Abstract
Abundant high-molecular-mass hyaluronic acid (HMM-HA) contributes to cancer resistance and possibly to the longevity of the longest-lived rodent—the naked mole-rat1,2. To study whether the benefits of HMM-HA could be transferred to other animal species, we generated a transgenic mouse overexpressing naked mole-rat hyaluronic acid synthase 2 gene (nmrHas2). nmrHas2 mice showed an increase in hyaluronan levels in several tissues, and a lower incidence of spontaneous and induced cancer, extended lifespan and improved healthspan. The transcriptome signature of nmrHas2 mice shifted towards that of longer-lived species. The most notable change observed in nmrHas2 mice was attenuated inflammation across multiple tissues. HMM-HA reduced inflammation through several pathways, including a direct immunoregulatory effect on immune cells, protection from oxidative stress and improved gut barrier function during ageing. These beneficial effects were conferred by HMM-HA and were not specific to the nmrHas2 gene. These findings demonstrate that the longevity mechanism that evolved in the naked mole-rat can be exported to other species, and open new paths for using HMM-HA to improve lifespan and healthspan.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Seluanov, A. et al. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc. Natl Acad. Sci. USA 106, 19352–19357 (2009).
Tian, X. et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346–349 (2013).
Lewis, K. N. & Buffenstein, R. in Handbook of the Biology of Aging (eds Kaeberlein, M. R. & Martin, G. M.) 179–204 (Elsevier, 2016).
Buffenstein, R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B 178, 439–445 (2008).
O’Connor, T. P., Lee, A., Jarvis, J. U. & Buffenstein, R. Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comp. Biochem. Physiol. A 133, 835–842 (2002).
Weissmann, B. & Meyer, K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical Cord. J. Am. Chem. Soc. 76, 1753–1757 (1954).
Fennouri, A. et al. Single molecule detection of glycosaminoglycan hyaluronic acid oligosaccharides and depolymerization enzyme activity using a protein nanopore. ACS Nano 6, 9672–9678 (2012).
Jiang, D., Liang, J. & Noble, P. W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91, 221–264 (2011).
Cyphert, J. M., Trempus, C. S. & Garantziotis, S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int. J. Cell Biol. 2015, 563818 (2015).
Wu, M. et al. A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J. 29, 1290–1298 (2015).
Zhang, G. et al. Colorectal cancer‐associated ~6 kDa hyaluronan serves as a novel biomarker for cancer progression and metastasis. FEBS J. 286, 3148–3163 (2019).
Ruppert, S., Hawn, T., Arrigoni, A., Wight, T. & Bollyky, P. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol. Res. 58, 186–192 (2014).
Strachan, R. K., Smith, P. & Gardner, D. L. Hyaluronate in rheumatology and orthopaedics: is there a role?. Ann. Rheum. Dis. 49, 949–952 (1990).
Muto, J., Yamasaki, K., Taylor, K. R. & Gallo, R. L. Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol. Immunol. 47, 449–456 (2009).
Šoltés, L. et al. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 7, 659–668 (2006).
Takasugi, M. et al. Naked mole-rat very-high-molecular-mass hyaluronan exhibits superior cytoprotective properties. Nat. Commun. 11, 2376 (2020).
Garantziotis, S. & Savani, R. C. Hyaluronan biology: a complex balancing act of structure, function, location and context. Matrix Biol. 78, 1–10 (2019).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A 69, S4–S9 (2014).
Fraser, J. & Laurent, T. The biology of hyaluronan. Ciba Found. Symp. 143, 41–59 (1989).
Ward, J. M. Lymphomas and leukemias in mice. Exp. Toxicol. Pathol. 57, 377–381 (2006).
Horvath, S. et al. DNA methylation clocks tick in naked mole rats but queens age more slowly than nonbreeders. Nat. Aging 2, 46–59 (2022).
Arneson, A. et al. A mammalian methylation array for profiling methylation levels at conserved sequences. Nat. Commun. 13, 783 (2022).
Mozhui, K. et al. Genetic loci and metabolic states associated with murine epigenetic aging. eLife 11, e75244 (2022).
Whitehead, J. C. et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J. Gerontol. A 69, 621–632 (2014).
Shiotsuki, H. et al. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods 189, 180–185 (2010).
Maddatu, T. P., Grubb, S. C., Bult, C. J. & Bogue, M. A. Mouse Phenome Database (MPD). Nucleic Acids Res. 40, D887–D894 (2012).
Chen, H., Zhou, X., Shoumura, S., Emura, S. & Bunai, Y. Age-and gender-dependent changes in three-dimensional microstructure of cortical and trabecular bone at the human femoral neck. Osteoporos. Int. 21, 627–636 (2010).
Tyshkovskiy, A. et al. Identification and application of gene expression signatures associated with lifespan extension. Cell Metab. 30, 573–593 (2019).
Tyshkovskiy, A. et al. Distinct longevity mechanisms across and within species and their association with aging. Cell 186, 2929–2949 (2023).
Schaum, N. et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020).
Lu, J. Y. et al. Comparative transcriptomics reveals circadian and pluripotency networks as two pillars of longevity regulation. Cell Metab. 34, 836–856 (2022).
Gubbels Bupp, M. R., Potluri, T., Fink, A. L. & Klein, S. L. The confluence of sex hormones and aging on immunity. Front. Immunol. 9, 01269 (2018).
Litwiniuk, M., Krejner, A., Speyrer, M. S., Gauto, A. R. & Grzela, T. Hyaluronic acid in inflammation and tissue regeneration. Wounds 28, 78–88 (2016).
Rayahin, J. E., Buhrman, J. S., Zhang, Y., Koh, T. J. & Gemeinhart, R. A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 1, 481–493 (2015).
Biragyn, A. & Ferrucci, L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 19, e295–e304 (2018).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466 (2017).
Sovran, B. et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. 9, 1437 (2019).
Bevins, C. L. & Salzman, N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Moorefield, E. C. et al. Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging 9, 1898 (2017).
Funk, M. C., Zhou, J. & Boutros, M. Ageing, metabolism and the intestine. EMBO Rep. 21, e50047 (2020).
DeJong, E. N., Surette, M. G. & Bowdish, D. M. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe 28, 180–189 (2020).
Yang, T. et al. Gut dysbiosis is linked to hypertension. Hypertension 65, 1331–1340 (2015).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Sibai, M. et al. Microbiome and longevity: high abundance of longevity-linked Muribaculaceae in the gut of the long-living rodent spalax leucodon. OMICS 24, 592–601 (2020).
Tung, V. W., Burton, T. J., Dababneh, E., Quail, S. L. & Camp, A. J. Behavioral assessment of the aging mouse vestibular system. J. Vis. Exp. https://doi.org/10.3791/51605 (2014).
Zhou, W., Triche Jr, T. J., Laird, P. W. & Shen, H. SeSAMe: reducing artifactual detection of DNA methylation by Infinium BeadChips in genomic deletions. Nucleic Acids Res. 46, e123 (2018).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Frankish, A. et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773 (2019).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Fransen, F. et al. β2→1-Fructans modulate the immune system in vivo in a microbiota-dependent and-independent fashion. Front. Immunol. 8, 154 (2017).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Acknowledgements
This work was supported by grants from the National Institutes of Health to V.N.G., A.S. and V.G.
Author information
Authors and Affiliations
Contributions
Z.Z., A.S. and V.G. designed research, analysed data and wrote the manuscript. Z.Z. performed most of the experiments. J.Y.L., A.T. and V.N.G. analysed RNA-seq data. X.T. designed research and generated the transgenic mouse strain. Z.Z., X.T., F.T.Z. and S.E. performed the ageing study. K.B. helped with immunofluorescence staining. J.A. performed the DMBA/TPA treatment. Q.L. helped with collecting and preparing faecal DNA for microbiome analysis. D.F. helped with the cell apoptosis assay. E.R. and S.A.B. helped with maintaining the mouse colony. S.H. performed the methylation clock assay. A.S. and V.G. supervised research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks F. Dilworth and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 nmrHAS2 mice exhibit resistance to spontaneous and induced cancer.
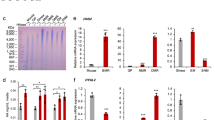
a. Representative stainings of HABP staining in organs of male nmrHAS2 and CreER mice. b. Pulse field gel shows that male nmrHAS2 mice have higher molecular weight and more abundant hyaluronic acid than CreER control mice. HA was extracted from 200 mg of pooled tissue from two individuals. HAase treated samples were run in parallel to confirm the specificity of HA staining. c. Quantification of relative HABP fluorescence intensity shown in b. n = 3. d. Old female nmrHAS2 mice have much lower spontaneous cancer incidence n = 47 for CreER and n = 49 for nmrHAS2. e. Old male nmrHAS2 mice have much lower spontaneous cancer incidence n = 27 for CreER and n = 32 for nmrHAS2. f. HABP staining shows that skin of nmrHAS2 mice has higher hyaluronan levels, n = 5. g. Quantification of papilloma formation in DMBA/TPA treated female mice. n = 3 for acetone treated mice, n = 5 for CreER, and n = 4 for nmrHAS2. h. Quantification of papilloma formation in DMBA/TPA treated male mice. n = 4 for acetone treated mice, n = 8 for CreER, and n = 7 for nmrHAS2. c,f,g. p-values were calculated by two-tailed unpaired t-test (p-values are indicated in the graphs). Bars represent the means, error bar displays the standard error, dots represent biological replicates. d-e. Old mice are older than 27 months. P-values were calculated by two tailed Chi-square test.
Extended Data Fig. 2 nmrHAS2 mice show extended lifespan and healthspan.
a. Female nmrHAS2 mice (n = 50) have extended median lifespan compared to female CreER mice (n = 54). p-value for median lifespan was calculated using two tailed log-rank test. b. Male nmrHAS2 (n = 34) mice have extended maximum lifespan compared to male CreER mice (n = 37). p-value for median lifespan was calculated using two tailed log-rank test. c. Old nmrHAS2 mice display younger epigenetic age. d-e. Old nmrHAS2 female mice (d) and old nmrHAS2 male mice (e) display lower age acceleration, or younger biological age than CreER controls. f. Mean methylation level of CpG sites that gain methylation during aging. Analysis was performed based on 9 animals. g. Mean methylation level of CpG sites that lose methylation during aging. Analysis was performed based on 9 animals. h. Old male nmrHAS2 mice (n = 11) have the same level of bone connectivity density compared to age-matched controls (n = 10). p-values were calculated by two-tailed unpaired t-test (p-values are indicated in the graphs). Bars represent the means, error bars show standard errors, dots represent biological replicates. d, e. The five-number summary on the boxplot displays the minimum, first quartile, median, third quartile, and maximum. p-values were calculated by two-tailed unpaired t-test. f, g. The five-number summary on the boxplot displays the minimum, first quartile, median, third quartile, and maximum. p-values were calculated by two-tailed paired t-test.
Extended Data Fig. 3 nmrHAS2 mice showed a distinct transcriptomic signature.
a. nmrHAS2 mice display an expression signature distinct form mice subjected to other pro-longevity interventions. A heatmap of correlation analysis performed on liver whole transcriptomes. b. Association between nmrHAS2 effect and signatures of lifespan-extending interventions and mammalian aging based on functional enrichment (GSEA) scores. Only functions enriched by at least one signature (adjusted p-value < 0.1) were used for the calculation. Exact adjusted p-values are shown in Extended Data Table 2. c. Functional enrichment (GSEA) of gene expression signatures associated with nmrHAS2, mammalian aging and established lifespan-extending interventions. Only functions significantly enriched by at least one signature (adjusted p-value < 0.1) are presented. Exact adjusted p-values are shown in Supplementary Table 4. d. Functional enrichment (Fisher exact test) of genes significantly associated with the effect of nmrHAS2, mammalian aging and established lifespan-extending interventions. Only functions enriched by at least one aggregated signature (adjusted p-value < 0.1) are shown. Proportion of pathway-associated genes is reflected by bubble size. Exact adjusted p-values are shown in Supplementary Table 5. b-d. ^ p.adjusted < 0.1; * p.adjusted < 0.05; ** p.adjusted < 0.01; *** p.adjusted < 0.001.
Extended Data Fig. 4 nmrHAS2 mice showed a younger transcriptomic state.
a. GSEA plots show that YOUNG gene set is upregulated, and OLD gene set is downregulated in all sequenced old nmrHAS2 mice of both sexes. * FDR<0.05, *** FDR<0.001. Exact FDR values are shown in Extended Data Fig. 4b–e. b. GSEA plots showing that OLD gene set is downregulated in WAT of old male nmrHAS2 mice. c. GSEA plots showing that that YOUNG gene set is upregulated, and OLD gene set is downregulated in the kidney of old male nmrHAS2 mice. d. GSEA plots showing that OLD gene set is downregulated in the muscle of old male nmrHAS2 mice. e. GSEA plots showing that that YOUNG gene set is upregulated, and OLD gene set is downregulated in the spleen of old male nmrHAS2 mice.
Extended Data Fig. 5 RNAseq shows reduced inflammation during aging in nmrHAS2 mice.
a-b. Enriched GO terms for upregulated genes in the spleens of CreER and nmrHAS2 female (a) and male mice (b) during aging. c-d. Enriched GO terms for upregulated genes in the WAT of CreER and nmrHAS2 female (c) and male (d) mice during aging. e-f. Enriched GO terms for upregulated genes in the muscle of CreER and nmrHAS2 female (e) and male (f) mice during aging. g-h. Enriched GO terms for upregulated genes in the kidneys of CreER and nmrHAS2 females (g) and males (h) during aging. i-j. Enriched GO terms for upregulated genes in the livers of old male (i) and female (j) nmrHAS2 mice.
Extended Data Fig. 6 Mean plasma concentrations of 36 inflammatory cytokines in nmrHAS2 and CreER male mice.
Mean plasma concentrations of 36 inflammatory cytokines and chemokines of young (5-months) and old (24-months) male mice. The heatmap is presented alongside the value chart. In the heatmap, the levels of each target were scale automatically using R.
Extended Data Fig. 7 Mean plasma concentrations of 36 inflammatory cytokines in nmrHAS2 and CreER female mice.
The mean plasma concentrations of 36 inflammatory cytokines and chemokines in young (5-months) and old (24-months) female mice. The heatmap is presented alongside the value chart. In the heatmap, the levels of each target were scaled automatically using R.
Extended Data Fig. 8 nmrHAS2 reduces pro-inflammatory response in vitro.
a. BMDM from nmrHAS2 mice have significantly upregulated HAS2 levels. BMDM were isolated from 5-months old female mice (n = 3). b. Raw264.7 cells overexpressing mHAS2 or nmrHAS2 show lower levels of pro-inflammatory cytokines and higher levels of anti-inflammatory cytokines. Data was normalized to Raw EV. n = 3. c. HYAL1 levels decrease after LPS treatment in BMDM from female mice. Normalization to CreER NT. n = 3. d. HYAL2 levels decrease after LPS treatment in BMDM from female mice. Normalization to CreER NT. n = 3. e. HYAL1 levels decrease after LPS treatment in BMDM from male mice. Normalization to CreER NT. n = 3. f. HYAL2 levels decrease after LPS treatment in BMDM from male mice. Normalization to CreER NT. n = 3. g. HAS2 levels decrease in LPS treated HAS2 expressing Raw264.7 cells. Normalization to Raw EV NT. n = 3. h. HYAL1 levels decrease after LPS treatment in HAS2 expressing Raw264.7 cells. Normalization to Raw EV NT. n = 3. i. HYAL2 levels decrease after LPS treatment in HAS2 expressing Raw264.7 cells. Normalization to Raw EV NT. n = 3. j. Raw264.7 cells overexpressing HAS2 produce more HA. HA ELISA was used to quantify the HA level in the media. n = 3. k. Raw264.7 cells overexpressing HAS2 produce more HMW-HA in the media after LPS treatment. Red square indicates the HMW-HA. Experiments were repeated for three times and showed a similar result. a-j. p values were calculated by two-tailed unpaired Student’s t-test (p values are indicated in the graphs). Bars represent the means, error bar displays the standard error, dots represent biological replicates. Adjustments were made for multiple comparisons.
Extended Data Fig. 9 nmrHAS2 reduces pro-inflammatory response in vivo and protects cells from oxidative stress.
a. nmrHAS2 mice produce significantly lower plasma TNFα levels 4 h and 24 h after LPS challenge in 5-months old female mice (n = 4). b. nmrHAS2 mice produce significantly lower plasma IL6 levels 4 h after LPS challenge in 5- months old female mice (n = 4). c. nmrHAS2 mice show lower IL1β and TNFα levels in liver 24 h post LPS challenge in 5-months old female mice (n = 4). d. nmrHAS2 mice show lower IL1β and TNFα levels in the spleen 24 h post LPS challenge in 5- months old female mice (n = 4). e. nmrHAS2 mice show lower IL1β and TNFα levels in kidney 24 h post LPS challenge in 5- months old female mice (n = 4). f. Pulse field gel shows nmrHAS2 skin fibroblasts produce more hyaluronic acid. compared to CreER fibroblasts. HAase treated samples were run in parallel to confirm the specificity of HA staining. Media from three different cell lines was pooled for HA extraction. Experiments were repeated for three times and showed a similar result. g. Levels of relative on gel HA intensity. The intensity of HA was quantified using ImageJ. Intensity of nmrHAS2 group was normalized to the CreER group. h. Skin fibroblasts isolated from nmrHAS2 mice are more resistant to H2O2 treatment. Fibroblasts were isolated from 5-months old female mice (n = 4). p-values were calculated using unpaired two-tailed t-test. a-e. p values were calculated by two-tailed unpaired t-test. Bars represent the means, error bars show the standard errors, dots represent biological replicates.
Extended Data Fig. 10 Overexpression of mouse or nmrHAS2 protects cells from oxidative stress.
a. Pulse field gel shows that mouse skin fibroblasts (MSF) overexpressing mouse HAS2 (mHAS2) or nmrHAS2 produce more hyaluronic acid compared to fibroblasts transfected with empty vector (EV). HAase-treated samples were run in parallel to confirm the specificity of HA staining. Media from three different cell lines was pooled for HA extraction. b. HA ELISA shows that mouse skin fibroblasts (MSF) overexpressing mHAS2 or nmrHAS2 produce more hyaluronic acid compared to fibroblasts transfected with empty vector (EV). p-values were calculated using unpaired two-tailed t-test, bars represent the means, error bars show standard errors, dots represent technical replicates. c. Mouse skin fibroblasts overexpressing mHAS2 or nmrHAS2 are more resistant to H2O2 treatment. p-values were calculated using unpaired two-tailed t-test, error bars show standard errors, dots represent technical replicates.
Extended Data Fig. 11 Old nmrHAS2 mice differ from age matched CreER controls in their gut microbiome composition.
a. Heatmap of genes involve in IFN, WNT, and Notch pathways. b. 16s rRNA sequencing shows old nmrHAS2 mice (n = 9) have a higher B/F ratio compared to age-matched controls (n = 10). Pooled females and males. c, d. 16s rRNA sequencing shows that at the phylum level old nmrHAS2 mice (n = 9) have more abundant Bacteroidetes (c) and less abundant Firmicutes (d) compared to age-matched controls (n = 10). 7- and 24-month-old mice were used. Pooled females and males. e-g. 16s rRNA sequencing shows that at family level, old nmrHAS2 mice (n = 9) have less abundant pro-inflammatory Streptococcaceae (e), Lachnospiraceae (f), and Deferribacteraceae (g) compared to age-matched controls (n = 10). 7- and 24-month-old mice were used. Pooled females and males. h. 16s rRNA sequencing shows that at family level, old nmrHAS2 mice (n = 9) have more Muribaculaceae compared to the age-matched controls (n = 10). Pooled females and males. b-h. p-values were calculated by two-tailed unpaired t-test (p-values are indicated in the graphs). Bars represent the means, error bars show standard errors, dots represent biological replicates. Adjustments were made for multiple comparisons.
Supplementary information
Supplementary Information
Supplementary Discussion, Supplementary References and Supplementary Table 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Tian, X., Lu, J.Y. et al. Increased hyaluronan by naked mole-rat Has2 improves healthspan in mice. Nature 621, 196–205 (2023). https://doi.org/10.1038/s41586-023-06463-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06463-0
This article is cited by
-
Aging research comes of age
Nature Methods (2024)
-
Axin2 depletion in macrophages alleviated senescence and increased immune response after myocardial infarction
Inflammation Research (2024)
-
A tool for optimizing messenger RNA sequence
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.