Abstract
Integrating human genomics and proteomics can help elucidate disease mechanisms, identify clinical biomarkers and discover drug targets1,2,3,4. Because previous proteogenomic studies have focused on common variation via genome-wide association studies, the contribution of rare variants to the plasma proteome remains largely unknown. Here we identify associations between rare protein-coding variants and 2,923 plasma protein abundances measured in 49,736 UK Biobank individuals. Our variant-level exome-wide association study identified 5,433 rare genotype–protein associations, of which 81% were undetected in a previous genome-wide association study of the same cohort5. We then looked at aggregate signals using gene-level collapsing analysis, which revealed 1,962 gene–protein associations. Of the 691 gene-level signals from protein-truncating variants, 99.4% were associated with decreased protein levels. STAB1 and STAB2, encoding scavenger receptors involved in plasma protein clearance, emerged as pleiotropic loci, with 77 and 41 protein associations, respectively. We demonstrate the utility of our publicly accessible resource through several applications. These include detailing an allelic series in NLRC4, identifying potential biomarkers for a fatty liver disease-associated variant in HSD17B13 and bolstering phenome-wide association studies by integrating protein quantitative trait loci with protein-truncating variants in collapsing analyses. Finally, we uncover distinct proteomic consequences of clonal haematopoiesis (CH), including an association between TET2-CH and increased FLT3 levels. Our results highlight a considerable role for rare variation in plasma protein abundance and the value of proteogenomics in therapeutic discovery.
Similar content being viewed by others
Main
Proteins circulating in the human bloodstream can provide a glimpse into an individual’s state of health1. These plasma proteins include critical regulators of cell signalling, transport, growth, repair and defence against infection, as well as proteins leaked from damaged cells throughout the body6. The dynamic nature of the plasma proteome and the accessibility of human blood makes these proteins valuable tools for diagnosing and predicting disease, identifying therapeutic targets and elucidating disease pathophysiology1,2,3,4. However, it is challenging to determine if changes in protein levels are directly linked to a disease or are simply markers of disease-related processes. Integrating proteomics with genomics to identify genetic variants associated with protein levels, called protein quantitative trait loci (pQTLs), can help to address this limitation.
Genetic variation, either in or near a gene that encodes a protein (cis) or in other parts of the genome (trans), can influence protein expression, folding, secretion and function. To date, most pQTLs have been discovered via genome-wide association studies (GWASs), which predominantly focus on common variants3,5,6,7,8. However, common variant associations are challenging to interpret because most are non-coding and tag linkage disequilibrium blocks. This can also confound the integration of pQTLs with disease-associated GWAS variants. Rarer protein-coding variants tend to confer larger biological effect sizes than common variants and are more directly interpretable. However, their influence on the plasma proteome is largely unknown because previous rare variant proteogenomic studies have been limited in scale9,10.
Here, we evaluated the potential role of rare variation in plasma protein abundance by analysing exome sequence data and plasma levels of 2,923 proteins measured in 49,736 UK Biobank (UKB) participants. We used variant- and gene-level association tests to map pQTLs across the allele frequency spectrum. We demonstrate how these associations can be used to discover biomarkers and allelic series. Moreover, we introduce a framework that includes protein-truncating variants (PTVs) and cis-acting missense pQTLs to bolster the discovery of gene–phenotype association studies.
UKB Pharma Plasma Proteome cohort characteristics
The UKB Pharma Plasma Proteome (UKB-PPP) cohort comprises plasma samples from 54,219 individuals, including 46,595 randomly selected participants, 6,376 consortium-chosen individuals and 1,268 participants from a COVID-19 repeat imaging study. Proteomic profiling on blood plasma was performed with the Olink Explore 3072 platform, which measures 2,941 protein analytes across 2,923 unique proteins (Supplementary Table 1). Exome sequencing data were available for 52,217 (96%) of these participants, which we processed through our previously published cloud-based pipeline11 (Extended Data Fig. 1a). We performed rigorous sample-level quality control (Methods), leaving 49,736 (92%) multi-ancestry samples with exomes for downstream analyses. Of these individuals, 46,327 (93%) were of European descent.
Variant-level associations
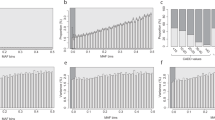
We first performed a variant-level exome-wide association study (ExWAS) between 2,923 plasma protein abundances and 617,073 variants with minor allele frequencies (MAFs) as low as 0.006% in individuals of European ancestries (Fig. 1a, Extended Data Fig. 1a, Supplementary Table 2 and Methods). Using an n-of-one permutation analysis as previously described11, we identified P ≤ 1 × 10−8 as an appropriate P value threshold (Methods and Supplementary Table 3). Genomic inflation was well-controlled (median λGC = 1.03; 95% range, 1.01–1.05; Supplementary Fig. 1 and Supplementary Table 4). In total, there were 151,491 significant genotype–protein associations, 5,433 of which corresponded to rare variants (MAF ≤ 0.1%).
a, Summary of significant (P ≤ 1 × 10−8) cis- and trans-pQTLs across the exome, limited to variants with MAF ≤ 0.1%. P values were generated via linear regression. If multiple variants in a gene were associated with the same protein, we displayed the most significant association for ease of visualization. The P values were not corrected for multiple testing; the study-wide significance threshold is P ≤ 1 × 10−8. b, Percentage of significant rare (MAF ≤ 0.1%) and common (MAF > 0.1%) ExWAS genotype–protein associations that were also significant in the UKB-PPP GWAS. c, The proportion of significant cis-CDS pQTLs per variant class across three MAF bins. ‘All tested variants’ refers to the total number of variants occurring in the genes corresponding to the proteins measured via the Olink platform that were included in the ExWAS. d, Effect sizes of significant rare pQTLs in each variant class. For all plots, if the same genotype–protein association was detected in multiple ExWAS models, we retained the association with the smallest P value.
In a separate array-based GWAS on the UKB-PPP cohort, we tested for the association between common variants (MAF > 0.1%) and 2,922 protein assays, resulting in 14,287 primary genetic associations5. The effect sizes (β) of nominally significant ExWAS pQTLs (P < 1 × 10−4) strongly correlated with the GWAS-derived pQTLs5 (r2 = 0.96; Supplementary Fig. 2). Furthermore, 90% of the more common study-wide associations (MAF > 0.1%) in our study were also significant in the GWAS (Fig. 1b). As expected, given the constraints of GWAS, only 19% of the rare (MAF ≤ 0.1%) associations from our ExWAS were significant in the UKB-PPP GWAS. There was also strong directional concordance between the ExWAS-derived pQTLs and pQTLs detected in an independent Icelandic population8, which had increased resolution for rarer variants (MAF > 0.01%; Supplementary Note).
We classified pQTLs as cis-CDS (that is, cis-coding sequence) if the variant occurred in or nearby a given protein and trans-CDS if the variant affected the abundance of a protein that was greater than 1 megabase (Mb) away (Extended Data Fig. 1b). There was a subset of trans associations that fell within 1 Mb of the gene encoding the protein whose level was altered, which we classified as ‘cis-position, trans-CDS’. Of the unique variants associated with at least one protein abundance measurement, 9,098 (18.7%) were cis-CDS, 14,127 (29.0%) were trans-CDS and 25,543 (52.3%) were cis-position, trans-CDS (Supplementary Table 2). The relative proportions of cis and trans associations were different among rare variants (MAF ≤ 0.1%), in which 2,227 (49.0%) were cis-CDS, 782 (17.2%) were trans-CDS and only 1,540 (33.8%) were cis-position, trans-CDS. Moreover, among the common cis-CDS pQTLs, the proportions of PTVs and missense, synonymous and non-coding variants closely matched the proportions observed for the total variants included in the ExWAS (that is, the expected null distribution; Fig. 1c). In comparison, PTVs and missense variants encompassed a significantly larger percentage of rare (MAF ≤ 0.1%) and ultra-rare (MAF ≤ 0.01%) cis-CDS pQTLs (Fig. 1c and Supplementary Table 5). These results illustrate how linkage disequilibrium contaminates common pQTLs, making it challenging to ascribe causality to these variants without fine-mapping.
Purifying selection keeps fitness-reducing variants at low frequencies in the population. As expected, the median absolute effect size (β) of rare cis-CDS pQTLs was 1.85, compared with 0.3 for common cis-CDS pQTLs (Wilcoxon P < 10−300). Similarly, the absolute effect sizes of rare trans-CDS pQTLs (median |β| = 1.26) were significantly larger than those of common trans-CDS pQTLs (median |β| = 0.08; Wilcoxon P < 10−300) (Extended Data Fig. 2a). Rare cis associations also had larger magnitudes of effect (median |β| = 1.86) than rare trans associations (median |β| = 1.26; Wilcoxon P < 10−300) (Extended Data Fig. 2b).
Of the 2,227 rare cis-CDS pQTLs, 538 (24.2%) were PTVs (Fig. 1d and Supplementary Table 5). As expected, nearly all the PTVs were associated with decreased protein abundances (n = 518 of 538; 96%). Ten of the 20 PTVs associated with increased protein abundances (50%) were predicted to escape nonsense-mediated decay because they occurred within the last exon, the penultimate exon within 50 base pairs (bp) of the 3′ junction or the first exon within the first 200 nucleotides of the coding sequence12. Four were annotated as loss of splice donor or acceptor sites, which can have more variable effects than frameshift and nonsense variants. Rare missense variants and in-frame indels also had more variable directions of effects than PTVs, although most still decreased protein abundances (n = 1,274 of 1,490; 86%). Trans associations were even more variable, with 26% (32 of 122) of PTVs and 26% (252 of 954) of missense variants/indels associated with decreased protein abundances (Fig. 1d).
Identifying allelic series, in which multiple variants in a gene associate with a phenotype with varying effect sizes, can help prioritize candidate drug targets13,14. Missense variants are of particular interest due to their spectrum of biological effects, ranging from complete or partial loss-of-function, to neutral, to gain-of-function. We explored how often missense variants within the same gene had a similar impact on protein abundance, focusing on the 50 genes with at least five rare (MAF ≤ 0.1%) missense cis-CDS pQTLs. Most often, rare missense variants within the same gene had a similar effect on protein abundance. For 47 of these 50 genes (94%), at least 75% of the significant missense pQTLs were associated with decreased protein abundance. The percentage of protein-lowering missense variants in the remaining three genes ranged from 14% to 50% (Supplementary Table 2).
Assessing epitope effects
Genetic variants can theoretically affect antibody binding due to changes in protein conformation. We sought to determine the extent to which these epitope effects might bias the ExWAS cis-CDS pQTLs. We first tested whether cis-CDS pQTLs were enriched for clinically relevant variants, as missense pQTLs independently associated with clinical phenotypes are more likely to reflect true biological effects. We found a significant enrichment of ClinVar15 pathogenic and likely pathogenic variants among the 1,484 significant rare missense and in-frame indel cis-CDS pQTLs (observed: 3.5%; expected: 0.54%; two-tail binomial P = 2.5 × 10−25; Extended Data Fig. 3a). By contrast, the 36,466 missense variants and in-frame indels that were not associated with cis changes in protein abundances (P > 1 × 10−4) were significantly depleted of ClinVar pathogenic/likely pathogenic variants (observed: 0.4%; expected: 0.54%; two-tail binomial P = 1.1 × 10−4). Moreover, the rare significant (P ≤ 1 × 10−8) and suggestive (1 × 10−8 < P ≤ 1 × 10−4) missense cis-CDS pQTLs were more likely to be predicted damaging by the in silico predictor, REVEL16 (Extended Data Fig. 3b).
Five of the Olink proteins harbouring cis-CDS pQTLs were independently measured via immunoturbidimetric assays in the UKB biomarker panel (APOA1, CST3, GOT1, LPA, SHBG). We compared the rare cis-CDS pQTLs (P < 1 × 10−4) in these genes with our independent variant-level associations for these markers measured in 470,000 UKB individuals11,17. In total, 13 of the 14 cis-CDS pQTLs (93%) replicated in the biomarker ExWAS (P ≤ 1 × 10−8), with effect sizes showing complete directional concordance (Supplementary Table 6). One missense variant in GOT1 had a suggestive association with reduced aspartate aminotransferase in the proteogenomic ExWAS. This variant did not achieve a P < 1 × 10−4 in the biomarker ExWAS, and its effect was in the opposite direction, suggesting a possible epitope effect. Finally, we assessed the concordance between the effect sizes of PCSK9 cis-CDS pQTLs (P < 1 × 10−4) and the effects of these variants on low-density lipoprotein. All six variants were also significantly (P ≤ 1 × 10−8) associated with low-density lipoprotein levels in the expected direction (Supplementary Table 7). These results collectively demonstrate that the cis-CDS pQTLs are enriched for biologically relevant signals.
Gene-level protein abundance associations
Because the power to identify statistically significant variant-level associations decreases with allele frequency, we next tested rare variants in aggregate via gene-level collapsing analyses. In this method, we identify rare variants that meet a predefined set of criteria (that is, ‘qualifying variants’ or ‘QVs’) in each gene and test for their aggregate effect on protein levels. Here, we used ten QV models introduced in our previous UKB phenome-wide association study (PheWAS), including one synonymous variant model as an empirical negative control11 (Extended Data Table 1; www.azphewas.com). Some examples include the ‘ptv’ model, which only includes rare PTVs, the recessive (‘rec’) model, which considers recessive and putative compound heterozygous signals, and the ultra-rare (‘UR’) model, which includes non-synonymous singleton variants. Another key advantage of this approach is that it mitigates against epitope effects that could confound variant-level tests.
We tested the association between 18,885 genes and 2,923 plasma protein levels in 46,327 individuals of European ancestry (Supplementary Table 8). The n-of-1 permutation analysis and the synonymous QV model converged on P ≤ 1 × 10−8 as an appropriately conservative P value threshold (Supplementary Table 3 and Methods). In total, there were 7,412 significant associations across the nine non-synonymous QV models (Fig. 2a). Of these, there were 1,962 unique gene–protein abundance associations, including 1,049 (53%) cis associations, 813 (41%) trans associations and 100 (5%) cis-position, trans-CDS signals. This relatively low percentage of cis-position, trans-CDS associations compared with the ExWAS (5% versus 52%) highlights the strength of collapsing analysis in mitigating contamination due to linkage disequilibrium. Some of the remaining cis-position, trans-CDS signals may indicate local co-regulation, which could be interrogated in future whole-genome sequencing studies18.
a, Miami plot of 1,962 unique gene–protein abundance associations across nine collapsing models. We excluded the empirical null synonymous model. The y axis is capped at 60. If the same gene–protein association was detected in multiple QV models, we retained the association with the smallest P value. The four labelled loci indicate trans-CDS pQTL hotspots. P values were generated via linear regression and were not corrected for multiple testing; the study-wide significance threshold is P ≤ 1 × 10−8. b, The number of unique significant (P ≤ 1 × 10−8) protein abundance associations per gene across the collapsing models. c, The effect sizes of significant gene–protein associations in each collapsing model are stratified by cis versus trans effects.
Notably, 501 (25.5%) of the 1,962 gene–protein abundance signals identified in the collapsing analysis did not reach significance in the ExWAS (Extended Data Fig. 4a). Among 1,084 gene–protein associations specifically arising from the ptv model, 667 (62%) were not significant in the ExWAS. Meanwhile, only 112 of the rare PTV signals from the ExWAS did not achieve significance in the PTV collapsing model (Extended Data Fig. 4b). Of the associations that only reached significance in the collapsing analysis, 101 (20.2%) were cis-CDS (Supplementary Table 8). These data demonstrate how collapsing analyses increase statistical power for discovering rare variant-driven associations.
The models that included PTVs alongside putatively damaging missense variants encompassed most of the 4,528 cis-CDS signals. The flexdmg model accounted for 854 signals (19%), the ptvraredmg model accounted for 804 signals (18%) and the ptv model accounted for 691 signals (15%). Moreover, there were only three significant gene-level cis-CDS pQTLs in the synonymous (syn) collapsing model19,20 (full definitions of collapsing models can be found in Extended Data Table 1).
Most pQTLs identified in the collapsing analysis were associated with changes in the abundance of a single protein (Fig. 2b). Among the 254 genes with trans-CDS associations, 87% were associated with three or fewer proteins. However, certain genes appeared to be pQTL ‘hotspots’, associated with over 20 different protein abundances, including ASGR1 (n = 186), STAB1 (n = 77), STAB2 (n = 41) and GNPTAB (n = 37) (Fig. 2a,b). ASGR1, which encodes a subunit of the asialoglycoprotein receptor, also emerged as a hotspot in the UKB-PPP GWAS and other large pQTL studies5,6,8. STAB1 and STAB2 are located on different chromosomes (3 and 12, respectively), but both encode related scavenger receptors expressed on macrophages and liver sinusoidal endothelial cells that mediate the clearance of aged plasma proteins and other waste molecules21,22. Interestingly, 20 of the 77 (26%) proteins associated with STAB1 variants were also associated with STAB2 variants.
GNPTAB encodes the alpha and beta subunits of GlcNAc-1-phosphotransferase, which selectively adds GlcNAc-1-phosphate to mannose residues of lysosomal hydrolases. Tagged lysosomal hydrolases are transported to the lysosome, whereas untagged hydrolases are secreted into the blood and extracellular space23. Recessive PTVs in GNPTAB are associated with mucolipidosis III, a severe lysosomal storage disorder (LSD)24. Of the 37 GNPTAB trans-CDS pQTLs detected in the collapsing model, 35 (95%) are lysosomal proteins25,26, 18 of which have been associated with other LSDs (Supplementary Table 9). Moreover, all 37 proteins were increased in PTV carriers, suggesting reduced lysosomal targeting. Notably, there are efforts to therapeutically increase GNPTAB to enhance the cellular uptake of lysosomal proteins involved in other LSDs and improve the efficacy of enzyme replacement therapies27.
As expected, 99% (n = 687 of 691) of the cis signals from the ptv model were associated with decreased protein levels (Fig. 2c). Meanwhile, only 77 (21%) of the 372 significant trans signals from the ptv model were associated with decreased protein levels. This signal was mostly driven by the hotspot loci ASGR1, GNPTAB, STAB1 and STAB2 (Fig. 2a). Among these four proteins, 99% (n = 191) of the 193 trans-pQTLs had positive effect sizes compared with only 58% (n = 104 of 149 pQTLs) for the remaining 104 genes. Some possible explanations for these signals include the loss of upstream regulators, reduced negative feedback or compensatory changes. For example, PTVs in EPOR, encoding the erythropoietin receptor, were associated with increased erythropoietin, an example of compensatory upregulation (‘ptv’ model; P = 3.5 × 10−24; β = 1.78; 95% confidence interval (CI), 1.43 to 2.12)28.
Two of the collapsing models (‘UR’ and ‘URmtr’) include ultra-rare (genome Aggregation Database (gnomAD) MAF = 0%, UKB MAF ≤ 0.005%) PTVs and damaging missense variants16 (Extended Data Table 1). The one difference between them is that ‘URmtr’ includes only missense variants that occur in genic subregions intolerant to missense variation, measured via the missense tolerance ratio (‘MTR’; Methods)29. The effect sizes of the ‘URmtr’-derived cis-CDS pQTLs (mean |β| = 1.62) were significantly larger than the ‘UR’ ones (mean |β| = 1.45; Wilcoxon P = 1.2 × 10−5) (Fig. 2c). This underscores the ability of population genetics-based methods to prioritize functional missense variants, offering complementary information to in silico pathogenicity predictors.
Pan-ancestry collapsing analysis
Including individuals of non-European ancestries in genetic studies promotes healthcare equity and boosts genetic discoveries11,30. We performed a pan-ancestry collapsing analysis on 49,736 UKB participants, including the original 46,327 European samples plus 3,409 individuals from African, Asian and other ancestries. In this combined analysis, there were 752 unique study-wide significant gene–protein abundance associations that were not significant in the European-only analyses. On the other hand, 228 associations that were significant in the European-only analyses did not reach study-wide significance in the pan-ancestry analysis (Supplementary Tables 8 and 10). Of the newly significant associations, 408 (54%) were cis, 327 (43%) were trans and 17 (2%) were cis-position, trans-gene (Supplementary Table 10).
One newly significant example was the trans association between PTVs in HBB and increased levels of the monocarboxylic acid transporter SLC16A1 (β = 1.85; 95% CI, 1.33 to 2.37; P = 3.2 × 10−12). This likely reflects the enrichment of HBB PTVs in individuals of South Asian ancestries, as observed in our UKB PheWAS11. Another example that only became significant in the pan-ancestry analysis was the trans association between PTVs in ATM, associated with ataxia telangiectasia and several cancers, and increased levels of alpha-fetoprotein (P = 1.8 × 10−9, β = 0.25, 95% CI, 0.17 to 0.33)31. These results illustrate the importance of increasing genetic diversity in proteogenomic studies.
Protein–protein interactions
Several trans associations from the collapsing analyses captured known protein–protein interactions. For example, there were significant associations between PTVs in PSAP and increased progranulin (GRN; P = 5.5 × 10−17; β = 2.60; 95% CI, 1.99 to 3.21) and cathepsin B (P = 1.2 × 10−11; β = 2.10; 95% CI, 1.49 to 2.70), and a nearly significant association with increased cathepsin D (P = 9.0 × 10−8; β = 1.61; 95% CI, 1.02 to 2.20) (Supplementary Table 8). Prosaposin and progranulin are key regulators of lysosomal function, and recessive mutations in either gene can cause separate LSDs32,33. Haploinsufficiency of GRN is also associated with frontotemporal lobar degeneration34. Prosaposin heterodimerizes with progranulin, regulating its levels and facilitating its transport to the lysosome35,36. Moreover, within the lysosome, prosaposin is cleaved by cathepsin D in the lysosome into four separate saposins. This example highlights the utility of proteogenomics in identifying existing and potentially novel protein–protein interactions.
There were also 22 trans-CDS associations between known ligand–receptor pairs37 (Supplementary Table 11). For example, there was a significant association between non-synonymous variants in TSHR, encoding the thyroid stimulating hormone receptor, and increased thyroid stimulating hormone (TSHB) (‘flexdmg’ model; P = 1.6 × 10−31; β = 0.66; 95% CI, 0.55 to 0.76). Likewise, we identified a trans association between mutations in FLT3, encoding the fms-related tyrosine kinase 3, and increased levels of the FLT3 ligand (FLT3LG; ‘ptvraredmg’ model; P = 1.2 × 10−22; β = 0.85; 95% CI, 0.68 to 1.02). Although we highlighted well-known ligand–receptor pairs here, this trans-CDS pQTL atlas may also help identify ligands of orphan receptors38 (https://astrazeneca-cgr-publications.github.io/pqtl-browser).
Insights into allelic series
Observing multiple pQTLs in one gene can help identify allelic series. For example, three rare protein-coding variants in NLRC4 were associated with significant changes in plasma levels of the proinflammatory cytokine IL-18 in the ExWAS (Extended Data Table 2 and Supplementary Table 2). NLRC4 is involved in inflammasome activation39, and rare, hypermorphic missense variants in this gene cause autosomal dominant infantile enterocolitis, characterized by recurrent flares of autoinflammation with elevated IL-18 and IL-1β levels40. The three NLRC4 pQTLs included one frameshift variant, one missense variant associated with reduced protein levels and one putatively gain-of-function missense variant associated with higher levels (Extended Data Table 2). Interestingly, there were no significant associations between these three variants and clinically relevant phenotypes in our published PheWAS of 470,000 UKB exomes (https://azphewas.com)11, suggesting that pharmacologic inhibition of NLRC4 may be safe. Moreover, these data show that some rare, putative gain-of-function mutations in this gene may not be sufficient to cause an observable phenotype, highlighting the value of this resource in clinical diagnostic settings.
Biomarker discovery
pQTLs offer a valuable resource for biomarker identification. For example, 29% of genes with at least one trans-CDS association in the collapsing analyses (n = 73 of 254) are targets of currently approved drugs listed in DrugBank (expected: 14%; two-tailed binomial P = 8.7 × 10−10)41. However, this pQTL atlas can also help discover putative biomarkers for candidate therapies that may not yet be approved. To demonstrate this, we examined the trans associations with a splice variant in HSD17B13 (rs72613567), known to protect against chronic liver disease42. In the ExWAS, this variant was associated with altered levels of ASS1 (P = 1.4 × 10−12; β = −0.05; 95% CI, −0.04 to −0.06), CES3 (P = 2.9 × 10−12; β = 0.07; 95% CI, 0.08 to 0.05), FUOM (P = 1.7 × 10−10; β = −0.05; 95% CI, −0.03 to −0.06), HYAL1 (P = 9.5 × 10−9; β = −0.04; 95% CI, −0.03 to −0.06), SHBG (P = 1.9 × 1−13; β = −0.05; 95% CI, −0.04 to −0.06) and SMPD1 (P = 2.7 × 10−11; β = −0.05; 95% CI, −0.03 to −0.06) (Supplementary Table 2). These associations not only serve as potential biomarkers, but could also inform future functional studies investigating the protective effect of this variant. Beyond biomarker discovery, this pQTL atlas may facilitate other components of drug development, including identifying novel genetic targets, safety profiling and drug repositioning opportunities (Extended Data Fig. 5a). To make these data broadly accessible, we provide the pQTL summary statistics in our PheWAS browser (https://azphewas.com) and publish a separate interactive portal (Extended Data Fig. 5b; https://astrazeneca-cgr-publications.github.io/pqtl-browser).
Clonal haematopoiesis pQTLs
The accumulation of somatic mutations with age can cause clonal expansion of haematopoietic stem cell populations (termed ‘clonal haematopoiesis’ or ‘CH’). CH has been associated with an increased risk of haematological cancer, cardiovascular disease, infection and other diseases43,44. We sought to identify plasma protein changes associated with CH to uncover potential disease mechanisms and biomarkers. We performed a gene-level collapsing analysis focused on clonal somatic variants in 15 genes recurrently mutated in CH and myeloid cancers (Methods and Supplementary Table 12).
We detected 36 trans associations with JAK2-CH, 15 with TET2-CH, eight with ASXL1-CH and four each with SF3B1-CH and SRSF2-CH (Fig. 3a–e and Supplementary Table 13). Strikingly, there was very little overlap between the protein abundances associated with each of these five genes, suggesting distinct downstream effects of the somatic events detected in each. The effect sizes were opposite in the two instances where the same protein was linked with CH events affecting two different genes. COL5A1 was positively associated with ASXL1-CH (P = 1.6 × 10−12; β = 0.75; 95% CI, 0.54 to 0.96) and negatively associated with TET2-CH (P = 5.9 × 10−9; β = −0.35; 95% CI, −0.47 to −0.23) (Fig. 3b,c). DKK1 was positively associated with JAK2-CH (P = 7.2 × 10−14; β = 1.57; 95% CI, 1.16 to 1.98) and negatively associated with SF3B1-CH (P = 1.8 × 10−10; β = −1.19; 95% CI, −0.47 to −0.23) (Fig. 3a,d).
a–e, Significant (P ≤ 1 × 10−8) trans-CDS pQTLs associated with somatic mutations in JAK2 (a), TET2 (b), ASXL1 (c), SF3B1 (d) and SRSF2 (e). Red lines indicate positive betas and black lines indicate negative betas. Line width is proportional to the absolute beta. We plotted significant associations for each gene in any of the four CH collapsing models.
Three of the JAK2 trans-CDS pQTLs are proteins involved in the integrin β2 pathway: FCGR2A, GP1BA and ICAM2 (ref. 45) (Fig. 3a). The most prevalent JAK2 missense variant associated with CH and myeloproliferative disorders (V617F) is thought to promote venous thrombosis by activating this pathway46.
Somatic mutations in TET2 were associated with increased levels of the receptor tyrosine kinase FLT3 (P = 3.4 × 10−16; β = 0.50; 95% CI, 0.38 to 0.62) and decreased levels of the FLT3 ligand, FLT3LG (P = 6.4 × 10−64; β = −0.97; 95% CI, −1.08 to −0.86) (Fig. 3b). FLT3 is a key regulator of haematopoietic stem cell proliferation and dendritic cell differentiation47. Roughly 30% of patients with acute myeloid leukaemia carry FLT3-activating mutations, the presence of which portends poor outcomes48. There are FLT3 inhibitors that improve the survival of patients with acute myeloid leukaemia49,50. If the relationship between TET2 and FLT3 is causal, this could suggest potential drug repositioning and precision medicine opportunities for TET2-CH. The three other proteins increased in abundance in TET2-CH were CD1C (a marker of conventional dendritic cells), CLEC4C (a marker of plasmacytoid dendritic cells) and CD86 (a marker of dendritic cells, monocytes and other antigen-presenting cells)51. Many of the downregulated proteins are markers of other haematopoietic lineages, such as KIR2DL2 and KIR2DL3 (natural killer cell activation)52, and PRG2 and PRG3 (constituents of eosinophil granules also involved in basophil stimulation)53,54. These results are consistent with the well-established association between CH and immune dysfunction55.
pQTL-augmented PheWAS
Defining appropriate QVs is critical to improving the signal-to-noise ratio in collapsing analysis. This is relatively straightforward for PTVs, but distinguishing between damaging and benign missense variants remains challenging. In silico tools help, but even the most well-performing predictors only modestly correlate with experimental measures of protein function56. We reasoned that incorporating both PTVs and missense cis-CDS pQTLs associated with decreased protein abundance could offer an orthogonal approach to defining QVs.
In our previous UKB PheWAS, the PTV collapsing models accounted for the greatest number of significant gene–phenotype relationships11. Here, we augmented our standard PTV model with missense variants associated with reduced protein abundance (that is, ExWAS cis-CDS pQTLs with β < 0 and P < 0.0001; Methods). We defined two new collapsing models: ‘ptvolink’, in which we included PTVs and missense pQTLs with a MAF < 0.1%, and ‘ptvolink2pcnt’, in which we relaxed the MAF of missense pQTLs to <2% (Methods, Fig. 4a and Extended Data Table 1). We then tested for associations between genes encoding the Olink proteins and 13,385 binary and 1,629 quantitative phenotypes (Supplementary Table 14).
a, Schematic representing the pQTL-informed collapsing framework. The purple diamonds represent missense pQTLs that would be included as QVs in the ptvolink model and ptvolink2pcnt model. PTVs, illustrated as X’s, are included in both models. b, The P values of gene-level associations for binary traits in which the P values improved in the ptvolink model compared with the ptv model. For comparison, we also include P values for the flexdmg model, which includes PTVs and rare (MAF < 0.1%) missense variants predicted to be damaging via REVEL (REVEL > 0.25), and the flexnonsyn model, which includes PTVs and missense variants without a REVEL cut-off. Only three genes that reached significance in the flexdmg model were not among the 25 genes significant across both ptvolink models. Of these, two were already captured by the standard ptv model. P values were generated via a two-tailed Fisher’s exact test and were not corrected for multiple testing. The dashed line indicates the study-wide significance threshold of P ≤ 1 × 10−8.
In the standard ptv model, 11 genes with at least one qualifying cis-CDS missense pQTL were significantly associated with at least one phenotype (Supplementary Table 15). These associations included ACVRL1 and ENG with hereditary haemorrhagic telangiectasia, GRN with dementia, NOTCH1 with chronic lymphocytic leukaemia, PCSK9 and ANGPTL3 with dyslipidaemia, and others (Fig. 4b and Supplementary Table 15). The P value of the association between PCSK9 and dyslipidaemia markedly improved from P = 4.02 × 10−17 (odds ratio (OR) = 0.35; 95% CI, 0.27 to 0.46) to P = 7.69 × 10−112 (OR = 0.63; 95% CI, 0.60 to 0.65) in the ptvolink2pcnt model. Likewise, the ANGPTL3-dyslipidaemia signal improved from P = 8.73 × 10−9 (OR = 0.58; 95% CI, 0.48 to 0.70) to P = 9.62 × 10−17 (OR = 0.57; 95% CI, 0.50 to 0.66) in the ptvolink model. Including these cis-CDS missense pQTLs, which tended to have more modest effects on protein abundance than PTVs, resulted in weaker effect sizes but increased statistical power. The signals from the remaining nine significant genes in the PTV-only model were attenuated in the plasma pQTL-informed missense models (Supplementary Table 15).
Impressively, 15 genes that were not significant in the standard ptv model became significant in at least one of the pQTL-informed models (Fig. 4b and Supplementary Table 15). Many of these examples are well-established, including LPL and hyperlipidaemia, PROC and thrombophilia, and VWF and Von Willebrand’s disease. There was also a newly significant association between TCN1, encoding a vitamin B12 binding protein, and vitamin B deficiency. Other examples include KEL with hypertrophic skin disorders, MICA with hypothyroidism, ANGPTL4 with hypercholesterolaemia, TNFRSF8 and protection from asthma and SPARC with special screening examinations (Fig. 4b and Supplementary Table 15). The second strongest association for SPARC was with basal cell carcinoma, suggesting that this signal arose from screening for skin cancer (ptvolink2pcnt P = 4.5 × 10−6; β = 2.9; 95% CI, 1.9 to 4.4).
Several quantitative trait associations also became more significant in these models (Extended Data Fig. 6 and Supplementary Table 16). Consistent with related binary phenotypes, the associations of PCSK9, ANGPTL4, LPL and ANGPTL3 with lipid-related traits all improved under the ptvolink and ptvolink2pcnt models. There was also an association between EPO and reduced haematocrit that was only significant in the ptvolink2pcnt model (P = 1.8 × 10−83; β = −0.24; 95% CI, −0.27 to −0.22). PTVs in this gene are a well-established cause of erythrocytosis57. Newly significant associations included PEAR1 (endothelial aggregation receptor) with decreased mean platelet volume (ptvolink2pcnt P = 6.9 × 10−27; β = −0.26; 95% CI, −0.31 to −0.21) and CA1 (carbonic anhydrase) with increased reticulocyte count (P = 1.0 × 10−19; β = 0.40; 95% CI, 0.32 to 0.49).
We compared the gene–phenotype associations that improved in the ptvolink model with our ‘flexnonsyn’ model, which includes rare PTVs and all rare missense variants. There were two associations where the flexnonsyn model outperformed the ptvolink model (PROC with thrombophlebitis and LCAT with HDL cholesterol; Fig. 4b and Extended Data Fig. 5). We next compared these results with the ‘flexdmg’ model, which includes PTVs and rare missense variants (MAF < 0.1%) predicted to be damaging (REVEL > 0.25). Only two of the seven binary trait associations and ten of the 92 quantitative associations were more significant in the flexdmg model than in the ptvolink model. These results demonstrate how including cis-CDS missense pQTLs can enhance conventional loss-of-function gene collapsing analyses. The approach is currently constrained by the UKB-PPP sample size used for pQTL discovery. As the number of proteogenomics samples increases, we will have more power to detect rarer missense pQTLs, further improving this approach.
Discussion
We performed an extensive rare variant proteogenomics study, including 2,923 plasma protein abundances measured in 49,736 UKB human exomes. Our results highlight the importance of exome sequencing for rare variant associations, as most rare variant pQTLs (MAF ≤ 0.1%) were not detected in the common variant analysis on the same cohort5. Moreover, rare cis- and trans-CDS pQTLs conferred significantly larger effect sizes than common variant pQTLs. cis-CDS pQTLs corresponding to PTVs were nearly always associated with decreased protein levels, highlighting the robustness of these associations. We showed that the rare missense cis-CDS pQTLs are enriched for pathogenic and predicted damaging missense variants. Although we detected one potential epitope effect in GOT1, epitope effects do not seem to systematically confound these coding pQTLs. In future studies, putatively novel associations should nonetheless be validated with orthogonal experiments.
We highlighted several examples of how this protein-coding pQTL atlas can address drug discovery and clinical pipeline challenges. We anticipate that this resource will provide novel insights into protein regulatory networks, upstream trans regulators of target genes whose inhibition could increase target protein levels, target safety assessments and drug repositioning opportunities (Extended Data Fig. 5a). Through our pQTL browser (https://astrazeneca-cgr-publications.github.io/pqtl-browser) and our previously published UKB PheWAS browser (azphewas.com), researchers can readily identify genetically anchored disease–protein abundance associations.
Methods
UKB cohort
The UKB is a prospective study of approximately 500,000 participants aged 40–69 years at recruitment. Participants were recruited in the United Kingdom between 2006 and 2010 and are continuously followed58. The average age at recruitment for sequenced individuals was 56.5 yr and 54% of the sequenced cohort comprises those of the female sex. Participant data include health records that are periodically updated by the UKB, self-reported survey information, linkage to death and cancer registries, collection of urine and blood biomarkers, imaging data, accelerometer data, genetic data and various other phenotypic end points59. All study participants provided informed consent.
Olink proteogenomics study cohort
Olink proteomic profiling was conducted on blood plasma samples collected from 54,967 UKB participants using the Olink Explore 3072 platform. This platform measured 2,923 protein analytes, reflecting 2,941 unique proteins measured across the Olink panels that comprise the 3072 panel (Cardiometabolic[II], Inflammation[II], Neurology[II] and Oncology[II]) (Supplementary Table 1). Details of UKB Proteomics participant selection (across the 46,673 randomized, the 6,365 consortia selected and the 1,268 individuals participating in the COVID-19 repeat imaging study) alongside the sample handling have been thoroughly documented in the Supplementary Information in Sun et al.5.
For whole-exome sequencing-based proteogenomic analyses, we analysed the 52,217 samples with available paired-exome sequence data. Next, we required that samples pass Olink NPX quality control as described in Sun et al.5, resulting in a test cohort reduction to 50,065 (96%). We then pruned this cohort for sample duplicates and first-degree genetic relatedness (no pair with a kinship coefficient exceeding 0.1769, n = 462), resulting in 49,736 (95%) participants available for the multi-ancestry analyses performed in this paper. Europeans are the most well-represented genetic ancestry in the UKB. We identified the participants with European genetic ancestry based on Peddy60 Pr(EUR) > 0.98 (n = 46,441). We then performed finer-scale ancestry pruning of these individuals, retaining those within 4 s.d. from the mean across the first four principal components, resulting in a final cohort of 46,327 (89%) individuals for the proteogenomic analyses.
Sequencing
Whole-exome sequencing data for UKB participants were generated at the Regeneron Genetics Center as part of a precompetitive data generation collaboration between AbbVie, Alnylam Pharmaceuticals, AstraZeneca, Biogen, Bristol-Myers Squibb, Pfizer, Regeneron and Takeda. Genomic DNA underwent paired-end 75-bp whole-exome sequencing at Regeneron Pharmaceuticals using the IDT xGen v1 capture kit on the NovaSeq6000 platform. Conversion of sequencing data in BCL format to FASTQ format and the assignments of paired-end sequence reads to samples were based on 10-base barcodes, using bcl2fastq v.2.19.0. Exome sequences from 469,809 UKB participants were made available to the Exome Sequencing consortium in May 2022. Initial quality control was performed by Regeneron and included sex discordance, contamination, unresolved duplicate sequences and discordance with microarray genotyping data checks61.
AstraZeneca Centre for Genomics Research bioinformatics pipeline
The 469,809 UKB exome sequences were processed at AstraZeneca from their unaligned FASTQ state. A custom-built Amazon Web Services (AWS) cloud computing platform running Illumina DRAGEN Bio-IT Platform Germline Pipeline v.3.0.7 was used to align the reads to the GRCh38 genome reference and to perform single-nucleotide variant (SNV) and insertion and deletion (indel) calling. SNVs and indels were annotated using SnpEFF v.4.3 (ref. 62) against Ensembl Build 38.92 (ref. 63). We further annotated all variants with their gnomAD MAFs (gnomAD v.2.1.1 mapped to GRCh38)64. We also annotated missense variants with MTR and REVEL scores16,29. The AstraZeneca pipeline output files including the variant call format files are available through UKB Showcase (https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=172).
ExWAS
We tested the 617,073 variants identified in at least four individuals from the 46,327 European ancestry UKB exomes that passed both exome and Olink sample quality checks. Variants were required to pass the following quality control criteria: minimum coverage 10×; percentage of alternate reads in heterozygous variants greater than or equal to 0.2; binomial test of alternate allele proportion departure from 50% in heterozygous state P > 1 × 10−6; genotype quality score (GQ) ≥ 20; Fisher’s strand bias score (FS) ≤ 200 (indels), FS ≤ 60 (SNVs); mapping quality score (MQ) ≥ 40; quality score (QUAL) ≥ 30; read position rank sum score (RPRS) ≥ −2; mapping quality rank sum score (MQRS) ≥ −8; DRAGEN variant status = PASS; the variant site is not missing (that is, less than 10× coverage) in 10% or more of sequences; the variant did not fail any of the aforementioned quality control in 5% or more of sequences; the variant site achieved tenfold coverage in 30% or more of gnomAD exomes; and, if the variant was observed in gnomAD exomes, 50% or more of the time those variant calls passed the gnomAD quality control filters (gnomAD exome AC/AC_raw ≥ 50%). In our previous UKB exome sequencing study we also created dummy phenotypes to correspond to each of the four exome sequence delivery batches to identify and exclude from analyses genes and variants that reflected sequencing batch effects; we provided these as a cautionary list resource for other UKB exome researchers as Supplementary Tables 25–27 in Wang et al.11. Since then, an additional fifth batch of exomes was released, for which we identified an additional 382 cautionary variants (Supplementary Table 17) on top of the original 8,365 previously described. We also report the ExWAS results from the 8,747 cautionary variants in Supplementary Table 17.
Variant-level pQTL P values were generated, adopting a linear regression (correcting for age, sex, age × sex, age × age, age × age × sex, principal component 1 (PC1), PC2, PC3, PC4, batch2, batch3, batch4, batch5, batch6, batch7 and a panel-specific measure of time between measurement and sampling). Three distinct genetic models were studied: genotypic (AA versus AB versus BB), dominant (AA + AB versus BB) and recessive (AA versus AB + BB), where A denotes the alternative allele and B denotes the reference allele. For ExWAS analysis, we used a significance cut-off of P ≤ 1 × 10−8. To support the use of this threshold, we performed an n-of-1 permutation on the full ExWAS pQTL analysis. In total, 24 of 5.4 billion permuted tests had P ≤ 1 × 10−8 (Supplementary Table 3). At this P ≤ 1 × 10−8 threshold, the expected number of ExWAS pQTL false positives is 24 out of the 328,975 observed significant associations (0.007%).
As an additional quality control check, we assessed the concordance of suggestive and significant ExWAS cis-CDS pQTLs (P < 1 × 10−4) corresponding to proteins that were measured in multiple Olink panels (CXCL8, TNF, IDO1 and LMOD1). Encouragingly, there was complete concordance across panels (Supplementary Table 18). Of note, IL-6 and SCRIB also were measured on multiple panels, but we did not observe any cis-CDS pQTLs with a P < 1 × 10−4 for these proteins. Ideally, potential epitope effects could be assessed by testing whether cis-CDS pQTLs preferentially overlap with known binding sites for the antibodies used on the Olink platform. These data were unavailable on request.
Collapsing analysis
As previously described, to perform collapsing analyses we aggregated variants within each gene that fitted a given set of criteria, identified as QVs11,65,66. In total, we performed nine non-synonymous collapsing analyses, including eight dominant and one recessive model, plus a tenth synonymous variant model that serves as an empirical negative control. In each model, for each gene, the proportion of cases was compared with the proportion of controls for individuals carrying one or more QVs in that gene. The exception is the recessive model, where a participant must have two qualifying alleles, either in homozygous or potential compound heterozygous form. Hemizygous genotypes for the X chromosome were also qualified for the recessive model. The QV criteria for each collapsing analysis model adopted in this study are in Extended Data Table 1. These models vary in terms of allele frequency (from private up to a maximum of 1%), predicted consequence (for example, PTV or missense) and REVEL and MTR scores. Based on SnpEff annotations, we defined synonymous variants as those annotated as ‘synonymous_variant’. We defined PTVs as variants annotated as exon_loss_variant, frameshift_variant, start_lost, stop_gained, stop_lost, splice_acceptor_variant, splice_donor_variant, gene_fusion, bidirectional_gene_fusion, rare_amino_acid_variant and transcript_ablation. We defined missense as: missense_variant_splice_region_variant and missense_variant. Non-synonymous variants included: exon_loss_variant, frameshift_variant, start_lost, stop_gained, stop_lost, splice_acceptor_variant, splice_donor_variant, gene_fusion, bidirectional_gene_fusion, rare_amino_acid_variant, transcript_ablation, conservative_inframe_deletion, conservative_inframe_insertion, disruptive_inframe_insertion, disruptive_inframe_deletion, missense_variant_splice_region_variant, missense_variant and protein_altering_variant.
For all models, we applied the following quality control filters: minimum coverage 10×; annotation in consensus coding sequence (CCDS) transcripts (release 22; approximately 34 Mb); at most 80% alternate reads in homozygous genotypes; percentage of alternate reads in heterozygous variants greater than or equal to 0.25 and less than or equal to 0.8; binomial test of alternate allele proportion departure from 50% in heterozygous state P > 1 × 10−6; GQ ≥ 20; FS ≤ 200 (indels), FS ≤ 60 (SNVs); MQ ≥ 40; QUAL ≥ 30; RPRS ≥ −2; MQRS ≥ −8; DRAGEN variant status = PASS; the variant site achieved tenfold coverage in ≥25% of gnomAD exomes; and, if the variant was observed in gnomAD exomes, the variant achieved exome z-score ≥ −2.0 and exome MQ ≥ 30.
The list of 18,885 studied genes and corresponding coverage statistics of how well each protein-coding gene is represented across all individuals by the exome sequence data is available in Supplementary Table 19. Moreover, we had previously created dummy phenotypes to correspond to each of the five exome sequence delivery batches to identify and exclude from analyses 46 genes that were enriched for exome sequencing batch effects; these cautionary lists are available in Supplementary Tables 25–27 of Wang et al.11. Gene-based pQTL P values were generated, adopting a linear regression (correcting for age, sex, age × sex, age × age, age × age × sex, PC1, PC2, PC3, PC4, batch1, batch2, batch3, batch4, batch5, batch6 and batch7). For the pan-ancestry analysis we included additional categorical covariates to capture broad ancestry (European, African, East Asian and South Asian).
For gene-based collapsing analyses, we used a significance cut-off of P ≤ 1 × 10−8. To support the use of this threshold, we ran the synonymous (empirical null) collapsing model and found only seven events achieved a signal below this threshold. Moreover, we performed an n-of-1 permutation on the full collapsing pQTL analysis. Only 4 of 499.9 million permuted tests had P ≤ 1 × 10−8 (Supplementary Table 3). At this P ≤ 1 × 10−8 threshold, the expected number of collapsing pQTL false positives is 4 out of the 7,412 (0.05%) observed significant associations.
Phenotypes
We studied two main phenotypic categories: binary and quantitative traits taken from the April 2022 data release that was accessed on 6 April 2022 as part of UKB applications 26041 and 65851. To parse the UKB phenotypic data, we adopted our previously described PEACOCK package, located at https://github.com/astrazeneca-cgr-publications/PEACOK11.
The PEACOK R package implementation focuses on separating phenotype matrix generation from statistical association tests. It also allows statistical tests to be performed separately on different computing environments, such as on a high-performance computing cluster or an AWS Batch environment. Various downstream analyses and summarizations were performed using R v.3.6.1 (https://cran.r-project.org). R libraries data.table (v.1.12.8; https://CRAN.R-project.org/package=data.table), MASS (7.3-51.6; https://www.stats.ox.ac.uk/pub/MASS4/), tidyr (1.1.0; https://CRAN.R-project.org/package=tidyr) and dplyr (1.0.0; https://CRAN.R-project.org/package=dplyr) were also used.
For UKB tree fields, such as the International Classification of Diseases tenth edition (ICD-10) hospital admissions (field 41202), we studied each leaf individually and studied each subsequent higher-level grouping up to the ICD-10 root chapter as separate phenotypic entities. Furthermore, for the tree-related fields, we restricted controls to participants who did not have a positive diagnosis for any phenotype contained within the corresponding chapter to reduce potential contamination due to genetically related diagnoses. A minimum of 30 cases were required for a binary trait to be studied. In addition to studying UKB algorithmically defined outcomes, we studied union phenotypes for each ICD-10 phenotype. These union phenotypes are denoted by a ‘Union’ prefix and the applied mappings are available in Supplementary Table 1 of Wang et al.11.
In total, we studied 13,385 binary and 1,629 quantitative phenotypes. As previously described, for all binary phenotypes, we matched controls by sex when the percentage of female cases was significantly different (Fisher’s exact two-sided P < 0.05) from the percentage of available female controls. This included sex-specific traits in which, by design, all controls would be the same sex as cases11. All phenotypes and corresponding chapter mappings for all phenotypes are provided in Supplementary Table 14.
Detecting CH somatic mutations
To detect putative CH somatic variants, we used the same GRCh38 genome reference aligned reads as for germline variant calling, and ran somatic variant calling with GATK’s Mutect2 (v.4.2.2.0)67. This analysis focused on the 74 genes previously curated as being recurrently mutated in myeloid cancers44. To remove potential recurrent artifacts, we filtered variants using a panel of normals created from 200 of the youngest UKB participants without a haematologic malignancy diagnosis. Subsequent filtering was performed with GATK’s FilterMutectCalls, including the filtering of read orientation artifacts using priors generated with LearnReadOrientationModel.
From the variant calls, clonal somatic variants were identified using a predefined list of gene-specific variant effects and specific missense variants (Supplementary Table 20). Only PASS variant calls with 0.03 ≤ variant allele frequency (VAF) ≤ 0.4 and allelic depth greater than or equal to 3 were included. For each gene, we validated the identified variants collectively as somatic by inspection of the age versus population prevalence profile (Supplementary Fig. 3), and limited further analysis to a set of 15 genes.
For the collapsing analysis, we considered four different VAF cut-offs (Supplementary Table 12). We excluded 359 individuals diagnosed with a haematological malignancy predating sample collection and included body mass index (BMI) and pack years of smoking as additional covariates. Most of the significant (P ≤ 1 × 10−8) associations arose with a VAF ≥ 10% cut-off (Supplementary Table 13).
Implementing the 470,000 missense pQTL-augmented PheWAS
In this study, we repeated our published PheWAS, adopting the now 469,809 available UKB exomes and 13,385 binary end points alongside 1,629 quantitative end points. We sought to test whether additional genotype–phenotype associations could be detected by augmenting our standard ptv model with cis-CDS missense pQTLs. Specifically, we included cis-acting missense variants nominally associated with reduced protein levels (that is, cis-CDS missense pQTLs with β < 0 and P < 0.0001). We identified 5,025 missense variants with cis-acting negative betas (P < 0.0001) among the genes encoding the 2,923 Olink protein analytes. In total, 1,487 (51%) distinct genes carried at least one of these 5,025 missense variants11. To assess improved signal detection over the baseline ptv collapsing model, we introduced two new collapsing models, ‘ptvolink’ and ‘ptvolink2pcnt’. ptvolink adopts the baseline ptv collapsing model with the only deviation being the inclusion of the 5,025 missense variants that also qualify the quality control and MAF criteria as adopted for the ptv collapsing model. ptvolink2pcnt is a repeat of the ptvolink collapsing model but permits missense variants with a MAF in the UKB population as high as 2%, as long as they were among the list of 5,025 missense variants identified to have a P < 0.0001 negative beta cis-CDS pQTL signal in the Olink ExWAS analyses. Full model descriptions are available in Extended Data Table 1. These new cis-CDS pQTL missense ptv augmented collapsing models were then compared with the standard collapsing models.
There may be instances where reduced protein levels reflect a disruption of antibody binding rather than a true biological signal. In the setting of collapsing analysis, in which we aggregate many variant effects in a gene, we expect these events to represent only a modest fraction of a gene’s complete allelic series. Moreover, in the context of this assessment, the inclusion of missense pQTLs would be expected to act conservatively (that is, diluting the value of including such missense in the PTV proteogenomic-augmented PheWAS collapsing analyses).
The UKB exomes cohort that was adopted for this refreshed PheWAS analysis was sampled from the available 469,809 UKB exome sequences. We excluded from analyses 118 (0.025%) sequences that achieved a VerifyBAMID freemix (contamination) level of 4% or higher68, and an additional five sequences (0.001%) where less than 94.5% of the CCDS (release 22) achieved a minimum of tenfold read depth69.
Using exome sequence-derived genotypes for 43,889 biallelic autosomal SNVs located in coding regions as input to the kinship algorithm included in KING v.2.2.3 (ref. 70), we generated pairwise kinship coefficients for all remaining samples. We used the ukb_gen_samples_to_remove() function from the R package ukbtools v.0.11.3 (ref. 71) to choose a subset of individuals within which no pair had a kinship coefficient exceeding 0.1769, to exclude predicted first-degree relatives. For each related pair, this function removes whichever member has the highest number of relatives above the provided threshold. Through this process, an additional 24,116 (5.1%) sequences were removed from downstream analyses. We predicted genetic ancestries from the exome data using Peddy v.0.4.2 with the ancestry-labelled 1000 Genomes Project as reference60. Of the 445,570 remaining UKB sequences, 24,790 (5.3%) had a Pr(EUR) ancestry prediction of less than 0.95. Focusing on the remaining 420,780 UKB participants, we further restricted the European ancestry cohort to those within ±4 s.d. across the top four principal component means. This resulted in 419,387 (89.3%) participants of European ancestry who were included in these cis-CDS pQTL modified analyses.
To remove potential concerns of circularity, we repeated the above ptvolink and ptvolink2pcnt collapsing model PheWAS; however, this time we removed UKB participants from the PheWAS analyses if they were part of the UKB Proteomics cohort of 46,327 individuals adopted to select the 5,025 cis-CDS pQTL missense variants. These results are included in Supplementary Tables 15 and 16, annotated as ‘ptvolinknoppp’ and ‘ptvolink2pcntnoppp’. The P values of the UKB-PPP excluded models and full models were highly correlated (R > 0.99; Supplementary Fig. 4).
Ethics declarations
The protocols for the UKB are overseen by the UKB Ethics Advisory Committee; for more information, see https://www.ukbiobank.ac.uk/ethics/ and https://www.ukbiobank.ac.uk/wp-content/uploads/2011/05/EGF20082.pdf.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Association statistics generated in this study are publicly available through our AstraZeneca Centre for Genomics Research (CGR) PheWAS Portal (http://azphewas.com/) and our pQTL browser (https://astrazeneca-cgr-publications.github.io/pqtl-browser). All whole-exome sequencing data described in this paper are publicly available to registered researchers through the UKB data access protocol. Exomes can be found in the UKB showcase portal: https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=170. The Olink proteomics data are also available under dataset https://biobank.ndph.ox.ac.uk/showcase/label.cgi?id=1838. Additional information about registration for access to the data is available at http://www.ukbiobank.ac.uk/register-apply/. Data for this study were obtained under Resource Application Number 26041. We also used data from DrugBank (https://go.drugbank.com), MTR (http://mtr-viewer.mdhs.unimelb.edu.au), REVEL (https://sites.google.com/site/revelgenomics), gnomAD (https://gnomad.broadinstitute.org) and ClinVar (https://www.ncbi.nlm.nih.gov/clinvar).
Code availability
PheWAS and ExWAS association tests were performed using a custom framework, PEACOK (PEACOK 1.0.7). PEACOK is available on GitHub: https://github.com/astrazeneca-cgr-publications/PEACOK/.
References
Suhre, K., McCarthy, M. I. & Schwenk, J. M. Genetics meets proteomics: perspectives for large population-based studies. Nat. Rev. Genet. 22, 19–37 (2021).
Zheng, J. et al. Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat. Genet. 52, 1122–1131 (2020).
Pietzner, M. et al. Mapping the proteo-genomic convergence of human diseases. Science 374, eabj1541 (2021).
Png, G. et al. Mapping the serum proteome to neurological diseases using whole genome sequencing. Nat. Commun. 12, 7042 (2021).
Sun, B. B. et al. Plasma proteomic associations with genetics and health in the UK Biobank. Nature https://doi.org/10.1038/s41586-023-06592-6 (2023).
Sun, B. B. et al. Genomic atlas of the human plasma proteome. Nature 558, 73–79 (2018).
Folkersen, L. et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat. Metab. 2, 1135–1148 (2020).
Ferkingstad, E. et al. Large-scale integration of the plasma proteome with genetics and disease. Nat. Genet. 53, 1712–1721 (2021).
Kierczak, M. et al. Contribution of rare whole-genome sequencing variants to plasma protein levels and the missing heritability. Nat. Commun. 13, 2532 (2022).
Katz, D. H. et al. Whole genome sequence analysis of the plasma proteome in Black adults provides novel insights into cardiovascular disease. Circulation 145, 357–370 (2022).
Wang, Q. et al. Rare variant contribution to human disease in 281,104 UK Biobank exomes. Nature 597, 527–532 (2021).
Coban-Akdemir, Z. et al. Identifying genes whose mutant transcripts cause dominant disease traits by potential gain-of-function alleles. Am. J. Hum. Genet. 103, 171–187 (2018).
Plenge, R. M., Scolnick, E. M. & Altshuler, D. Validating therapeutic targets through human genetics. Nat. Rev. Drug. Discov. 12, 581–594 (2013).
Nag, A. et al. Human genetics uncovers MAP3K15 as an obesity-independent therapeutic target for diabetes. Sci. Adv. 8, eadd5430 (2022).
Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 46, D1062–D1067 (2018).
Ioannidis, N. M. et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 99, 877–885 (2016).
Nag, A. et al. Effects of protein-coding variants on blood metabolite measurements and clinical biomarkers in the UK Biobank. Am. J. Hum. Genet. 110, 487–498 (2023).
Albert, F. W., Bloom, J. S., Siegel, J., Day, L. & Kruglyak, L. Genetics of trans-regulatory variation in gene expression. eLife https://doi.org/10.7554/eLife.35471 (2018).
Shen, X., Song, S., Li, C. & Zhang, J. Synonymous mutations in representative yeast genes are mostly strongly non-neutral. Nature 606, 725–731 (2022).
Dhindsa, R. S. et al. A minimal role for synonymous variation in human disease. Am. J. Hum. Genet. 109, 2105–2109 (2022).
Schledzewski, K. et al. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J. Clin. Invest. 121, 703–714 (2011).
Manta, C. P. et al. Targeting of scavenger receptors Stabilin-1 and Stabilin-2 ameliorates atherosclerosis by a plasma proteome switch mediating monocyte/macrophage suppression. Circulation 146, 1783–1799 (2022).
Coutinho, M. F., Prata, M. J. & Alves, S. Mannose-6-phosphate pathway: a review on its role in lysosomal function and dysfunction. Mol. Genet. Metab. 105, 542–550 (2012).
Raas-Rothschild, A. et al. Molecular basis of variant pseudo-hurler polydystrophy (mucolipidosis IIIC). J. Clin. Invest. 105, 673–681 (2000).
Ponnaiyan, S., Akter, F., Singh, J. & Winter, D. Comprehensive draft of the mouse embryonic fibroblast lysosomal proteome by mass spectrometry based proteomics. Sci. Data 7, 68 (2020).
Mosen, P., Sanner, A., Singh, J. & Winter, D. Targeted quantification of the lysosomal proteome in complex samples. Proteomes https://doi.org/10.3390/proteomes9010004 (2021).
Liu, L., Lee, W. S., Doray, B. & Kornfeld, S. Engineering of GlcNAc-1-phosphotransferase for production of highly phosphorylated lysosomal enzymes for enzyme replacement therapy. Mol. Ther. Methods Clin. Dev. 5, 59–65 (2017).
Harlow, C. E. et al. Identification and single-base gene-editing functional validation of a cis-EPO variant as a genetic predictor for EPO-increasing therapies. Am. J. Hum. Genet. 109, 1638–1652 (2022).
Traynelis, J. et al. Optimizing genomic medicine in epilepsy through a gene-customized approach to missense variant interpretation. Genome Res. 27, 1715–1729 (2017).
Petrovski, S. & Goldstein, D. B. Unequal representation of genetic variation across ancestry groups creates healthcare inequality in the application of precision medicine. Genome Biol. 17, 157 (2016).
Waldmann, T. A. & McIntire, K. R. Serum-α-fetoprotein levels in patients with ataxia-telangiectasia. Lancet 2, 1112–1115 (1972).
Kretz, K. A. et al. Characterization of a mutation in a family with saposin B deficiency: a glycosylation site defect. Proc. Natl Acad. Sci. USA 87, 2541–2544 (1990).
Smith, K. R. et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 90, 1102–1107 (2012).
Baker, M. et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 (2006).
Zhou, X., Sullivan, P. M., Sun, L. & Hu, F. The interaction between progranulin and prosaposin is mediated by granulins and the linker region between saposin B and C. J. Neurochem. 143, 236–243 (2017).
Nicholson, A. M. et al. Prosaposin is a regulator of progranulin levels and oligomerization. Nat. Commun. 7, 11992 (2016).
Ramilowski, J. A. et al. A draft network of ligand-receptor-mediated multicellular signalling in human. Nat. Commun. 6, 7866 (2015).
Elenbaas, J. S. et al. SVEP1 is an endogenous ligand for the orphan receptor PEAR1. Nat. Commun. 14, 850 (2023).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 (2006).
Romberg, N. et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 46, 1135–1139 (2014).
Wishart, D. S. et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 34, D668–672 (2006).
Abul-Husn, N. S. et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N. Engl. J. Med. 378, 1096–1106 (2018).
Abelson, S. et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559, 400–404 (2018).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Schaefer, C. F. et al. PID: the Pathway Interaction Database. Nucleic Acids Res. 37, D674–679 (2009).
Edelmann, B. et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J. Clin. Invest. 128, 4359–4371 (2018).
Small, D. et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc. Natl Acad. Sci. USA 91, 459–463 (1994).
Ravandi, F. et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk. Res. 34, 752–756 (2010).
Perl, A. E. et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N. Engl. J. Med. 381, 1728–1740 (2019).
Stone, R. M. et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 377, 454–464 (2017).
Collin, M. & Bigley, V. Human dendritic cell subsets: an update. Immunology 154, 3–20 (2018).
Colonna, M. & Samaridis, J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science 268, 405–408 (1995).
O’Donnell, M. C., Ackerman, S. J., Gleich, G. J. & Thomas, L. L. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J. Exp. Med. 157, 1981–1991 (1983).
Plager, D. A. et al. Comparative structure, proximal promoter elements, and chromosome location of the human eosinophil major basic protein genes. Genomics 71, 271–281 (2001).
Belizaire, R., Wong, W. J., Robinette, M. L. & Ebert, B. L. Clonal haematopoiesis and dysregulation of the immune system. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-023-00843-3 (2023).
Frazer, J. et al. Disease variant prediction with deep generative models of evolutionary data. Nature 599, 91–95 (2021).
Zmajkovic, J. et al. A gain-of-function mutation in EPO in familial erythrocytosis. N. Engl. J. Med. 378, 924–930 (2018).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Pedersen, B. S. & Quinlan, A. R. Who’s who? Detecting and resolving sample anomalies in human DNA sequencing studies with Peddy. Am. J. Hum. Genet. 100, 406–413 (2017).
Van Hout, C. V. et al. Exome sequencing and characterization of 49,960 individuals in the UK Biobank. Nature 586, 749–756 (2020).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6, 80–92 (2012).
Howe, K. L. et al. Ensembl 2021. Nucleic Acids Res. 49, D884–D891 (2021).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Petrovski, S. et al. An exome sequencing study to assess the role of rare genetic variation in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 196, 82–93 (2017).
Povysil, G. et al. Rare-variant collapsing analyses for complex traits: guidelines and applications. Nat. Rev. Genet. 20, 747–759 (2019).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013).
Jun, G. et al. Detecting and estimating contamination of human DNA samples in sequencing and array-based genotype data. Am. J. Hum. Genet. 91, 839–848 (2012).
Pujar, S. et al. Consensus coding sequence (CCDS) database: a standardized set of human and mouse protein-coding regions supported by expert curation. Nucleic Acids Res. 46, D221–D228 (2018).
Manichaikul, A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010).
Hanscombe, K. B., Coleman, J. R. I., Traylor, M. & Lewis, C. M. ukbtools: an R package to manage and query UK Biobank data. PLoS ONE 14, e0214311 (2019).
Acknowledgements
We thank the participants and investigators of the UK Biobank study who made this work possible (Resource Application Numbers 26041 and 65851). We are grateful to the research and development leadership teams at the 13 participating UKB-PPP member companies (Alnylam Pharmaceuticals, Amgen, AstraZeneca, Biogen, Bristol-Myers Squibb, Calico, Genentech, Glaxo Smith Klein, Janssen Pharmaceuticals, Novo Nordisk, Pfizer, Regeneron and Takeda) for funding the study. We thank the Legal and Business Development teams at each company for overseeing the contracting of this complex, precompetitive collaboration, with particular thanks to E. Olson of Amgen, A. Walsh of GSK and F. Middleton of AstraZeneca. We thank the UKB Exome Sequencing Consortium (UKB-ESC) members: AbbVie, Alnylam Pharmaceuticals, AstraZeneca, Biogen, Bristol-Myers Squibb, Pfizer, Regeneron and Takeda for funding the generation of the data, and Regeneron Genetics Center for completing the sequencing and initial quality control of the exome sequencing data. We are also grateful to the AstraZeneca Centre for Genomics Research Analytics and Informatics team for processing and analysis of sequencing data.
Author information
Authors and Affiliations
Consortia
Contributions
R.S.D., O.S.B., Q.W. and S.P. designed the study. R.S.D., O.S.B., B.B.S., B.P.P., D. Matelska, E.W., J.M., E.O., K.R.S., K.C., D.V. and Q.W. performed statistical analyses and data interpretation. S.W. and Q.W. performed bioinformatic processing. D. Matelska and D.V. developed the pQTL portal. R.S.D., O.S.B., E.W., J.M. and V.A.H. generated figures. R.S.D. and S.P. wrote the manuscript. O.S.B., B.B.S., A.R.H., D.S.P., M.A.F., H.R., C.V., B.C., A.P., E.A.A., C.D.W. and M.N.P. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.S.D., O.S.B., B.P.P., D. Matelska, E.W., J.M., E.O., V.A.H., K.R.S., K.C., S.W., A.R.H., D.S.P., M.A.F., C.V., B.C., A.P., D.V., M.N.P., Q.W. and S.P. are current employees and/or stockholders of AstraZeneca. B.B.S., C.D.W. and H.R. are employees and/or stockholders of Biogen. E.A.A. is a founder of Personalis, Inc., DeepCell, Inc. and Svexa Inc.; a founding advisor of Nuevocor; a non-executive director at AstraZeneca; and an advisor to SequenceBio, Novartis, Medical Excellence Capital, Foresite Capital and Third Rock Ventures.
Peer review
Peer review information
Nature thanks Yukinori Okada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Study design.
(a) Schematic depicting the overall study design and sample sizes for the variant-level ExWAS and the gene-level collapsing analyses. The number of significant gene-level pQTLs corresponds to the number of unique genes associated with at least one protein abundance. (b) Depiction of cis-, trans-, and cis-position trans-CDS pQTLs.
Extended Data Fig. 2 ExWAS pQTL effect sizes.
(a) Effect size distributions of cis- versus trans-CDS pQTLs stratified by allele frequency. (b) Effect sizes of rare (MAF ≤ 0.1%) pQTLs.
Extended Data Fig. 3 Missense cis-CDS pQTLs.
(a) Enrichment of ClinVar pathogenic and likely pathogenic (P/LP) variants among missense cis-CDS pQTLs. P-values calculated via two-tailed binomial test and are uncorrected. (b) REVEL scores of cis-CDS missense pQTLs. P-values were calculated with the Mann-Whitney U test (two-sided) and are not corrected for multiple testing. The appropriate Bonferroni-adjusted p-value threshold is p < 0.017. The boxplots show the median (centre line) and interquartile ranges (IQR) (box limits).
Extended Data Fig. 4 Overlap between pQTLs detected in the ExWAS and collapsing analysis.
(a) Number of unique gene-phenotype associations among non-synonymous pQTLs in the ExWAS versus the collapsing analysis. (b) Number of unique gene-phenotype associations among rare (MAF ≤ 0.1%) PTV-driven pQTLs in the ExWAS and ptv collapsing model.
Extended Data Fig. 5 pQTL atlas and interactive browser.
(a) Illustration of potential applications of this trans-CDS pQTL atlas to drug development. The chord diagram represents trans-CDS pQTLs detected in the collapsing analysis (p ≤ 1 × 10−8). Created using biorender.com (b) The AstraZeneca pQTL browser, highlighting LDLR as an example query. Users can browse pQTLs from both the ExWAS and gene-based collapsing analyses using an intuitive range of parameters and thresholds.
Extended Data Fig. 6 pQTL-informed collapsing analyses.
The p-values of gene-level associations for quantitative traits in which the p-values improved in the ptvolink model compared to the ptv model. For comparison, we also include p-values for the flexdmg model, which includes PTVs and rare (MAF < 0.1%) missense variants predicted to be damaging via REVEL (REVEL > 0.25), and the flexnonsyn model, which includes PTVs and missense variants without a REVEL cutoff. An additional 17 genes were not among the 87 significantly associated genes in the ptvolink models, and only 9 of these were not already captured by the ptv model. P-values were generated via linear regression and were not corrected for multiple testing. The dashed line indicates the study-wide significance threshold of p ≤ 1 × 10-8.
Supplementary information
Supplementary Information
Supplementary Note, Figs. 1–4, detailed descriptions of Datasets 1–20 and references.
Supplementary Table
Supplementary Datasets 1–20.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dhindsa, R.S., Burren, O.S., Sun, B.B. et al. Rare variant associations with plasma protein levels in the UK Biobank. Nature 622, 339–347 (2023). https://doi.org/10.1038/s41586-023-06547-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06547-x
This article is cited by
-
Plasma proteomic profiles predict future dementia in healthy adults
Nature Aging (2024)
-
The transition from genomics to phenomics in personalized population health
Nature Reviews Genetics (2024)
-
Harnessing the power of proteomics in precision diabetes medicine
Diabetologia (2024)
-
Protein-truncating variants in BSN are associated with severe adult-onset obesity, type 2 diabetes and fatty liver disease
Nature Genetics (2024)
-
A genetic atlas of the human blood proteome
Nature Reviews Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.