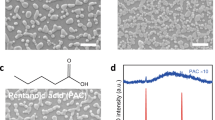
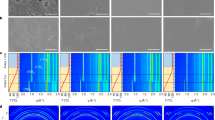
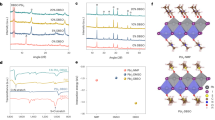
Abstract
Achieving both high efficiency and long-term stability is the key to the commercialization of perovskite solar cells (PSCs)1,2. However, the diversity of perovskite (ABX3) compositions and phases makes it challenging to fabricate high-quality films3,4,5. Perovskite formation relies on the reaction between AX and BX2, whereas most conventional methods for film-growth regulation are based solely on the interaction with the BX2 component. Herein, we demonstrate an alternative approach to modulate reaction kinetics by anion–π interaction between AX and hexafluorobenzene (HFB). Notably, these two approaches are independent but work together to establish ‘dual-site regulation’, which achieves a delicate control over the reaction between AX and BX2 without unwanted intermediates. The resultant formamidinium lead halides (FAPbI3) films exhibit fewer defects, redshifted absorption and high phase purity without detectable nanoscale δ phase. Consequently, we achieved PSCs with power conversion efficiency (PCE) up to 26.07% for a 0.08-cm2 device (25.8% certified) and 24.63% for a 1-cm2 device. The device also kept 94% of its initial PCE after maximum power point (MPP) tracking for 1,258 h under full-spectrum AM 1.5 G sunlight at 50 ± 5 °C. This method expands the range of chemical interactions that occur in perovskite precursors by exploring anion–π interactions and highlights the importance of the AX component as a new and effective working site to improved photovoltaic devices with high quality and phase purity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary information.
Code availability
The code used for this study is available from the corresponding authors on reasonable request.
References
Rao, M. K. et al. Review on persistent challenges of perovskite solar cells’ stability. Sol. Energy 218, 469–491 (2021).
Mohd Yusoff, A. R. B. et al. Passivation and process engineering approaches of halide perovskite films for high efficiency and stability perovskite solar cells. Energy Environ. Sci. 14, 2906–2953 (2021).
Chen, B., Rudd, P. N., Yang, S., Yuan, Y. & Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 48, 3842–3867 (2019).
Yin, W.-J., Shi, T. & Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 104, 063903 (2014).
Correa-Baena, J.-P. et al. Homogenized halides and alkali cation segregation in alloyed organic-inorganic perovskites. Science 363, 627–631 (2019).
National Renewable Energy Laboratory (NREL). Best Research-Cell Efficiencies. NREL https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf (2023).
Khenkin, M. V. et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 5, 35–49 (2020).
Li, W. et al. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nat. Rev. Mater. 2, 16099 (2017).
Ma, J.-P. et al. Defect-triggered phase transition in cesium lead halide perovskite nanocrystals. ACS Mater. Lett. 1, 185–191 (2019).
Yuan, Y. & Huang, J. Ion migration in organometal trihalide perovskite and its impact on photovoltaic efficiency and stability. Acc. Chem. Res. 49, 286–293 (2016).
Slotcavage, D. J., Karunadasa, H. I. & McGehee, M. D. Light-induced phase segregation in halide-perovskite absorbers. ACS Energy Lett. 1, 1199–1205 (2016).
Frohna, K. et al. Nanoscale chemical heterogeneity dominates the optoelectronic response of alloyed perovskite solar cells. Nat. Nanotechnol. 17, 190–196 (2022).
Wright, A. D. et al. Intrinsic quantum confinement in formamidinium lead triiodide perovskite. Nat. Mater. 19, 1201–1206 (2020).
Macpherson, S. et al. Local nanoscale phase impurities are degradation sites in halide perovskites. Nature 607, 294–300 (2022).
Doherty, T. A. S. et al. Stabilized tilted-octahedra halide perovskites inhibit local formation of performance-limiting phases. Science 374, 1598–1605 (2021).
Yang, W. S. et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348, 1234–1237 (2015).
Lee, J.-W. et al. Tuning molecular interactions for highly reproducible and efficient formamidinium perovskite solar cells via adduct approach. J. Am. Chem. Soc. 140, 6317–6324 (2018).
Kim, M. et al. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule 3, 2179–2192 (2019).
Jeong, J. et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 592, 381–385 (2021).
Lu, H. et al. Vapor-assisted deposition of highly efficient, stable black-phase FAPbI3 perovskite solar cells. Science 370, eabb8985 (2020).
Lee, J.-W., Kim, H.-S. & Park, N.-G. Lewis acid–base adduct approach for high efficiency perovskite solar cells. Acc. Chem. Res. 49, 311–319 (2016).
Schottel, B. L., Chifotides, H. T. & Dunbar, K. R. Anion-π interactions. Chem. Soc. Rev. 37, 68–83 (2008).
Sun, X. et al. Halide anion–fullerene π noncovalent interactions: n-doping and a halide anion migration mechanism in p–i–n perovskite solar cells. J. Mater. Chem. A 5, 20720–20728 (2017).
Kan, C. et al. Mitigating ion migration by polyethylene glycol-modified fullerene for perovskite solar cells with enhanced stability. ACS Energy Lett. 6, 3864–3872 (2021).
Garau, C. et al. Cation–π versus anion–π interactions: energetic, charge transfer, and aromatic aspects. J. Phys. Chem. A 108, 9423–9427 (2004).
Anstöter, C. S., Rogers, J. P. & Verlet, J. R. R. Spectroscopic determination of an anion–π bond strength. J. Am. Chem. Soc. 141, 6132–6135 (2019).
Stevenson, J. et al. Mayer bond order as a metric of complexation effectiveness in lead halide perovskite solutions. Chem. Mater. 29, 2435–2444 (2017).
Shargaieva, O., Kuske, L., Rappich, J., Unger, E. & Nickel, N. H. Building blocks of hybrid perovskites: a photoluminescence study of lead-iodide solution species. ChemPhysChem 21, 2327–2333 (2020).
Barrit, D. et al. Room-temperature partial conversion of α-FAPbI3 perovskite phase via PbI2 solvation enables high-performance solar cells. Adv. Funct. Mater. 30, 1907442 (2020).
Zhang, H. et al. Bottom-up quasi-epitaxial growth of hybrid perovskite from solution process-achieving high-efficiency solar cells via template-guided crystallization. Adv. Mater. 33, 2100009 (2021).
Stoumpos, C. C., Malliakas, C. D. & Kanatzidis, M. G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52, 9019–9038 (2013).
Yoo, J. J. et al. Efficient perovskite solar cells via improved carrier management. Nature 590, 587–593 (2021).
Gaussian 16, Revision C.01 (Gaussian, Inc., 2016).
Salzner, U. & Aydin, A. Improved prediction of properties of π-conjugated oligomers with range-separated hybrid density functionals. J. Chem. Theory Comput. 7, 2568–2583 (2011).
Guha, S., Goodson, F. S., Corson, L. J. & Saha, S. Boundaries of anion/naphthalenediimide interactions: from anion–π interactions to anion-induced charge-transfer and electron-transfer phenomena. J. Am. Chem. Soc. 134, 13679–13691 (2012).
Giese, M., Albrecht, M. & Rissanen, K. Anion–π interactions with fluoroarenes. Chem. Rev. 115, 8867–8895 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 52125206, 51972004 and 52202241), the National Key Research and Development Program of China (grant no. 2020YFB1506400) and the Tencent Foundation through the Xplorer Prize. We would like to thank the staff at beamlines BL14B1 and BL11B at the Shanghai Synchrotron Radiation Facility (SSRF) for their help with GIWAXS and EXAFS characterizations, and H. Zhengguan (SSRF) for designing the in situ GIWAXS measurement. We acknowledge Y. Chen (Peking University) for the discussion on DFT calculations, Y. Jiang (Peking University) for the assistance in designing C–V measurement and C. Zhu (Beijing Institute of Technology) for the assistance with the material structure analysis. We acknowledge support from T. Yang (Jiangnan University) for the help with the GIWAXS test and L. Xiong and Z. Yan (Jiangnan University) for the help with the QCM-D test.
Author information
Authors and Affiliations
Contributions
Z.H. and H.Zhou conceived the idea and designed the experiments. Z.H. fabricated and characterized the perovskite films. Z.H. and Y.B. fabricated the solar cell devices. Y.W. ensured device encapsulation and preservation and participates in efficiency certification. Y.B. performed the SIMS mapping test. X.H. and L.W. performed the TEM measurement. J.L. and T.L. performed and helped to analyse the DFT calculations. Y.C. helped with the scheme design. K.L. performed the SEM measurements. X.N. conducted the XPS, EQE and PL mapping measurements. N.L. carried out the FTIR measurements. Y.Z. and H.Zai contributed to the fabrication of high-performance PSCs. H.Zhou, Q.C. and Z.H. wrote and revised the manuscript. All authors were involved in the discussion of data analysis and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Interaction between FAI and HFB.
a, FTIR spectra of FAI, HFB or mixed FAI and HFB. b, 1H NMR of FAI and FAI with HFB in DMSO-d6. c, C–V measurement of iodine ion activity of FAI or FAI with HFB. The electrode oxidation potential \({{\rm{E}}}_{{I}^{-}}\) of FAI (0.2 mg ml−1) in IPA at 293 K is +0.245 V, which dropped to +0.197 V of FAI with the addition of HFB \(({{\rm{E}}}_{{I}^{-}-{\rm{HFB}}})\). The activity of iodide without \(({{\rm{\gamma }}}_{{I}^{-}})\) and with \(({{\rm{\gamma }}}_{{I}^{-}-{\rm{HFB}}})\) HFB could be compared using the Nernst equation \({{\rm{E}}}_{{I}^{-}}-{{\rm{E}}}_{{I}^{-}-{\rm{HFB}}}=RT{\rm{ln}}({{\rm{\gamma }}}_{{I}^{-}}/{{\rm{\gamma }}}_{{I}^{-}-{\rm{HFB}}})/F\). The \({{\rm{\gamma }}}_{{I}^{-}}/{{\rm{\gamma }}}_{{I}^{-}-{\rm{HFB}}}\) ratio is determined to be 2.2, indicating the substantially reduced activity of iodide ions. The decrease in the ion activity ultimately leads to a decrease in the chemical potential of FAI, which retards the reaction between FAI and PbI2.
Extended Data Fig. 2 DFT calculations for the FAI⋯HFB⋯PbI2 three-component system.
DFT-calculated Gibbs energy of three reactions in gas phase (a) and the implicit solvent model (solvent = DMF) (b). According to the implicit solvent model, we observed that the Gibbs energy of reaction 1 (−3.19 kcal mol−1) is still lower than that of reaction 2 (6.00 kcal mol−1) in solvent. Notably, reaction 3 (ΔG = −3.97 kcal mol−1) became more favourable in solvent, which is consistent with the UV–vis absorption spectra of the three-component system (Fig. 1d). This was probably because of the coordination of solvent molecules in the implicit solvent model. But the energy of reaction 3 in solvent is still not enough to cancel out the energy difference between reaction 1 and reaction 2. Therefore, DFT calculations in gas phase as well as in the implicit solvent model (solvent = DMF) yield consistent results, that HFB reacts with FAI to form [FAI⋯HFB] and retards the reaction between FAI and PbI2.
Extended Data Fig. 3 Anion–π interaction between the AX component of perovskite and HFB.
UV–vis absorption spectra for HFB, various salts with different cations and anions FAI (a), MAI (b), CsI (c), MACl (d), MABr (e), MASCN (f) and their mixture. Similar redshift about the absorption edge from 280 nm to more than 320 nm was found after FAI, MAI or CsI solution mixing with HFB, indicating that this polarization appeared regardless of the cation species35. Besides, the absorption edge of HFB at 280 nm shifted to 285 nm and 300 nm after mixing with Br− and SCN−, respectively. These evidences implied that such polarization also existed between HFB and bromide or even SCN−. It should be noted that there is no redshift observed when HFB is mixed with chloride, indicative of no polarization, which is probably because of the small radius of chlorine. However, because anion–π interactions also consisted of the contribution from electrostatic force, the strong electrostatic force between Cl− and HFB also leads to anion–π interactions, as reported previously36. These results indicate that the anion–π interaction is widely present between HFB and various types of AX in perovskite.
Extended Data Fig. 4 Effects of other strong electron-deficient molecules.
UV–vis absorption (a) and PL spectra (b) of control perovskite and perovskite fabricated with HFB, 2,4,6-trifluoro-1,3,5-triazine (labelled π-I), s-tetrazine (labelled π-II) and pentafluoropyridine (labelled π-III). PCE (c), FF (d), VOC (e) and JSC (f) of PSCs fabricated with HFB, π-I, π-II and π-III.
Supplementary information
Supplementary Information
This file contains 23 Supplementary Figures and legends for the two Supplementary Videos.
Supplementary Video 1
IPA evaporation rate test. One millilitre of IPA was added dropwise to a watch glass and, after 938 s, IPA volatilized 20% of its initial mass. Video played with 15× acceleration.
Supplementary Video 2
HFB evaporation rate test. One millilitre of HFB was added dropwise to a watch glass (the watch glass specification was the same as that used in Supplementary Video 1) and, after 334 s, the HFB volatilized 20% of its initial mass. This shows that the volatilization rate of HFB is higher than that of IPA under the same conditions. Video played with 15× acceleration.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Z., Bai, Y., Huang, X. et al. Anion–π interactions suppress phase impurities in FAPbI3 solar cells. Nature 623, 531–537 (2023). https://doi.org/10.1038/s41586-023-06637-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06637-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.