Abstract
Distinct morphologies of the mitochondrial network support divergent metabolic and regulatory processes that determine cell function and fate1,2,3. The mechanochemical GTPase optic atrophy 1 (OPA1) influences the architecture of cristae and catalyses the fusion of the mitochondrial inner membrane4,5. Despite its fundamental importance, the molecular mechanisms by which OPA1 modulates mitochondrial morphology are unclear. Here, using a combination of cellular and structural analyses, we illuminate the molecular mechanisms that are key to OPA1-dependent membrane remodelling and fusion. Human OPA1 embeds itself into cardiolipin-containing membranes through a lipid-binding paddle domain. A conserved loop within the paddle domain inserts deeply into the bilayer, further stabilizing the interactions with cardiolipin-enriched membranes. OPA1 dimerization through the paddle domain promotes the helical assembly of a flexible OPA1 lattice on the membrane, which drives mitochondrial fusion in cells. Moreover, the membrane-bending OPA1 oligomer undergoes conformational changes that pull the membrane-inserting loop out of the outer leaflet and contribute to the mechanics of membrane remodelling. Our findings provide a structural framework for understanding how human OPA1 shapes mitochondrial morphology and show us how human disease mutations compromise OPA1 functions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All of the 3D cryo-EM data supporting the findings of this study have been deposited in Electron Microscopy Data Bank under accession codes EMD-26977 and EMDB-26984. The model coordinates have been deposited at the PDB under accession codes 8CT1 and 8CT9. Protein sequence data for sequence alignments are available from UniProt (see the figure legends for accession codes). OPA1 sequences used in this study are as follows: human (UniProt: O60313), Chlorocebus sabaeus (green monkey; UniProt: A0A0D9R952), Macaca mulatta (rhesus macaque; UniProt: F6Y1N8), Pan troglodytes (Chimpanzee; UniProt: A0A2I3SKT2), Gorilla gorilla (gorilla; UniProt: G3S1U3), Pan paniscus (bonobo; UniProt: A0A2R9BDG8), Papio anubis (baboon; UniProt: A0A096N399), Callithrix jacchus (marmoset; UniProt: A0A2R8PC53), Oryctolagus cuniculus (rabbit; UniProt: G1TAB7), Ictidomys tridecemlineatus (squirrel; UniProt: I3MI89), Cavia porcellus (guinea pig; UniProt: H0V6M3), Mus musculus (mouse; UniProt: P58281), Rattus norvegicus (rat; UniProt: Q2TA68), Canis familiaris (dog, UniProt: F1PK93), Vulpes vulpes (red fox, UniProt: A0A3Q7T0T6), Felis catus (cat; UniProt: A0A337SN50), Ailuropoda melanoleuco (cat; UniProt: G1MBN4), Sus scrofa (pig; UniProt: A0A5G2QQR2), Loxodonta africana (African elephant; UniProt: G3SNG0), Equus caballus (horse; UniProt: F6Z2C8), Vicugna pacos (alpaca; UniProt: A0A6I9I1B0), Bos taurus (cow; UniProt: E1BBC4), Capra hircus (goat; UniProt: A0A452EKR4), Ovis aries (sheep; UniProt: A0A6P7D299), Desmodus rotundus (vampire bat; UniProt: K9J3D6), Tursiops truncatus (dolphin; UniProt: A0A2U4ACH9), Delphinapterus leucas (beluga whale; UniProt: A0A2Y9MT19), Danio rerio (zebrafish; UniProt: Q5U3A7), Oncorhynchus masou (salmon; UniProt: O93248), Gallus gallus (chicken; UniProt: Q5F499) and Meleagris gallopavo (wild turkey; UniProt: G3UT81). Full versions of all of the gels and blots are provided in Supplementary Fig. 1. Source data are provided with this paper.
References
Wai, T. & Langer, T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metab. 27, 105–117 (2016).
Quintana-Cabrera, R. & Scorrano, L. Determinants and outcomes of mitochondrial dynamics. Mol. Cell 83, 857–876 (2023).
Eisner, V., Picard, M. & Hajnóczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 20, 755–765 (2018).
Olichon, A. et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 278, 7743–7746 (2003).
Griparic, L., van der Wel, N. N., Orozco, I. J., Peters, P. J. & van der Bliek, A. M. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J. Biol. Chem. 279, 18792–18798 (2004).
Carlton, J. G., Jones, H. & Eggert, U. S. Membrane and organelle dynamics during cell division. Nat. Rev. Mol. Cell Biol. 21, 151–166 (2020).
Stefan, C. J. et al. Membrane dynamics and organelle biogenesis—lipid pipelines and vesicular carriers. BMC Biol. 15, 102 (2017).
Aydin, H., Sultana, A., Li, S., Thavalingam, A. & Lee, J. E. Molecular architecture of the human sperm IZUMO1 and egg JUNO fertilization complex. Nature 534, 562–565 (2016).
Giacomello, M., Pyakurel, A., Glytsou, C. & Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 21, 204–224 (2020).
Kalia, R. et al. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature 558, 401–405 (2018).
Cao, Y.-L. et al. MFN1 structures reveal nucleotide-triggered dimerization critical for mitochondrial fusion. Nature 542, 372–376 (2017).
Chan, D. C. Mitochondrial dynamics and its involvement in disease. Annu. Rev. Pathol. Mech. Dis. 15, 235–259 (2020).
Alexander, C. et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 26, 211–215 (2000).
Delettre, C. et al. Nuclear gene OPA1, encoding a mitochondrial dynamin- related protein, is mutated in dominant optic atrophy. Nat. Genet. 26, 4 (2000).
Cipolat, S., de Brito, O. M., Dal Zilio, B. & Scorrano, L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc. Natl Acad. Sci. USA 101, 15927–15932 (2004).
Cogliati, S. et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171 (2013).
Wai, T. et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350, aad0116 (2015).
Herkenne, S. et al. Developmental and tumor angiogenesis requires the mitochondria-shaping protein Opa1. Cell Metab. 31, 987–1003 (2020).
Gómez-Valadés, A. G. et al. Mitochondrial cristae-remodeling protein OPA1 in POMC neurons couples Ca2+ homeostasis with adipose tissue lipolysis. Cell Metab. 33, 1820–1835 (2021).
Chen, L. et al. OPA1 mutation and late-onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J. Am. Heart Assoc. 1, e003012 (2012).
Zerem, A. et al. Metabolic stroke in a patient with bi-allelic OPA1 mutations. Metab. Brain Dis. 34, 1043–1048 (2019).
Bonneau, D. et al. Early-onset Behr syndrome due to compound heterozygous mutations in OPA1. Brain 137, e301 (2014).
Carelli, V. et al. Syndromic Parkinsonism and dementia associated with OPA1 missense mutations. Ann. Neurol. 78, 21–38 (2015).
Del Dotto, V. et al. OPA1 isoforms in the hierarchical organization of mitochondrial functions. Cell Rep. 19, 2557–2571 (2017).
Mishra, P., Carelli, V., Manfredi, G. & Chan, D. C. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 19, 630–641 (2014).
Anand, R. et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 204, 919–929 (2014).
Ban, T., Heymann, J. A. W., Song, Z., Hinshaw, J. E. & Chan, D. C. OPA1 disease alleles causing dominant optic atrophy have defects in cardiolipin-stimulated GTP hydrolysis and membrane tubulation. Hum. Mol. Genet. 19, 2113–2122 (2010).
Song, Z., Chen, H., Fiket, M., Alexander, C. & Chan, D. C. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 178, 749–755 (2007).
Glytsou, C. et al. Optic atrophy 1 is epistatic to the core MICOS component MIC60 in mitochondrial cristae shape control. Cell Rep. 17, 3024–3034 (2016).
Cipolat, S. et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126, 163–175 (2006).
Ban, T. et al. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat. Cell Biol. 19, 856–863 (2017).
Zhang, D. et al. Cryo-EM structures of S-OPA1 reveal its interactions with membrane and changes upon nucleotide binding. eLife 9, e50294 (2020).
Ge, Y. et al. Two forms of Opa1 cooperate to complete fusion of the mitochondrial inner-membrane. eLife 9, e50973 (2020).
Yu, C. et al. Structural insights into G domain dimerization and pathogenic mutation of OPA1. J. Cell Biol. 219, e201907098 (2020).
Faelber, K. et al. Structure and assembly of the mitochondrial membrane remodelling GTPase Mgm1. Nature 571, 429–433 (2019).
Yan, L. et al. Structural analysis of a trimeric assembly of the mitochondrial dynamin-like GTPase Mgm1. Proc. Natl Acad. Sci. USA 117, 4061–4070 (2020).
Rujiviphat, J. et al. Mitochondrial genome maintenance 1 (Mgm1) protein alters membrane topology and promotes local membrane bending. J. Mol. Biol. 427, 2599–2609 (2015).
Faelber, K. et al. Crystal structure of nucleotide-free dynamin. Nature 477, 556–560 (2011).
Low, H. H., Sachse, C., Amos, L. A. & Löwe, J. Structure of a bacterial dynamin-like protein lipid tube provides a mechanism for assembly and membrane curving. Cell 139, 1342–1352 (2009).
Ramachandran, R. & Schmid, S. L. Real-time detection reveals that effectors couple dynamin’s GTP-dependent conformational changes to the membrane. EMBO J. 27, 27–37 (2008).
Daumke, O. et al. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 449, 923–927 (2007).
Low, H. H. & Löwe, J. Dynamin architecture—from monomer to polymer. Curr. Opin. Struct. Biol. 20, 791–798 (2010).
Gao, S. et al. Structural basis of oligomerization in the stalk region of dynamin-like MxA. Nature 465, 502–506 (2010).
Alvarez, F. J. D. et al. CryoEM structure of MxB reveals a novel oligomerization interface critical for HIV restriction. Sci. Adv. 3, e1701264 (2017).
Yu-Wai-Man, P. et al. Multi-system neurological disease is common in patients with OPA1 mutations. Brain 133, 771–786 (2010).
Liu, T. et al. CHCHD10-regulated OPA1-mitofilin complex mediates TDP-43-induced mitochondrial phenotypes associated with frontotemporal dementia. FASEB J. 34, 8493–8509 (2020).
Chappie, J. S. et al. A pseudoatomic model of the dynamin polymer identifies a hydrolysis-dependent powerstroke. Cell 147, 209–222 (2011).
Antonny, B. et al. Membrane fission by dynamin: what we know and what we need to know. EMBO J. 35, 2270–2284 (2016).
Studier, F. W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 41, 207–234 (2005).
Ardail, D. et al. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 265, 18797–18802 (1990).
Bennett, J. A., Steward, L. R., Rudolph, J., Voss, A. P. & Aydin, H. The structure of the human LACTB filament reveals the mechanisms of assembly and membrane binding. PLoS Biol. 20, e3001899 (2022).
von der Malsburg, A., Abutbul-Ionita, I., Haller, O., Kochs, G. & Danino, D. Stalk domain of the dynamin-like MxA GTPase protein mediates membrane binding and liposome tubulation via the unstructured L4 Loop. J. Biol. Chem. 286, 37858–37865 (2011).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Manicki, M. et al. Structure and functionality of a multimeric human COQ7:COQ9 complex. Mol. Cell 82, 4307–4323 (2022).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
He, S. & Scheres, S. H. W. Helical reconstruction in RELION. J. Struct. Biol. 198, 163–176 (2017).
Shaikh, T. R. et al. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protoc. 3, 1941–1974 (2008).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D 75, 861–877 (2019).
Alford, R. F. et al. The Rosetta all-atom energy function for macromolecular modeling and design. J. Chem. Theory Comput. 13, 3031–3048 (2017).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Jurrus, E. et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 27, 112–128 (2018).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Holm, L. & Rosenström, P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Bui, K. H. et al. Integrated structural analysis of the human nuclear pore complex scaffold. Cell 155, 1233–1243 (2013).
Leitner, A. et al. Expanding the chemical cross-linking toolbox by the use of multiple proteases and enrichment by size exclusion chromatography. Mol. Cell. Proteom. 11, M111.014126 (2012).
Bern, M., Kil, Y. J. & Becker, C. Byonic: advanced peptide and protein identification software. Curr. Protoc. Bioinform. 40, 13.20.1–13.20.14 (2012).
Combe, C. W., Fischer, L. & Rappsilber, J. xiNET: cross-link network maps with residue resolution. Mol. Cell. Proteom. 14, 1137–1147 (2015).
Kosinski, J. et al. Xlink Analyzer: software for analysis and visualization of cross-linking data in the context of three-dimensional structures. J. Struct. Biol. 189, 177–183 (2015).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Fischer, C. A. et al. MitoSegNet: easy-to-use deep learning segmentation for analyzing mitochondrial morphology. iScience 23, 101601 (2020).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Lee, J. & Lentz, B. R. Outer leaflet-packing defects promote poly(ethylene glycol)-mediated fusion of large unilamellar vesicles. Biochemistry 36, 421–431 (1997).
Acknowledgements
We thank L. Doan for reagent preparation; the staff of the W.M. Keck Foundation Advanced Microscopy laboratory of the University of California, San Francisco, A. Myasnikov, D. Bulkley and Z. Yu for help with data collection; E. Paraskevi Tsiolaki for technical assistance with mass photometry; P. Thomas for computational support; E. Krause for assistance with cell sorting; S. Jungbluth for acquiring the TEM images of cells; G. Morgan and C. Ozzello for electron microscopy training and support; the staff at the Shared Instrument Pool (SIP) core facility (SCR_018986) of the Department of Biochemistry at the University of Colorado Boulder for the use of the shared research instrumentation infrastructure; A. Erbse for assistance with biophysical instruments and support; the members of the Biofrontiers Advanced Light Microscopy Core for the use of laser confocal microscopes; J. Dragavon for training and support; K. Luger and J. Rudolph for their support and for sharing the microplate reader for the fluorescence-based assays; M. Ford, K. Faelber, V. Gama and C. Hayes for reading the manuscript; and O. Daumke and the members of the Aydin and Kasinath laboratories for technical advice and discussions. This work was supported in part by American Heart Association Postdoctoral Fellowship 23POST1020756 (to K.E.Z.), Boettcher Foundation Webb-Waring Biomedical Research Award (H.A.), a National Institute of Health grant R35 GM150942 (to H.A.), Deutsche Forschungsgemeinschaft (DFG), Collaborative Research Center 894, project A20 (to M.v.d.L.), a National Institute of Health grant R01 GM127673 (to A.F.), a Faculty Scholar Grant from the HHMI (to A.F.) and a QBI-FUN Collaborative Integrative Structural Biology Grant (to A.F.). A.F. is an alumni investigator of the Chan Zuckerburg Biohub.
Author information
Authors and Affiliations
Contributions
A.v.d.M. cloned the siRNA-resistant OPA1 constructs and performed mammalian cell culture experiments, prepared samples for in vitro fluorescence microscopy imaging, and performed immunoblot analysis, imaging and analysis. H.A., G.M.S., K.E.Z. and J.A.B. performed cloning, mutagenesis, biochemical and biophysical characterizations, negative-stain EM, cryo-EM experiments and analysis, determined the cryo-EM structures, and conducted model building, refinement and validation of the cryo-EM structures. L.A.A. and M.D.P. performed molecular dynamics simulations and data analysis. F.R.M. assisted with liposome preparation and cryo-EM experiments. A.v.A. assisted with chemical cross-linking sample preparation and analysis. R.K. contributed to cryo-EM image analysis, model building and discussions. M.v.d.L., A.v.d.M., A.F. and H.A. designed and supervised the research. All of the authors analysed the data, discussed the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.F. is a shareholder and employee of Altos Labs and a shareholder and consultant for Relay Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Thomas Langer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
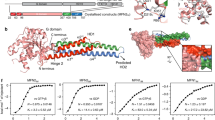
Extended Data Fig. 1 Purification and functional characterization of the human S-OPA1 construct.
Using a recombinant Escherichia coli expression system, we expressed and purified human S-OPA1 (residues 252-960) in the presence of detergents by Ni2+-affinity and gel filtration chromatography. a, A representative size exclusion chromatogram of the human S-OPA1. b, Purified S-OPA1 constructs on an SDS-PAGE gel stained with Coomassie blue. Performed in technical triplicate. c, Mass photometry profile of nucleotide-free human S-OPA1 reveals an apparent molecular mass of 82 σ 9.8 kDa, which corresponds to a monomeric state. d, Kyte and Doolittle hydropathy plot of full-length human OPA1 isoform 1. Hydrophobic regions corresponding to the transmembrane (TM) region and fusion loop are highlighted in blue and red, respectively. The red line indicates the zero baseline on the hydropathy scale. e, To assess the proper folding and nucleotide-dependent dimerization of recombinant S-OPA1, we incubated the sample with the non-hydrolysable analogue GMPPCP, Mg2+, and K+. Then, using negative-stain transmission electron microscopy (TEM), we observed that S-OPA1 forms dimers via GTPase domain interactions. Representative negative-stain EM 2D class averages of human S-OPA1 from the negative-stain data collected on Tecnai T12 microscope equipped with CCD camera showing that particles have well-defined shapes with modular domain architecture. Monomeric human S-OPA1 forms G domain dimers in the presence of non-hydrolysable GTP analogue GMPPCP.
Extended Data Fig. 2 Reconstitution of human S-OPA1 assemblies and analysis by cryo-EM.
a, Representative motion-corrected electron micrograph of membrane nanotube-bound S-OPA1 filaments. b, Gallery of 2D class averages calculated from the cryo-EM data showing the S-OPA1 monomers assembled on the surface of cardiolipin-enriched lipid nanotubes. c, g, Slices through the unsharpened density map at distinct levels are shown in the top view. d, h, Angular distribution of the membrane-bound S-OPA1 filament for all particle images included in the calculation of the final 3D reconstruction of the membrane-proximal (d) and the membrane-distal conformations (h). e, i, Fourier shell coefficient (FSC) curves (threshold of 0.143) between two independently refined half maps before and after post-processing. f, j, Final 3D electron density maps of both structures are coloured according to local resolution and are shown in horizontal and vertical slices through cryo-EM densities. Local resolution was calculated by ResMap.
Extended Data Fig. 3 Flowchart for cryo-EM data processing of membrane-bound S-OPA1 assembly.
Details can be found in the image analysis and 3D reconstruction section of the Methods.
Extended Data Fig. 4 Crosslinks between all OPA1 subunits in membrane-bound conformation.
a, b, Chemical cross-linking and mass spectrometry reveals protein-protein interaction (PPI) maps of the human S-OPA1 polymers. Circular plot showing the distribution of DSG (a) and DSS (b) chemical crosslinks mapped to human OPA1 represented as coloured circles with amino acid positions labelled. Orange bars indicate the positions of lysine residues, and crosslinks connecting the lysine residues are represented by black lines between the corresponding amino acid pairs. c, Crosslinks that are gained (red) in the S-OPA1 polymer upon membrane binding. Identified inter-molecular crosslinks are mapped on the S-OPA1 subunits. CX-MS reveals a cluster of contacts between S-OPA1 paddle domains, indicating strong interactions in membrane-bound conformation. d, Crosslinks between all OPA1 domains. Domains are arranged based on sequence (coloured blocks). The DSG (red) and DSS (blue) crosslinks that satisfy the distance restraint (30 Å) are mapped onto the membrane-bound cryo-EM structure of human S-OPA1. Crosslinks identified in both DSG and DSS datasets are shown in purple.
Extended Data Fig. 5 Human S-OPA1 map quality, model building, and structural comparison with the yeast Mgm1.
a, Isolated S-OPA1 monomer EM density from post-processed maps showing the quality of the map, build and fit. A single view of the cryo-EM density is depicted as a semi-transparent mesh and superimposed upon the model. Examples of model fit within B-factor sharpened cryo-EM density for the alpha-helices of S-OPA1 BSE (red), stalk (blue), paddle (green) are shown in the context of the atomic model with side chains are shown as sticks and the backbone as ribbons. b, A comparative analysis of the human S-OPA1 to yeast structures deposited in the PDB using topology independent comparison server CLICK revealed that the S-OPA1 structure displays a similar topology for the GTPase, BSE, and stalk domains; however, the paddle domain adopts a novel architecture with the addition of α3P helix, which facilitates the formation of interface 7. Structural comparison of membrane-proximal conformation of human S-OPA1 (beige) to S. cerevisiae (Pink, PDB ID: 6JSJ) and C. thermophilum (Light blue, PDB ID: 6QL4) Mgm1 crystal structures. Overall, the membrane-proximal conformation of human S-OPA1 and S. cerevisiae Mgm1 align with an r.m.s.d. of 8.46 Å, and human S-OPA1 and C. thermophilum Mgm1 align with an r.m.s.d. of 10.30 Å over all Cα atoms. c, Superimposition of membrane-distal conformation of human S-OPA1 (beige), S. cerevisiae (Pink), and C. thermophilum (Light blue) Mgm1 structures. Overall, the membrane-distal conformation of human S-OPA1 superimposes with S. cerevisiae Mgm1 and C. thermophilum Mgm1 with a rmsd of ~7.3 Å over all Cα atoms. d, Multiple sequence alignment of human S-OPA1, S. cerevisiae S-Mgm1, and C. thermophilum S-Mgm1. There is ~21% sequence conservation between human and yeast proteins. Most of the residues involved in binding to membranes are not conserved (highlighted with an asterisk).
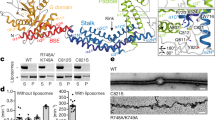
Extended Data Fig. 6 Oligomerization and liposome deformation activity of wild-type S-OPA1 and mutants visualized with EM.
To gain further insights into the molecular mechanism of OPA1-mediated membrane remodelling, we reconstituted the human S-OPA1 polymer assembly in the presence of GMPPCP and CL-enriched membranes. Negative-stain electron micrographs showing the liposome binding and remodelling activity of wild-type and mutant S-OPA1 on cardiolipin-enriched liposomes and membrane nanotubes. S-OPA1 forms well-ordered filaments that wrapped around membrane tubules. Wild-type and mutant proteins were incubated with liposomes and membrane nanotubes for four hours at room temperature prior to grid preparation. Mutations to positively charged and hydrophobic residues within the membrane docking region, membrane-inserting loop (MIL), membrane-facing surface, and interface 7 resulted in severe defects in membrane binding and remodelling activity of human S-OPA1. Inset, close-up views of selected liposomes and membrane nanotubes. Images were taken in technical triplicate. Scale bars, 100 nm.
Extended Data Fig. 7 Liposome co-sedimentation experiments.
a, Representative SDS-PAGE gels showing the sedimentation of S-OPA1 WT and mutants in the presence and absence of CL-enriched liposomes. The samples were derived from the same experiment and analysed using multiple gels in parallel. P, pellet; S, supernatant; WT, wild type; MIL, membrane-inserting loop. b, c, Gel quantification depicting the relative amount of S-OPA1 (%) for pellet and supernatant fractions without liposomes (b) and with liposomes (c) in the co-sedimentation assays. The bar graphs represent the quantification of the Coomassie-stained protein bands from three biological replicates and expressed as mean ± s.e.m. Two-tailed unpaired t-tests were performed on each S-OPA1 variant comparing pellet verse supernatant. P < 0.0001 (****); P = 0.0002(***) or P = 0.0003(***); P = 0.001 (**); P = 0.01 (*); ns, not significant.
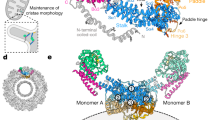
Extended Data Fig. 8 Mitochondrial morphology analyses for the WT, fusion loop, and disease mutants of OPA1.
a, Fluorescence microscopy images of wild-type (WT), membrane docking (K738E, R858E), membrane-inserting loop (W771A, W775A, L776A, K779E), and disease (R781E, R824E) mutants in mitochondrial morphology analyses. HeLa cells were transfected with empty vector (E.V.) or the indicated siRNA-resistant OPA1 constructs for 24h and then subjected to RNA interference using the indicated siRNAs for 72h. The mitochondrial network was visualized by staining for the outer membrane protein TOMM22 (green), and co-transfected mCherry-NLS (red) was used to identify transfected cells. Performed in biological triplicate. Scale bar, 20 µm. b, Expression and stability of the siRNA-resistant OPA1 proteins were assessed by immunoblot analysis of Triton X-100 extracts derived from HeLa cells transfected with either empty vector (E.V.) or the indicated siRNA-resistant OPA1 constructs 24h before treatment with the indicated siRNAs for 72h. 20 µg per lane (n = 3 independent experiments). c, Quantification of microscopy images of wild-type, empty vector (E.V.), and mutants. Mitochondrial phenotypes observed in HeLa cells transiently expressing human OPA1 variants as described in a. Cells expressing the control siRNA E.V. (n = 280), OPA1 siRNA E.V. (n = 269 cells), wild-type OPA1 (n = 238 cells), OPA1 K738E (n = 235 cells), OPA1 W771A (n = 281 cells), OPA1 W775A (n = 264 cells), OPA1 W776A (n = 255 cells), OPA1 K779E (n = 282 cells), OPA1 R858E (n = 245 cells), OPA1 R781E (n = 262 cells), and OPA1 R824E (n = 283 cells) over three experimental replicates. Data points represent the average percentage of cells across three experimental replicates. Error bars indicate s.e.m. d, Human OPA1 mutations associated with optic atrophy, cerebellar ataxia, and other diseases mapped onto the paddle domain of OPA1 as solid spheres and numbered. Back and side views of the molecular structure of human S-OPA1 LBD (coloured in green).
Extended Data Fig. 9 OPA1 Mutations disrupt cristae architecture and overall mitochondrial morphology as compared to the WT.
a, Mitochondrial surface area (μm2) was calculated from ultrathin sections of cells transfected with control siRNA empty vector (E.V.) (n = 312), OPA1 siRNA E.V. (n = 444), OPA1 WT (n = 543), OPA1 membrane-inserting loop (MIL) mutant (n = 523), and OPA1 K819E (n = 516). The standard error of the mean is reported with the mean of each dataset (n = 3 technical triplicates). Due to the variation in numbers for each dataset, two-tailed Mann-Whitney statistical test was utilized to calculate the statistical significance between control siRNA E.V. to each other sample. P < 0.0001 (****). b, Quantification of the number of cristae per mitochondrion. The mean number of cristae was calculated and reported with the standard error of the mean for each sample (n = 3 technical triplicates). The control siRNA E.V. and other samples were subjected to a two-tailed Mann-Whitney test to determine the statistical significance. P = 0.0176 (*); P < 0.0001 (****). c, Quantification of the mitochondrial morphology in WT and mutant OPA1 cells. The mitochondrial shape was assigned to four different classes, oval, ellipsoidal, polygonal, or elongated, and the relative distribution of each shape was reported as a bar graph. The percentage of each shape is reported for each sample. d, Quantification of cristae morphology in WT and mutant OPA1 cells. A bar graph representing the distribution of four different cristae morphology (normal, swollen, short, or disordered) observed in respective samples as percentages. e, Representative TEM images of the mitochondrion showing different types of cristae morphology assigned to four classes: normal, swollen, short, and disordered. Images were taken in technical triplicate. Scale bar, 500 nm.
Extended Data Fig. 10 S-OPA1 membrane binding and remodelling activities cause lipid bilayer deformations.
a, Representative fluorescent microscopy images show the co-localization of Alexa Fluor 488 labelled WT S-OPA1 onto the Texas Red-DHPE-containing liposomes after approximately 30min. Experiments performed in technical triplicate. Scale bar, 0.5 µm. b, Membrane deformation assays with WT S-OPA1 and liposomes containing 0.25% Nile Red; n = 5 biologically independent experiments and expressed as mean, error bars as ± s.e.m. Statistical analysis was performed using an unpaired two-tailed Student t-test (P = <0.0001, ****), and the Grubbs test removed one outlier from each dataset. c-f, Negative stain TEM analysis of membrane reconstitution assays with c, S-OPA1 alone; d, liposomes with 0.25% Nile Red alone; e, BSA with liposomes containing 0.25% Nile Red; and f, WT S-OPA1 with liposomes containing 0.25% Nile Red. g, Membrane deformation assay with WT S-OPA1 and liposomes containing 1% NBD-PC; n = 5 biologically independent experiments and expressed as mean error bars as ± s.e.m. An unpaired two-tailed Student t-test was performed on the NBD-PC dataset (P = <0.0001, ****). h, liposomes with 1% NBD-PC alone; i, BSA with liposomes containing 1% NBD-PC; and j, WT S-OPA1 with liposomes containing 1% NBD-PC. Inset, close-up view of selected liposomes from negative-stain TEM images. Scale bar, 100 nm. It has been proposed that the outer leaflet perturbations of CL-containing large unilamellar vesicles (LUV) result in vesicle fusion82. Atomic force microscopy (AFM) and fluorescence microscopy, moreover, have revealed that the incubation of yeast S-Mgm1 with labelled liposomes results in increased membrane “roughness” on the surface of liposomes37. These observations indicate that the yeast ortholog Mgm1 may utilize a similar mechanism to perturb membrane properties. Together, our structural and functional analyses suggest that OPA1-mediated leaflet perturbations support the membrane remodelling activity of the protein. We note that these conclusions are based on our current structural knowledge and are limited to lipids in synthetic membranes and reconstitution assays in vitro. To what extent these findings apply to mitochondrial membrane remodelling in human cells remains elusive. Our work provides the foundation to further study the complex mechanisms that regulate mitochondrial morphology and function.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Supplementary Table 1.
Supplementary Data 1
Raw data behind Supplementary Fig. 3f.
Supplementary Data 2
Raw data behind Supplementary Fig. 4f.
Supplementary Data 3
Raw data behind MitoSegNet segmentation analyses in Supplementary Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
von der Malsburg, A., Sapp, G.M., Zuccaro, K.E. et al. Structural mechanism of mitochondrial membrane remodelling by human OPA1. Nature 620, 1101–1108 (2023). https://doi.org/10.1038/s41586-023-06441-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06441-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.