Abstract
Lysine residues in histones and other proteins can be modified by post-translational modifications that encode regulatory information1. Lysine acetylation and methylation are especially important for regulating chromatin and gene expression2,3,4. Pathways involving these post-translational modifications are targets for clinically approved therapeutics to treat human diseases. Lysine methylation and acetylation are generally assumed to be mutually exclusive at the same residue. Here we report cellular lysine residues that are both methylated and acetylated on the same side chain to form Nε-acetyl-Nε-methyllysine (Kacme). We show that Kacme is found on histone H4 (H4Kacme) across a range of species and across mammalian tissues. Kacme is associated with marks of active chromatin, increased transcriptional initiation and is regulated in response to biological signals. H4Kacme can be installed by enzymatic acetylation of monomethyllysine peptides and is resistant to deacetylation by some HDACs in vitro. Kacme can be bound by chromatin proteins that recognize modified lysine residues, as we demonstrate with the crystal structure of acetyllysine-binding protein BRD2 bound to a histone H4Kacme peptide. These results establish Kacme as a cellular post-translational modification with the potential to encode information distinct from methylation and acetylation alone and demonstrate that Kacme has all the hallmarks of a post-translational modification with fundamental importance to chromatin biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data from ChIP–seq, CUT&RUN, TT-TL-seq and STL-seq experiments have been deposited in the NCBI Gene Expression Omnibus (GEO) database under accession number GSE182204. The mass spectrometry proteomics data have been deposited in the Mass Spectrometry Interactive Virtual Environment (MassIVE) database under accession number MSV000087982. The crystallography data have been deposited in the RCSC Protein Data Bank under accession number 8SB6. Data used in, but not generated in, this study include SRX150481, SRX6913085 and SRX6913048 from ChIP-Atlas, and PDB 2DVQ and 6ONY.
Code availability
Pipelines used for analysis of ChIP–seq and CUT&RUN data (https://bitbucket.org/mattsimon9/simonlabchip) and for analysis of TT-TL-seq data (https://bitbucket.org/mattsimon9/timelapse_pipeline) are publicly available.
References
Wang, Z. A. & Cole, P. A. The chemical biology of reversible lysine post-translational modifications. Cell Chem. Biol. 27, 953–969 (2020).
Allfrey, V. G., Faulkner, R. & Mirsky, A. E. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl Acad. Sci. USA 51, 786–794 (1964).
Allis, C. D. & Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487–500 (2016).
Muller, M. M. & Muir, T. W. Histones: at the crossroads of peptide and protein chemistry. Chem. Rev. 115, 2296–2349 (2015).
Fuhs, S. R. et al. Monoclonal 1- and 3-phosphohistidine antibodies: new tools to study histidine phosphorylation. Cell 162, 198–210 (2015).
Hori, T. et al. Histone H4 Lys 20 monomethylation of the CENP-A nucleosome is essential for kinetochore assembly. Dev. Cell 29, 740–749 (2014).
Jorgensen, S., Schotta, G. & Sorensen, C. S. Histone H4 lysine 20 methylation: key player in epigenetic regulation of genomic integrity. Nucleic Acids Res. 41, 2797–2806 (2013).
Chen, Y. et al. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol. Cell. Proteomics 6, 812–819 (2007).
Garcia, B. A. et al. Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat. Protoc. 2, 933–938 (2007).
Hseiky, A., Crespo, M., Kieffer-Jaquinod, S., Fenaille, F. & Pflieger, D. Small mass but strong information: diagnostic ions provide crucial clues to correctly identify histone lysine modifications. Proteomes 9, 18 (2021).
Muroski, J. M., Fu, J. Y., Nguyen, H. H., Ogorzalek Loo, R. R. & Loo, J. A. Leveraging immonium ions for targeting acyl-lysine modifications in proteomic datasets. Proteomics 21, e2000111 (2021).
Wan, N. et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat. Methods 19, 854–864 (2022).
Green, E. M., Mas, G., Young, N. L., Garcia, B. A. & Gozani, O. Methylation of H4 lysines 5, 8 and 12 by yeast Set5 calibrates chromatin stress responses. Nat. Struct. Mol. Biol. 19, 361–363 (2012).
Mahat, D. B., Salamanca, H. H., Duarte, F. M., Danko, C. G. & Lis, J. T. Mammalian heat shock response and mechanisms underlying its genome-wide transcriptional regulation. Mol. Cell 62, 63–78 (2016).
Schofield, J. A., Duffy, E. E., Kiefer, L., Sullivan, M. C. & Simon, M. D. TimeLapse-seq: adding a temporal dimension to RNA sequencing through nucleoside recoding. Nat. Methods 15, 221–225 (2018).
Zimmer, J. T., Rosa-Mercado, N. A., Canzio, D., Steitz, J. A. & Simon, M. D. STL-seq reveals pause-release and termination kinetics for promoter-proximal paused RNA polymerase II transcripts. Mol. Cell 81, 4398–4412 (2021).
Lasko, L. M. et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 550, 128–132 (2017).
Lu, X. et al. The effect of H3K79 dimethylation and H4K20 trimethylation on nucleosome and chromatin structure. Nat. Struct. Mol. Biol. 15, 1122–1124 (2008).
Shogren-Knaak, M. et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311, 844–847 (2006).
Beaver, J. E. & Waters, M. L. Molecular recognition of Lys and Arg methylation. ACS Chem. Biol. 11, 643–653 (2016).
McCullough, C. E. & Marmorstein, R. Molecular basis for histone acetyltransferase regulation by binding partners, associated domains, and autoacetylation. ACS Chem. Biol. 11, 632–642 (2016).
Filippakopoulos, P. & Knapp, S. The bromodomain interaction module. FEBS Lett. 586, 2692–2704 (2012).
Zaware, N. & Zhou, M. M. Bromodomain biology and drug discovery. Nat. Struct. Mol. Biol. 26, 870–879 (2019).
Brand, M. et al. Small molecule inhibitors of bromodomain-acetyl-lysine interactions. ACS Chem. Biol. 10, 22–39 (2015).
Filippakopoulos, P. et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149, 214–231 (2012).
LeRoy, G., Rickards, B. & Flint, S. J. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell 30, 51–60 (2008).
Umehara, T. et al. Structural basis for acetylated histone H4 recognition by the human BRD2 bromodomain. J. Biol. Chem. 285, 7610–7618 (2010).
Umehara, T. et al. Structural implications for K5/K12-di-acetylated histone H4 recognition by the second bromodomain of BRD2. FEBS Lett. 584, 3901–3908 (2010).
Kent, L. N. & Leone, G. The broken cycle: E2F dysfunction in cancer. Nat. Rev. Cancer 19, 326–338 (2019).
Brehm, A. et al. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391, 597–601 (1998).
Luo, R. X., Postigo, A. A. & Dean, D. C. Rb interacts with histone deacetylase to repress transcription. Cell 92, 463–473 (1998).
Magnaghi-Jaulin, L. et al. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature 391, 601–605 (1998).
Nicolas, E., Ait-Si-Ali, S. & Trouche, D. The histone deacetylase HDAC3 targets RbAp48 to the retinoblastoma protein. Nucleic Acids Res. 29, 3131–3136 (2001).
Coffey, K. et al. Characterisation of a Tip60 specific inhibitor, NU9056, in prostate cancer. PLoS ONE 7, e45539 (2012).
Nguyen, D. P., Garcia Alai, M. M., Kapadnis, P. B., Neumann, H. & Chin, J. W. Genetically encoding Nϵ-methyl-l-lysine in recombinant histones. J. Am. Chem. Soc. 131, 14194–14195 (2009).
Nikolovska-Coleska, Z. et al. Design and characterization of bivalent Smac-based peptides as antagonists of XIAP and development and validation of a fluorescence polarization assay for XIAP containing both BIR2 and BIR3 domains. Anal. Biochem. 374, 87–98 (2008).
Amblard, M., Fehrentz, J. A., Martinez, J. & Subra, G. Methods and protocols of modern solid phase peptide synthesis. Mol. Biotechnol. 33, 239–254 (2006).
Pinilla, C., Appel, J. R., Judkowski, V. & Houghten, R. A. Identification of B cell and T cell epitopes using synthetic peptide combinatorial libraries. Curr. Protoc. Immunol. Chapter 9, 9.5.1–9.5.16 (2012).
Peptide Competition Assay (PCA) (Abcam) (accessed March 2019); http://docs.abcam.com/pdf/protocols/peptide_competition_assay_protocol.pdf.
Sidoli, S., Bhanu, N. V., Karch, K. R., Wang, X. & Garcia, B. A. Complete workflow for analysis of histone post-translational modifications using bottom-up mass spectrometry: from histone extraction to data analysis. J. Vis. Exp. 17, 54112 (2016).
Hentges, P., Van Driessche, B., Tafforeau, L., Vandenhaute, J. & Carr, A. M. Three novel antibiotic marker cassettes for gene disruption and marker switching in Schizosaccharomyces pombe. Yeast 22, 1013–1019 (2005).
Kao, L. R. & Megraw, T. L. RNAi in cultured Drosophila cells. Methods Mol. Biol. 247, 443–457 (2004).
Machyna, M., Kiefer, L. & Simon, M. D. Enhanced nucleotide chemistry and toehold nanotechnology reveals lncRNA spreading on chromatin. Nat. Struct. Mol. Biol. 27, 297–304 (2020).
Bowman, S. K. et al. Multiplexed Illumina sequencing libraries from picogram quantities of DNA. BMC Genom. 14, 466 (2013).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet 17, 10–12 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-seq (MACS). Genome Biol. 9, R137 (2008).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Quinlan, A. R. BEDTools: the Swiss-Army tool for genome feature analysis. Curr. Protoc. Bioinform. 47, 11.12.11–11.12.34 (2014).
Simovski, B. et al. Coloc-stats: a unified web interface to perform colocalization analysis of genomic features. Nucleic Acids Res. 46, W186–W193 (2018).
Welch, R. P. et al. ChIP-Enrich: gene set enrichment testing for ChIP-seq data. Nucleic Acids Res. 42, e105 (2014).
Zou, Z., Ohta, T., Miura, F. & Oki, S. ChIP-Atlas 2021 update: a data-mining suite for exploring epigenomic landscapes by fully integrating ChIP-seq, ATAC-seq and Bisulfite-seq data. Nucleic Acids Res. 50, W175–W182 (2022).
Stark, R. B. G. DiffBind: differential binding analysis of ChIP-seq peak data (2013); http://bioconductor.org/packages/release/bioc/vignettes/DiffBind/inst/doc/DiffBind.pdf.
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9, e1003118 (2013).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Vock, I. W. & Simon, M. D. bakR: uncovering differential RNA synthesis and degradation kinetics transcriptome-wide with Bayesian hierarchical modeling. RNA 29, 958–976 (2023).
Duffy, E. E. et al. Tracking distinct RNA populations using efficient and reversible covalent chemistry. Mol. Cell 59, 858–866 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Abmayr, S. M., Yao, T., Parmely, T. & Workman, J. L. Preparation of nuclear and cytoplasmic extracts from mammalian cells. Curr. Protoc. Mol. Biol. Chapter 12, 12.1.1–12.1.10 (2006).
Wysocka, J. Identifying novel proteins recognizing histone modifications using peptide pull-down assay. Methods 40, 339–343 (2006).
Kabsch, W. Xds. Acta Crystallogr. D 66, 125–132 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
Faivre, E. J. et al. Selective inhibition of the BD2 bromodomain of BET proteins in prostate cancer. Nature 578, 306–310 (2020).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Turnbull, W. B. & Daranas, A. H. On the value of c: can low affinity systems be studied by isothermal titration calorimetry? J. Am. Chem. Soc. 125, 14859–14866 (2003).
Acknowledgements
We thank D. Schatz, K. Shokat and Y. Jacob, and the members of the Simon laboratory for comments on this Article; and the staff at the Yale Center for Genome Analysis. This work was supported by NIH T32GM007223 (to W.J.L.-C.), NIH T32GM007205 (to W.J.L.-C.), T32GM67543 (to I.W.V.), NIH AI118891, CA196539 and AG031862 (to B.A.G.), NIH R01GM141313 (to F.B.), and NIH DP2HD083992, NIH R01GM137117 and the DeLuca Center for Innovation in Hematology Research (to M.D.S.).
Author information
Authors and Affiliations
Contributions
M.D.S. conceived the project with input from W.J.L.-C. W.J.L.-C. made the initial discovery. Peptide synthesis, antibody development, antibody characterization and immunoblot analyses were performed, analysed and interpreted by W.J.L.-C., L.J.C., B.T.R. and M.D.S. Proteomic validation of Kacme in cells was performed, analysed and interpreted by Y.X., N.V.B. and B.A.G. Genomic distribution and sequencing experiments (ChIP, CUT&RUN, TT-TL-seq and STL-seq) were performed, analysed and interpreted by W.J.L.-C., L.J.C., M.M., A.P.-U., J.T.Z., I.W.V. and M.D.S. In vitro enzymatic and inhibitor experiments were performed, analysed and interpreted by W.J.L.-C., L.J.C., Y.X., B.T.R., A.P.-U., B.A.G. and M.D.S. S. pombe set5Δ strains were generated by M.C.K. Structural and biophysical characterization was performed, analysed and interpreted by L.J.C., B.E.E., B.T.R., F.B. and M.D.S. W.J.L.-C., L.J.C. and M.D.S. wrote the manuscript with input and edits from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Bing Zhu, Jinrong Min and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Development and characterization of Kacme antisera.
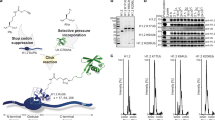
a, Chemical structures of lysine post-translational modifications referenced in this work. b, Scheme for synthesis of Fmoc-Lys(Ac,Me)-OH for peptide synthesis. i, CuCO3·Cu(OH)2, Ac2O; ii, 8-hydroxyquinoline; iii, NaHCO3, Fmoc-OSu. c, Chemical structure of the peptide library used to generate Kacme antisera. Yellow box indicates the Kacme residue. d, Peptide dot blots of Kacme antisera with synthetic histone peptides. Yellow boxes indicate Kacme peptides. e, Coomassie stain of Kacme antisera after Protein G purification and SDS-PAGE. Gels were repeated independently twice with similar results. f, Peptide dot blots of Kacme antisera with synthetic peptides. Yellow boxes indicate Kacme peptides. g, Peptide dot blots of purified Kacme antisera with synthetic peptides. Yellow boxes indicate Kacme peptides. h, Western blot of recombinant histone H4 and extracted histones from HEK293T cells with Kacme antisera. i, Western blots of histones extracted from indicated cell types using Kacme antisera pre-incubated with indicated competitor peptide library. Dm, Drosophila melanogaster; Hs, Homo sapiens; Mm, Mus musculus. h-i, Blots were repeated independently twice with similar results.
Extended Data Fig. 2 Identification of H4K5acme diagnostic ions by mass spectrometry of synthetic standards.
a, Proteomic workflow to identify and validate the existence of Kacme in cells using synthetic isotopically labelled peptides and endogenous histones40. Note that peptides were processed with standard propionylation methods. b-h, MS/MS spectra of isotopically labelled synthetic H4Kacme and H4Kpr peptides. See Table S1 for peptide sequences, Table S2 for target ions, and Tables S3–S5 for calculated and observed y- and b-ions. Diagnostic ions for each modification are indicated in zoomed-in inlays by colours (red and blue for H4K5acme, green for H4Kpr). b, H4K5acme. c, H4K5pr. d, H4K5acmeK8acK12acK16ac. e, H4K5prK8acK12acK16ac. f, H4K5acmeK8acK12acmeK16ac. g, H4K5prK8acK12prK16ac. h, H4K12acme. d10 indicates peptide isotopically labeled with deuterated leucine.
Extended Data Fig. 3 Validation of diagnostic ions for H4K5acme and H4Kpr.
a, Schemes of proposed diagnostic ion formation from synthetic Kacme and Kpr peptide standards. b, Extracted ion chromatograms of diagnostic ions for synthetic H4K5acme and H4K5pr peptide standards. Chromatogram peaks are labelled with retention times. d10 indicates peptide isotopically labeled with deuterated leucine.
Extended Data Fig. 4 Proteomic identification and abundance of H4K5acme in cells.
a, Extracted ion chromatograms for targeted ions from histones extracted from HEK293T cells (endogenous, m/z 775.9543), the isotopically labelled synthetic H4K5acme peptide (m/z 780.9857), and their mixture. b, MS spectra for the extracted ion chromatograms in a. c, Extracted ion chromatograms of diagnostic ion 157.1335 for H4K5acme from a synthetic standard (left) and endogenous HEK293T histones (right). d-h, MS/MS spectra of endogenous targeted peptides. See Table S2 for target ions and Tables S5–S7 for calculated and observed y- and b-ions. i, Standard curves of extracted ion chromatogram peak areas of diagnostic ions for synthetic H4K5acme (270.1812 and 157.1335) and H4Kpr (140.1070) peptide standards. Points show the mean for three independent replicates, and error bars show standard deviation. Note that peptides were processed with standard propionylation methods. d10 indicates peptide isotopically labeled with deuterated leucine.
Extended Data Fig. 5 Genomic distribution of Kacme in fly and human cells by ChIP-seq and CUT&RUN, and the association of Kacme with increased transcription initiation.
a, ChIP-seq density of Kacme and H4Kac across Kacme ChIP-seq peaks. b, Colocalization of Kacme peaks with regions of enrichment from ChIP experiments from the MODENCODE database using the GenometriCorr colocalization ratio. c, Kacme ChIP-qPCR from two biological replicates after incubation of Kacme antisera with indicated competitor peptide at indicated concentrations. d, Comparison of binned Kacme ChIP-seq density with and without pre-incubation of Kacme antisera with H4Kac competitor peptide (75 nM). e, Comparison of binned Kacme ChIP-seq density with and without pre-incubation of Kacme antisera with H4Kacme competitor peptide (75 nM). f, Genome browser tracks of Kacme ChIP-seq after incubation of Kacme antisera with indicated competitor peptide (75 nM). g, Kacme and H4Kac ChIP-seq reads across transcription start sites (TSS) for all genes in K562 cells. h, Kacme, H4Kac, and H3K4me3 CUT&RUN density across TSS for all genes in K562 cells. i, Colocalization of Kacme peaks with regions of enrichment from ChIP experiments in human cells using the Interval Stats Ratio. j, Kacme ChIP-qPCR in HEK293T cells after incubation of Kacme antisera with indicated competitor peptide (75 nM). Bar plots show the mean of three biological replicates. Statistical analysis by two-tailed unpaired t-test. p < 0.0001; NS = not significant. k, Kacme ChIP-seq read counts in HEK293T cells after incubation of Kacme antisera with indicated competitor peptide (75 nM). Box plots show the distribution of mean read counts for each peak from three biological replicates. Distributions were compared using a two-tailed unpaired t-test. l, Relative gene-wide transcriptional activities as determined by TT-TL-seq. Normalized read counts were binned by the ratio of Kacme-to-H4Kac ChIP-seq signal. m, Relative transcriptional activity for genes in the 25th or 75th percentiles of Kacme levels as binned by H4Kac ChIP-seq signal. n, Estimates of the half-lives of promoter-proximal paused transcripts for genes in the 25th or 75th percentiles of Kacme levels determined by STL-seq. Data are binned by H4Kac ChIP-seq signal as in m. o, Relative promoter-proximal pause site occupancy as determined by STL-seq for genes in the 25th or 75th percentiles of Kacme ChIP-seq signal. Data is binned by H4Kac ChIP-seq signal. l-o, Distribution means compared with two-tailed unpaired Wilcoxon test for two biological replicates. k-o, The box plots indicate the median (centre line), the third and first quartiles (box limits), 1.5x interquartile range (IQR) above and below the box (whiskers), and the 95% confidence interval (notches). Outlier points are not plotted. * p < 0.05; *** p < 0.001; NS = not significant. n = 8429 (k), 4089 (l), 614 (m), 367 (n-o). p, HOMER motif analysis for promoters of genes with the highest ratios of Kacme over H4Kac ChIP-seq signal. Yellow boxes indicate enrichment of motifs associated with E2F family transcription factors.
Extended Data Fig. 6 Characterization of H4Kacme antisera.
a, Chemical structure of the peptide used to generate H4Kacme antisera. Yellow box indicates the H4K5acme residue. b, Peptide dot blots of H4Kacme antisera with synthetic histone peptides. Yellow boxes indicate H4Kacme peptides, and the green box indicates a H3K27acme peptide. c, Coomassie stain of H4Kacme antisera after Protein A and affinity purification and SDS-PAGE. Gels were repeated independently twice with similar results. d, Peptide dot blots of purified H4Kacme antisera with synthetic histone peptides. Yellow boxes indicate H4Kacme peptides, and the green box indicates a H3K27acme peptide. e, Western blot of histones extracted from two biological replicates of HEK-293T cells. f, Western blot of whole nuclear proteins from two biological replicates of HEK293T cells using H4Kacme antisera pre-incubated with indicated competitor peptide. g, Western blots of histones extracted from two biological replicates of HEK293T cells using H4Kacme antisera pre-incubated with indicated competitor peptides. h, Western blots of histones extracted from HEK-293T cells transfected with GFP-H4 plasmids with the indicated genotypes. Blots were repeated independently twice with similar results. i, H4Kacme ChIP-qPCR in HEK293T cells after incubation of H4Kacme antisera with indicated competitor peptide (100 nM). Bar plots show the mean of two biological replicates. j, H4Kacme ChIP-seq read counts in HEK293T cells after incubation of H4Kacme antisera with indicated competitor peptide (100 nM). Box plots show the distribution of mean read counts for each peak from two biological replicates. Distributions were compared using a two-tailed unpaired t-test. The box plots indicate the median (centre line), the third and first quartiles (box limits), 1.5x interquartile range (IQR) above and below the box (whiskers), and the 95% confidence interval (notches). Outlier points are not plotted. *** p < 2.2e−16; NS = not significant. n = 478. k, Genome browser tracks of Kacme and H4Kacme ChIP-seq after incubation of Kacme or H4Kacme antisera with indicated competitor peptide (75 nM for Kacme, 100 nM for H4Kacme). Yellow boxes highlight Kacme competitor peptide tracks.
Extended Data Fig. 7 H4Kacme ChIP-seq in human cells supports conclusions from Kacme ChIP-seq, and the relationship of Kacme with KATs and the in vitro acetylation of H4 peptides.
a, Genome browser tracks of Kacme and H4Kacme ChIP-seq from two biological replicates. b, ENCODE transcription factor enrichment analysis for genes marked by H4Kacme. Yellow boxes indicate enrichment of motifs associated with E2F family transcription factors. c, KEGG pathways that are enriched for genes marked by H4Kacme. d, MSigDB pathways that are enriched for genes marked by H4Kacme. e, Relative gene-wide transcriptional activities as determined by TT-TL-seq. Normalized read counts were binned by the ratio of H4Kacme-to-H4Kac ChIP-seq signal. f, Relative transcriptional activity for genes in the 25th or 75th percentiles of H4Kacme levels as binned by H4Kac ChIP-seq signal. g, Estimates of the half-lives of promoter-proximal paused transcripts for genes in the 25th or 75th percentiles of H4Kacme levels determined by STL-seq. Data are binned by H4Kac ChIP-seq signal as in f. h, Relative promoter-proximal pause site occupancy as determined by STL-seq for genes in the 25th or 75th percentiles of H4Kacme ChIP-seq signal. Data is binned by H4Kac ChIP-seq signal. e-h, Distribution means compared with two-tailed unpaired Wilcoxon test for two biological replicates. The box plots indicate the median (centre line), the third and first quartiles (box limits), 1.5x interquartile range (IQR) above and below the box (whiskers), and the 95% confidence interval (notches). Outlier points are not plotted. * p < 0.05; *** p < 0.001; NS = not significant. n = 2876 (e), 430 (f), 285 (g-h). i, ENCODE transcription factor enrichment analysis of genes marked by Kacme using Enrichr56. Yellow boxes indicate enrichment of motifs associated with p300. j, ChIP-seq density of p300 (SRX150481) across Kacme ChIP-seq peaks. k, p300 ChIP-qPCR enrichment at selected loci identified by Kacme ChIP-seq. Bar plot shows the mean of three biological replicates of HEK-293T cells, and error bars show standard deviation. Statistical analysis by two-tailed unpaired t-test. * p < 0.05; ** p = 0.007. GD = gene desert. l-m, Extracted ion chromatograms of H4 (l) and H4Kac (m) peptides after in vitro acetylation by p300. n-o, Extracted ion chromatograms of H4 (n) and H4Kac (o) peptides after in vitro acetylation by Tip60. Extracted ion chromatograms of H4Kac (m, o) are representative of all acetylated species. p, Extracted ion chromatograms for H4Kme1 peptides after in vitro acetylation with p300 or Tip60. Note that unlike H4 peptides, the majority of H4Kme1 peptides are not consumed in the reaction, suggesting H4K peptides are better substrates for p300 than H4Kme1 peptides. q, Kacme dot blot of H4Kme1 and H3K27me1 peptides after in vitro acetylation with p300. Data represent two independent experiments. Yellow boxes indicate Kacme peptides.
Extended Data Fig. 8 Kacme is affected by acetylation and methylation pathways, and in vitro deacetylation of H4 peptides.
a, Representative western blots of two biological replicates of HEK293T histones treated with A-485 and NU9056 (10 μM for 24 h). b, Bar plot of normalized western blot signal for Kacme in two biological replicates of HEK293T histones treated with A-485 (10 μM for 24 h). Bar plot shows the mean of the individual normalized values. c, Kacme (top) and H3K27ac (bottom) ChIP-qPCR in HEK293T cells after A-485 treatment (10 μM for 24 h). Bar plots show the mean of two biological replicates. GD = gene desert. d, qPCR results showing relative expression levels of indicated transcripts in HEK293T cells after A-485 treatment (10 μM for 24 h). Bar plots show the mean of two biological replicates. e, Replicate blots for Kacme in wild type and set5 knockout (KO) S. pombe. Blots were repeated independently three times with similar results. f, qPCR results showing relative expression levels of D. melanogaster Smyd3 and Smyd5 after RNAi knockdown. g, Western blots of histones extracted from D. melanogaster cells after RNAi knockdown, with * indicating a lane from an unsuccessful knockdown. Blots were repeated independently twice with similar results. h, Distribution of H4K5acme peptides from HEK293T histones with and without trichostatin A (TSA) treatment (400 nM for 18 h). K5acmeKac1, 2, and 3 represent H4 4-17 peptides with K5acme and 1, 2, or 3 acetyl modifications at remaining lysine residues (K8, K12, K16). i, Extracted ion chromatograms of H4Kac (left) and H4K (right) peptides after in vitro deacetylation by HDAC1. j, Extracted ion chromatograms of H4Kacme (left) and H4Kme1 (right) peptides after in vitro deacetylation by HDAC1. k, Extracted ion chromatograms of H4Kac (left) and H4K (right) peptides after in vitro deacetylation by HDAC3. l, Extracted ion chromatograms of H4Kacme (left) and H4Kme1 (right) peptides after in vitro deacetylation by HDAC3. m, Relative transcriptional activity after TSA treatment (400 nM for 18 h) for genes in the 25th or 75th percentiles of H4Kacme levels as binned by H4Kac ChIP-seq signal. n, Relative transcriptional activity after TSA treatment (400 nM for 18 h) for genes in the 25th or 75th percentiles of H4Kac levels as binned by H4Kacme ChIP-seq signal. m-n, Distribution means compared with two-tailed unpaired Wilcoxon test for two biological replicates. The box plots indicate the median (centre line), the third and first quartiles (box limits), 1.5x interquartile range (IQR) above and below the box (whiskers), and the 95% confidence interval (notches). Outlier points are not plotted. * p = 0.019; *** p < 0.001; NS = not significant. n = 389 (m-n).
Extended Data Fig. 9 Chromatin proteins can bind H4Kacme, and proposed models for biological roles of Kacme.
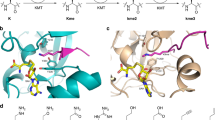
a-c, Western blots of bromo- and YEATS domain-containing proteins enriched by biotinylated peptides incubated with HEK293T nuclear extract. Blots were repeated independently twice with similar results. See Table S1 for peptide sequences. The input for GAS41 is shown from a different exposure to avoid overexposure. d, ChIP-seq density of BRD2 (SRX6913085) and BRD3 (SRX6913048) across Kacme ChIP-seq peaks. e-g, Crystal structure of BRD2 BD1 bound to H4Kacme peptide (PDB 8SB6). Views demonstrate the binding pocket and local residues (e), hydrogen bond interactions (f), and overlay with structure of BRD2 BD1 bound to a H4Kac peptide (g, PDB 2DVQ27). The repositioned waters are outlined in yellow. h, ITC traces of H4, H4K5me1, and H4K5pr peptides binding to BRD2 BD1. Data are representative of three independent replicates. i, ITC traces of H4 peptides binding to BRD2 BD1 N156A. Data are representative of two independent replicates and are corrected for heats of dilution. j-k, Peptide dot blots of Kac and Kacme peptides with selected antibodies. In j, the data represent two independent replicates, and the experiment in k was performed once. Yellow boxes indicate Kacme peptides. See Methods for antibody identification. l, Kacme may be recognized by specific Kacme-binding proteins. m, Kacme may be recognized by some but not other Kac-binding proteins providing differential binding at Kacme versus Kac marked loci. n, Kacme may mask Kme1 marks allowing KATs and HDACs to mask and reveal the methylation, thereby providing a way to effectively install and remove methylation without KMT/KDM enzymes. o, Regardless of whether they share downstream functions, Kacme and Kac demonstrate altered kinetics of installation and removal by KATs and HDACs, providing differential regulation of Kacme versus Kac marked loci.
Supplementary information
41586_2023_6565_MOESM1_ESM.pdf
Supplementary Tables 1–9, Supplementary Tables 13 and 14 and Supplementary Fig. 1, and legends for Supplementary Tables 10–12.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu-Culligan, W.J., Connor, L.J., Xie, Y. et al. Acetyl-methyllysine marks chromatin at active transcription start sites. Nature 622, 173–179 (2023). https://doi.org/10.1038/s41586-023-06565-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06565-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.