion, [(Me3Si)3NSi(H)Me2]+, by silylium-catalyzed methyl/hydrogen exchange reactions. Organometallics 33, 3223–3226 (2014).
Omann, L. et al. Thermodynamic versus kinetic control in substituent redistribution reactions of silylium ions steered by the counteranion. Chem. Sci. 9, 5600–5607 (2018).
Bähr, S. & Oestreich, M. A neutral RuII hydride complex for the regio- and chemoselective reduction of N-silylpyridinium ions. Chem. Eur. J. 24, 5613–5622 (2018).
Torigoe, T., Ohmura, T. & Suginome, M. Utilization of a trimethylsilyl group as a synthetic equivalent of a hydroxyl group via chemoselective C(sp3)–H borylation at the methyl group on silicon. J. Org. Chem. 82, 2943–2056 (2017).
Kajita, D. et al. Design and synthesis of silicon-containing fatty acid amide derivatives as novel peroxisome proliferator-activated receptor (PPAR) agonists. Bioorg. Med. Chem. Lett. 25, 3350–3354 (2015).
Lehmann, M., Schulz, A. & Villinger, A. Bissilylated halonium ions: [Me3Si–X–SiMe3][B(C6F5)4] (X=F, Cl, Br, I). Angew. Chem. Int. Ed. 48, 7444–7447 (2009).
Budanow, A. et al. Two-coordinate gallium ion [tBu3Si-Ga-SitBu3]+ and the halonium ions [tBu3Si-X-SitBu3]+ (X = Br, I): sources of the supersilyl cation [tBu3Si]+. Organometallics 31, 7298–7301 (2012).
Budanow, A., Bolte, M., Wagner, M. & Lerner, H.-W. The ion-like supersilylium compound tBu3Si–F–Al[OC(CF3)3]3. Eur. J. Inorg. Chem. 2524–2527 (2015).
Merk, A. et al. Intramolecular halo stabilization of silyl cations—silylated halonium- and bis-halo-substituted siliconium borates. Chem. Eur. J. 27, 3496–3503 (2021).
Klare, H. F. T. et al. Silylium ions: from elusive reactive intermediates to potent catalysts. Chem. Rev. 121, 5889–5985 (2021).
Allemann, O., Duttwyler, S., Romanato, P., Baldridge, K. K. & Siegel, J. S. Proton-catalyzed, silane-fueled Friedel–Crafts coupling of fluoroarenes. Science 332, 574–577 (2011).
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC 2008/1-390540038, UniSysCat). M.O. is indebted to the Einstein Foundation Berlin for an endowed professorship.
Author information
Authors and Affiliations
Contributions
T.H., H.F.T.K. and M.O. conceived the work and designed the experiments. T.H. performed the experiments and analysed the data. T.H., H.F.T.K. and M.O. discussed the results and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Chuan He, Shigeki Matsunaga and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, T., Klare, H.F.T. & Oestreich, M. Arenium-ion-catalysed halodealkylation of fully alkylated silanes. Nature 623, 538–543 (2023). https://doi.org/10.1038/s41586-023-06646-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06646-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.