Abstract
Single-atom catalysts (SACs) have well-defined active sites, making them of potential interest for organic synthesis1,2,3,4. However, the architecture of these mononuclear metal species stabilized on solid supports may not be optimal for catalysing complex molecular transformations owing to restricted spatial environment and electronic quantum states5,6. Here we report a class of heterogeneous geminal-atom catalysts (GACs), which pair single-atom sites in specific coordination and spatial proximity. Regularly separated nitrogen anchoring groups with delocalized π-bonding nature in a polymeric carbon nitride (PCN) host7 permit the coordination of Cu geminal sites with a ground-state separation of about 4 Å at high metal density8. The adaptable coordination of individual Cu sites in GACs enables a cooperative bridge-coupling pathway through dynamic Cu–Cu bonding for diverse C–X (X = C, N, O, S) cross-couplings with a low activation barrier. In situ characterization and quantum-theoretical studies show that such a dynamic process for cross-coupling is triggered by the adsorption of two different reactants at geminal metal sites, rendering homo-coupling unfeasible. These intrinsic advantages of GACs enable the assembly of heterocycles with several coordination sites, sterically congested scaffolds and pharmaceuticals with highly specific and stable activity. Scale-up experiments and translation to continuous flow suggest broad applicability for the manufacturing of fine chemicals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the manuscript or in the Supplementary information. The data for the LCA analysis are deposited in the Zenodo repository: https://doi.org/10.5281/zenodo.8277667.
References
Cui, X., Li, W., Ryabchuk, P., Junge, K. & Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 1, 385–397 (2018).
Li, W.-H., Yang, J., Wang, D. & Li, Y. Striding the threshold of an atom era of organic synthesis by single-atom catalysis. Chem 8, 119–140 (2022).
Yan, H., Su, C., He, J. & Chen, W. Single-atom catalysts and their applications in organic chemistry. J. Mater. Chem. A 6, 8793–8814 (2018).
Giannakakis, G., Mitchell, S. & Pérez-Ramírez, J. Single-atom heterogeneous catalysts for sustainable organic synthesis. Trends Chem. 4, 264–276 (2022).
Lang, R. et al. Single-atom catalysts based on the metal–oxide interaction. Chem. Rev. 120, 11986–12043 (2020).
Shan, J. et al. Metal-metal interactions in correlated single-atom catalysts. Sci. Adv. 8, eabo0762 (2022).
Kessler, F. K. et al. Functional carbon nitride materials—design strategies for electrochemical devices. Nat. Rev. Mater. 2, 17030 (2017).
Hai, X. et al. Scalable two-step annealing method for preparing ultra-high-density single-atom catalyst libraries. Nat. Nanotechnol. 17, 174–181 (2022).
Bolm, C. & Beller, M. Transition Metals for Organic Synthesis Vol. 1 (Wiley, 2004).
Brandsma, L., Vasilevsky, S. F. & Verkruijsse, H. D. Application of Transition Metal Catalysts in Organic Synthesis (Springer, 2012).
De Meijere, A., Bräse, S. & Oestreich, M. Metal-Catalyzed Cross-Coupling Reactions and More Vol. 1 (Wiley, 2014).
Sheldon, R. A. & Van Bekkum, H. Fine Chemicals Through Heterogeneous Catalysis (Wiley, 2008).
Biffis, A., Centomo, P., Del Zotto, A. & Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: a critical review. Chem. Rev. 118, 2249–2295 (2018).
Vásquez-Céspedes, S., Betori, R. C., Cismesia, M. A., Kirsch, J. K. & Yang, Q. Heterogeneous catalysis for cross-coupling reactions: an underutilized powerful and sustainable tool in the fine chemical industry? Org. Process Res. Dev. 25, 740–753 (2021).
Tsubogo, T., Oyamada, H. & Kobayashi, S. Multistep continuous-flow synthesis of (R)- and (S)-rolipram using heterogeneous catalysts. Nature 520, 329–332 (2015).
Benaglia, M. & Puglisi, A. Catalyst Immobilization: Methods and Applications (Wiley, 2019).
Kalz, K. F. et al. Future challenges in heterogeneous catalysis: understanding catalysts under dynamic reaction conditions. ChemCatChem 9, 17–29 (2017).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Chen, Z. et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol. 13, 702–707 (2018).
Kaiser, S. K., Chen, Z., Faust Akl, D., Mitchell, S. & Pérez-Ramírez, J. Single-atom catalysts across the periodic table. Chem. Rev. 120, 11703–11809 (2020).
Li, Z. et al. Well-defined materials for heterogeneous catalysis: from nanoparticles to isolated single-atom sites. Chem. Rev. 120, 623–682 (2019).
Regalbuto, J. R. & Datye, A. K. All the lonely atoms, where do they all belong? Nat. Nanotechnol. 17, 110–111 (2022).
Tyborski, T. et al. Crystal structure of polymeric carbon nitride and the determination of its process-temperature-induced modifications. J. Phys. Condens. Matter 25, 395402 (2013).
Fina, F., Callear, S. K., Carins, G. M. & Irvine, J. T. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 27, 2612–2618 (2015).
Stuart, B. H. Infrared Spectroscopy: Fundamentals and Applications (Wiley, 2004).
Sendzik, M., Pushie, M. J., Stefaniak, E. & Haas, K. L. Structure and affinity of Cu(I) bound to human serum albumin. Inorg. Chem. 56, 15057–15065 (2017).
Worrell, B., Malik, J. & Fokin, V. V. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science 340, 457–460 (2013).
Tang, Y., Wang, Y.-G. & Li, J. Theoretical investigations of Pt1@CeO2 single-atom catalyst for CO oxidation. J. Phys. Chem. C 121, 11281–11289 (2017).
Zuo, J.-M., Kim, M., O’Keeffe, M. & Spence, J. Direct observation of d-orbital holes and Cu–Cu bonding in Cu2O. Nature 401, 49–52 (1999).
Zhang, W. et al. Emerging dual‐atomic‐site catalysts for efficient energy catalysis. Adv. Mater. 33, 2102576 (2021).
Campos, J. Bimetallic cooperation across the periodic table. Nat. Rev. Chem. 4, 696–702 (2020).
Klapars, A., Antilla, J. C., Huang, X. & Buchwald, S. L. A general and efficient copper catalyst for the amidation of aryl halides and the N-arylation of nitrogen heterocycles. J. Am. Chem. Soc. 123, 7727–7729 (2001).
Satyanarayana, K., Srinivas, K., Himabindu, V. & Reddy, G. M. A scaleable synthesis of dutasteride: a selective 5α-reductase inhibitor. Org. Process Res. Dev. 11, 842–845 (2007).
Kim, D., Jun, H., Lee, H., Hong, S.-S. & Hong, S. Development of new fluorescent xanthines as kinase inhibitors. Org. Lett. 12, 1212–1215 (2010).
Zheng, J., Cui, W.-J., Zheng, C. & You, S.-L. Synthesis and application of chiral spiro Cp ligands in rhodium-catalyzed asymmetric oxidative coupling of biaryl compounds with alkenes. J. Am. Chem. Soc. 138, 5242–5245 (2016).
Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Bhunia, S., Pawar, G. G., Kumar, S. V., Jiang, Y. & Ma, D. Selected copper‐based reactions for C–N, C–O, C–S, and C–C bond formation. Angew. Chem. Int. Ed. 56, 16136–16179 (2017).
Gutmann, B., Cantillo, D. & Kappe, C. O. Continuous‐flow technology—a tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 54, 6688–6728 (2015).
Du, Y. et al. XAFCA: a new XAFS beamline for catalysis research. J. Synchrotron Radiat. 22, 839–843 (2015).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Frisch, M. J. et al. Gaussian 16 (Gaussian, Inc., 2016).
Francl, M. M. et al. Self‐consistent molecular orbital methods. XXIII. A polarization‐type basis set for second‐row elements. J. Chem. Phys. 77, 3654–3665 (1982).
Hay, P. J. & Wadt, W. R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 82, 299–310 (1985).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098 (1988).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
ISO 14040:2006. Environmental management — life cycle assessment — principles and framework. International Organization for Standardization (ISO) (2006).
ISO 14044:2006. Environmental management — life cycle assessment — requirements and guidelines. International Organization for Standardization (ISO) (2006).
Wernet, G. et al. The ecoinvent database version 3 (part I): overview and methodology. Int. J. Life Cycle Assess. 21, 1218–1230 (2016).
Forster, P. et al. in Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (eds Masson-Delmotte, V. et al.) Ch. 7, 923–1054 (Cambridge Univ. Press, 2021).
Huijbregts, M. A. et al. ReCiPe2016: a harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess. 22, 138–147 (2017).
Mutel, C. Brightway: an open source framework for life cycle assessment. J. Open Source Softw. 2, 236 (2017).
Acknowledgements
J. Lu acknowledges support from MOE Tier 2 (MOE-T2EP50121-0008) and the Agency for Science, Technology and Research (A*STAR) under its MTC IRG grant (project nos. M22K2c0082 and A20E5c0096). Y. Zhu acknowledges support from Pharma Innovation Programme Singapore (PIPS, A*STAR-IAF-PP A19B3a0016 and A20B3a0107). M.J.K. acknowledges support from MOE Tier 2 (MOE-T2EP10122-0003). R.Z. acknowledges financial support from the National Natural Science Foundation of China (grant 51825201). S.M., V.T., G.G.-G. and J.P.-R. acknowledge funding from NCCR Catalysis (grant 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation. Jun Li acknowledges financial support from the National Natural Science Foundation of China (grant 22033005), NSFC Center for Single-Atom Catalysis, National Key R&D Project (nos. 2022YFA1503900 and 2022YFA1503000) and the Guangdong Provincial Key Laboratory of Catalysis (no. 2020B121201002). Q.Y. acknowledges financial support by the Natural Science Basic Research Program of Shaanxi (2021JCW-20 and 2022KJXX-18). We acknowledge the computational resources supported by the Center for Computational Science and Engineering (SUSTech), Tsinghua National Laboratory for Information Science and Technology and the National Supercomputing Centre (NSCC) Singapore. Q.Y. is grateful for the hospitality of Tsinghua University during her sabbatical visit.
Author information
Authors and Affiliations
Contributions
J. Lu supervised the project and organized the collaboration. X.H. and J. Lu conceived the research and proposed geminal-atom catalysis. X.H. designed the experiments and synthesized the materials. Jun Li and Q.Y. conceived the Cu–Cu bonding reaction mechanism and carried out quantum-chemical calculations. N.G. and C.Z. carried out theoretical calculations of the periodic structures. Y. Zhe., X.H. and Y. Zhu discovered the catalytic activity. M.J.K. and X. Luo conducted the comparison studies and synthetic applications. S.X. performed the XAFS measurement and structural analysis. X.Z. and Y.C. performed the electron microscopy experiments and data analysis. X.S. and X.P. performed the scanning tunnelling microscope experiments. J.W., X. Lo and M.W. performed the continuous-flow synthesis. V.T., J.P.-R. and G.G.-G. performed the LCA analysis and interpreted the results. S.M. and J.P.-R. advised on the experiments, methodologies and data presentation. L.R., Jing Li, P.H., H.L., J.S. and R.Z. assisted with materials characterization and data analysis. X.H. wrote the draft, with the assistance of Q.Y., Y. Zhe. and S.M. All authors discussed the results and edited and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jagadeesh Rajenahally and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
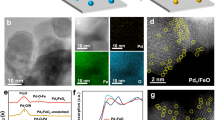
Extended Data Fig. 1 STM characterization of Cug/PCN.
a, Overview STM image after annealing PCN on Cu(111) at 370 K, V = 2.0 V, I = 20 pA. b, Corresponding constant-height STM image with a CO-functionalized tip (V = 5 mV, Δz = −20 pm; set point before turning off feedback, V = 5 mV, I = 200 pA).
Extended Data Fig. 2 Characterization of Cug/PCN.
a, High-resolution TEM image with the corresponding Fourier transform diffraction pattern inset. b, ADF-STEM image and corresponding elemental maps. c, Atomic force microscopy image of Cug/PCN with height profile inset. d, XRD patterns of PCN and Cug/PCN. e, Cu 2p XPS spectra of Cug/PCN. The peaks at 932.8 eV and 952.7 eV are assigned to Cu(I). The solid red line and dotted black line represent the experiment and fitting curves, respectively. f, EPR spectra of Cug/PCN and the reference CuCl2.
Extended Data Fig. 3 Comparison of the experimental and modelled Cu K-edge XANES spectra.
The corresponding DFT-modelled atomic structures are shown in the insets. Colour code: C, grey; N, blue; O, green; Cu, orange; H, white.
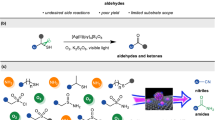
Extended Data Fig. 4 Substrate scope of Cug/PCN-catalysed cross-coupling and cycloaddition.
Product isolated yields obtained in Cug/PCN-catalysed C–N (52–72) and C–O bond formations (73–82). Products yields for Cug/PCN-catalysed azide–alkyne cycloadditions (83–90) determined by gas chromatography.
Extended Data Fig. 5 Cug/PCN-catalysed pharmaceutical and biorelevant molecules.
a, Synthesis of pharmaceutical compounds (91–93) in multisteps and one-pot manner. b, Synthesis of biorelevant molecules (94–96) from natural products.
Extended Data Fig. 6 Characterization of Cu1/PCN with different Cu contents.
ADF-STEM images (scale bar, 1 nm) (a), FTIR spectra (b) and Fourier-transformed EXAFS spectra (c) of Cu1/PCN samples with different Cu contents. Red and yellow dashed lines encircle GAC and SAC sites, respectively.
Extended Data Fig. 7 Electronic structure of Cu1/PCN with different Cu contents.
Cu K-edge XANES (a) and Cu 2p XPS (b) spectra of Cu1/PCN samples with different Cu contents. c,d, Charge density distributions and PDOS of monomeric (c) and geminal (d) Cu sites, respectively. Colour code: Cu, pink; C, grey; N, blue; H, white.
Extended Data Fig. 8 In situ XANES and EPR spectra during C–O coupling reaction.
a,b, In situ Cu K-edge XANES (a) and Fourier-transformed EXAFS (b) spectra of Cug/PCN catalyst measured before and in the C–O coupling reaction, respectively. c, In situ EPR spectra of Cug/PCN recorded at different times in the C–O coupling reaction. The chemical state change of Cug/PCN during the C–O cross-coupling reaction cycle was clearly seen from Cu K-edge XANES. A distinct weakening of the feature peak at 8,983 eV and an increase of the main peak at 8,996 eV indicate that the valence state of Cu increases during the reaction, which is related to the successful adsorption of 4-iodotoluene or methanol. Correspondingly, the intensity of the main peak related to the first coordination sphere in the Fourier-transformed EXAFS spectra increases, evidencing the increased coordination number of Cu. The change in the chemical valence state of Cu during the reaction cycle is further explained by in situ EPR spectroscopy. EPR-silent Cu(I) is first oxidized to EPR-sensitive Cu(II) with a gradually enhanced signal intensity and then reduced to the original Cu(I) state during a complete reaction process, in line with the in situ XAFS results.
Extended Data Fig. 9 Stability of Cug/PCN in C–O coupling.
a, Cycling test for the coupling of 4-iodotoluene with ethanol to form C–O bond over Cug/PCN. FTIR spectra (b), XRD pattern (c), XPS spectra (d), Cu K-edge Fourier-transformed EXAFS (e) and XANES (f) spectra of the as-prepared Cug/PCN and the catalyst recovered after nine reaction cycles.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23, Supplementary Tables 1–7 and NMR data.
Supplementary Video 1
Animation following the path for the C–O coupling reaction with the Cu GACs calculated by DFT.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hai, X., Zheng, Y., Yu, Q. et al. Geminal-atom catalysis for cross-coupling. Nature 622, 754–760 (2023). https://doi.org/10.1038/s41586-023-06529-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06529-z
This article is cited by
-
Artificial kagome lattices of Shockley surface states patterned by halogen hydrogen-bonded organic frameworks
Nature Communications (2024)
-
Well-defined diatomic catalysis for photosynthesis of C2H4 from CO2
Nature Communications (2024)
-
Advances in heterogeneous single-cluster catalysis
Nature Reviews Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.