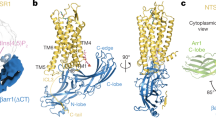
Abstract
Phosphorylation of G-protein-coupled receptors (GPCRs) by GPCR kinases (GRKs) desensitizes G-protein signalling and promotes arrestin signalling, which is also modulated by biased ligands1,2,3,4,5,6. The molecular assembly of GRKs on GPCRs and the basis of GRK-mediated biased signalling remain largely unknown owing to the weak GPCR–GRK interactions. Here we report the complex structure of neurotensin receptor 1 (NTSR1) bound to GRK2, Gαq and the arrestin-biased ligand SBI-5537. The density map reveals the arrangement of the intact GRK2 with the receptor, with the N-terminal helix of GRK2 docking into the open cytoplasmic pocket formed by the outward movement of the receptor transmembrane helix 6, analogous to the binding of the G protein to the receptor. SBI-553 binds at the interface between GRK2 and NTSR1 to enhance GRK2 binding. The binding mode of SBI-553 is compatible with arrestin binding but clashes with the binding of Gαq protein, thus providing a mechanism for its arrestin-biased signalling capability. In sum, our structure provides a rational model for understanding the details of GPCR–GRK interactions and GRK2-mediated biased signalling.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The density maps and structure coordinates have been deposited to the Electron Microscopy Data Bank (EMDB) and the Protein Data Bank (PDB) with accession numbers EMD-36474 and 8JPB, respectively, for the NTSR1–GRK2–Gαq complex 1; EMD-36475 and 8JPC, respectively, for the NTSR1–GRK2–Gαq complex 2; EMD-36476 and 8JPD, respectively, for the focused refinement structure of GRK2; EMD-36477 and 8JPE, respectively, for the focused refinement structure of Gαq; and EMD-36478 and 8JPF, respectively, for the focused refinement structure of NTSR1.
References
Pitcher, J. A., Freedman, N. J. & Lefkowitz, R. J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 67, 653–692 (1998).
Gurevich, E. V., Tesmer, J. J., Mushegian, A. & Gurevich, V. V. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol. Ther. 133, 40–69 (2012).
Gurevich, V. V. & Gurevich, E. V. GPCR signaling regulation: the role of GRKs and arrestins. Front. Pharmacol. 10, 125 (2019).
Hodavance, S. Y., Gareri, C., Torok, R. D. & Rockman, H. A. G protein-coupled receptor biased agonism. J. Cardiovasc. Pharmacol. 67, 193–202 (2016).
Rankovic, Z., Brust, T. F. & Bohn, L. M. Biased agonism: an emerging paradigm in GPCR drug discovery. Bioorg. Med. Chem. Lett. 26, 241–250 (2016).
Seyedabadi, M., Gharghabi, M., Gurevich, E. V. & Gurevich, V. V. Structural basis of GPCR coupling to distinct signal transducers: implications for biased signaling. Trends Biochem. Sci. 47, 570–581 (2022).
Slosky, L. M. et al. β-Arrestin-biased allosteric modulator of NTSR1 selectively attenuates addictive behaviors. Cell 181, 1364–1379.e1314 (2020).
Benovic, J. L., DeBlasi, A., Stone, W. C., Caron, M. G. & Lefkowitz, R. J. β-Adrenergic receptor kinase: primary structure delineates a multigene family. Science 246, 235–240 (1989).
Mushegian, A., Gurevich, V. V. & Gurevich, E. V. The origin and evolution of G protein-coupled receptor kinases. PLoS ONE 7, e33806 (2012).
Sulon, S. M. & Benovic, J. L. Targeting G protein-coupled receptor kinases (GRKs) to G protein-coupled receptors. Curr. Opin. Endocr. Metab. Res. 16, 56–65 (2021).
Ribas, C. et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim. Biophys. Acta 1768, 913–922 (2007).
Komolov, K. E. et al. Structure of a GRK5–calmodulin complex reveals molecular mechanism of GRK activation and substrate targeting. Mol. Cell 81, 323–339 e311 (2021).
Chen, Q. et al. Structures of rhodopsin in complex with G-protein-coupled receptor kinase 1. Nature 595, 600–605 (2021).
Beautrait, A. et al. Mapping the putative G protein-coupled receptor (GPCR) docking site on GPCR kinase 2: insights from intact cell phosphorylation and recruitment assays. J. Biol. Chem. 289, 25262–25275 (2014).
Baameur, F. et al. Role for the regulator of G-protein signaling homology domain of G protein-coupled receptor kinases 5 and 6 in beta 2-adrenergic receptor and rhodopsin phosphorylation. Mol. Pharmacol. 77, 405–415 (2010).
Komolov, K. E. et al. Structural and functional analysis of a β2-adrenergic receptor complex with GRK5. Cell 169, 407–421.e416 (2017).
Lodowski, D. T., Pitcher, J. A., Capel, W. D., Lefkowitz, R. J. & Tesmer, J. J. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300, 1256–1262 (2003).
Tesmer, V. M., Kawano, T., Shankaranarayanan, A., Kozasa, T. & Tesmer, J. J. Snapshot of activated G proteins at the membrane: the Gαq–GRK2–Gβγ complex. Science 310, 1686–1690 (2005).
He, Y. et al. Molecular assembly of rhodopsin with G protein-coupled receptor kinases. Cell Res. 27, 728–747 (2017).
Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature 477, 549–555 (2011).
Kang, Y. et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567 (2015).
Zhou, X. E. et al. Identification of phosphorylation codes for arrestin recruitment by G protein-coupled receptors. Cell 170, 457–469.e413 (2017).
Yin, W. et al. A complex structure of arrestin-2 bound to a G protein-coupled receptor. Cell Res. 29, 971–983 (2019).
Huang, W. et al. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579, 303–308 (2020).
Staus, D. P. et al. Structure of the M2 muscarinic receptor–β-arrestin complex in a lipid nanodisc. Nature 579, 297–302 (2020).
Lee, Y. et al. Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor. Nature 583, 862–866 (2020).
Besserer-Offroy, E. et al. The signaling signature of the neurotensin type 1 receptor with endogenous ligands. Eur. J. Pharmacol. 805, 1–13 (2017).
Rostene, W. H. & Alexander, M. J. Neurotensin and neuroendocrine regulation. Front. Neuroendocrinol. 18, 115–173 (1997).
Inagaki, S. et al. G protein-coupled receptor kinase 2 (GRK2) and 5 (GRK5) exhibit selective phosphorylation of the neurotensin receptor in vitro. Biochemistry 54, 4320–4329 (2015).
Kato, H. E. et al. Conformational transitions of a neurotensin receptor 1–Gi1 complex. Nature 572, 80–85 (2019).
Barnea, G. et al. The genetic design of signaling cascades to record receptor activation. Proc. Natl Acad. Sci. USA 105, 64–69 (2008).
Dixon, A. S. et al. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol. 11, 400–408 (2016).
Duan, J. et al. Cryo-EM structure of an activated VIP1 receptor–G protein complex revealed by a NanoBiT tethering strategy. Nat. Commun. 11, 4121 (2020).
Cato, M. C. et al. The open question of how GPCRs interact with GPCR kinases (GRKs). Biomolecules 11, 447 (2021).
Homan, K. T. & Tesmer, J. J. Molecular basis for small molecule inhibition of G protein-coupled receptor kinases. ACS Chem. Biol. 10, 246–256 (2015).
Pellegrini, E., Signor, L., Singh, S., Boeri Erba, E. & Cusack, S. Structures of the inactive and active states of RIP2 kinase inform on the mechanism of activation. PLoS ONE 12, e0177161 (2017).
Underwood, K. W. et al. Catalytically active MAP KAP kinase 2 structures in complex with staurosporine and ADP reveal differences with the autoinhibited enzyme. Structure 11, 627–636 (2003).
White, J. F. et al. Structure of the agonist-bound neurotensin receptor. Nature 490, 508–513 (2012).
Komolov, K. E., Bhardwaj, A. & Benovic, J. L. Atomic structure of GRK5 reveals distinct structural features novel for G protein-coupled receptor kinases. J. Biol. Chem. 290, 20629–20647 (2015).
Pitcher, J. A. et al. Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science 257, 1264–1267 (1992).
Smrcka, A. V. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell. Mol. Life Sci. 65, 2191–2214 (2008).
Draper-Joyce, C. J. et al. Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature 597, 571–576 (2021).
Egyed, A., Kiss, D. J. & Keseru, G. M. The impact of the secondary binding pocket on the pharmacology of class A GPCRs. Front. Pharmacol. 13, 847788 (2022).
Duan, J. et al. Structure of a G protein-coupled receptor with GRK2 and a biased ligand. Preprint at bioRxiv https://doi.org/10.1101/2022.10.19.512855 (2022).
Krumm, B. E. et al. Neurotensin receptor allosterism revealed in complex with a biased allosteric modulator. Biochemistry 62, 1233–1248 (2023).
Bouley, R. A. et al. A new paroxetine-based GRK2 inhibitor reduces internalization of the μ-opioid receptor. Mol. Pharmacol. 97, 392–401 (2020).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D 74, 519–530 (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2020).
Acknowledgements
The cryo-EM data were collected at the Advanced Center for Electron Microscopy, Shanghai Institute of Materia Medica (SIMM). The authors thank the staff at the Advanced Center for Electron Microscopy for their technical support. This work was partially supported by CAS Strategic Priority Research Program (XDB37030103 to H.E.X.); Shanghai Municipal Science and Technology Major Project (2019SHZDZX02 to H.E.X.); Shanghai Municipal Science and Technology Major Project (to H.E.X.); The National Natural Science Foundation of China (32130022 to H.E.X., 82121005 to H.E.X. and D.Y., 82273961 to M.-W.W., 82273985 to D.Y. and 82204474 to F.Z.); the Lingang Laboratory (no. LG-GG-202204-01 to H.E.X.); the National Key R&D Program of China (2018YFA0507002 to H.E.X. and 2018YFA0507000 to M.-W.W.); Young Elite Scientists Sponsorship Program by CAST (2022QNRC001 to J.D.); and Shanghai Sailing Program (23YF1456800 to J.D.).
Author information
Authors and Affiliations
Contributions
J.D. designed the expression constructs, purified the proteins, performed cryo-EM grid preparation and data collection, participated in functional studies and participated in figure and manuscript preparation. H.L. and Q.Y. performed cryo-EM data calculations and model building and participated in figure preparation. F.Z., Y. Ji and X. Cai performed functional studies. X.H. performed molecular dynamic simulations supervised by X. Cheng. J.L. and K.W. participated in cryo-EM data collection and calculations. X.L., W.Y., S.Z., S.L. and T.G. participated in the experiments. Y. Jiang and M.-W.W. supervised the studies. D.Y. supervised F.Z., X. Cai and S.L. for the functional studies. H.E.X. and J.D. conceived the project, analysed the structures and wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 NTSR1–GRK2–Gαq complex assembly.
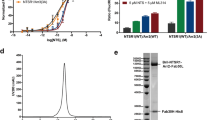
a, Screening for GPCR–GRK2 complexes by tango assay. RLU, relative luciferase units, which was normalized to the values of NTSR1. Data were processed as mean ± S.D. from three independent experiments (n = 3), performed in triplicates. Statistical significance of differences between NTSR1 and other receptor was determined by two-sided one-way ANOVA. **P < 0.01 and *** P < 0.001 versus NTSR1 (P < 0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001 from left to right). b, NTS and SBI-553 improve NTSR1-GRK2 interaction determined by Tango assay. Data were processed as mean ± S.D. from three independent experiments (n = 3), performed in triplicates. Statistical significance was determined by two-sided one-way ANOVA. **P < 0.01 and ***P < 0.001 (P = 0.0024, 0.0027, <0.0001, 0.0001, <0.0001, <0.0001 from left to right). c-e, SDS-PAGE of the complexes. NTSR1–GRK2–Gαq–Gβγ complex (c), NTSR1_LgBiT–GRK2_HiBiT–Gαq–Gβγ complex (d), NTSR1–GRK2–Gαq complex before crosslinking (left panel of e) and NTSR1–GRK2–Gαq complex crosslinked by BS3 (right panel of e). For gel source data, see Supplementary Data Fig. 1. Representative Figures from at least three independent experiments were shown. f, Size-exclusion chromatography elution profile of the NTSR1–GRK2–Gαq complex. Red star indicates the monomer peak of the complex. g, Cryo-EM micrograph of the NTSR1–GRK2–Gαq complex. Representative Cryo-EM micrograph from 57,477 movies was shown. Particles picked for 3D classifications were highlighted in red circles.
Extended Data Fig. 2 Single-particle reconstruction of the NTSR1–GRK2–Gαq complex.
a, Flowchart of cryo-EM data analysis of the NTSR1–GRK2–Gαq complex. b, Micrograph of the reference-free 2D class averages. c-d, Global Cryo-EM maps of the NTSR1–GRK2–Gαq complexes were generated and colored by local resolutions from 2.4 Å (blue) to 4.5 Å (red). e-g, Local Cryo-EM maps focusing on NTSR1, GRK2 bound with Gαq and only Gαq protein were generated and colored by local resolutions from 2.4 Å (blue) to 4.5 Å (red) respectively. h-l, The “Gold-standard” Fourier shell correlation (FSC) curve indicates that the resolution of the global electron density map of the NTSR1–GRK2–Gαq complex 1 is 3.08 Å (h), the NTSR1–GRK2–Gαq complex 2 is 3.10 Å (i), and the resolution of the local electron density maps of NTSR1 (contains a partial of GRK2), GRK2 bound with Gαq and only Gαq protein from complex 1 are 2.91 Å (j), 2.81 Å (k) and 3.02 Å (l), respectively.
Extended Data Fig. 3 Cryo-EM density maps with all transmembrane helices, and H8 of NTSR1, NTS, staurosporine, GDP·Mg2+·AlF4− and αN of GRK2.
The density map is shown at contour levels of 0.36, 0.34, 0.29, 0.30, 0.30, 0.29, 0.41, 0.32, 0.22, 0.83, 0.28 and 0.43, respectively, with carve radii of 2.0 Å.
Extended Data Fig. 4 Structural comparison of the NTSR1–GRK2–Gαq complex 1 and 2.
Comparison of these two complexes reveals that they have very similar NTSR1 structure but a swing of GRK2 and Gαq of ~5–10 Å related to NTSR1. The red arrows are referring to the movement between two structures.
Extended Data Fig. 5 Structural comparison of the GRK2-Gαq from the cryo-EM structure NTSR1–GRK2–Gαq complex with the crystal structure of GRK2–Gαq–Gβγ.
Comparing the GRK2 structure from the NTSR1 complex to the crystal structure of GRK2 from the complex with Gαq and Gβγ reveals three major differences. The GRK2 structure from the NTSR1 complex contains a N-terminal helix that is packed onto the kinase domain, has a breakage in the ionic lock between its RHD from the KD, and adopts a closed conformation in its KD by 2-3 Å shifts of the KD relative to the KD of GRK2 from the crystal structure. The density map is shown at a level of 0.28. The red arrows are referring to the movement between two structures.
Extended Data Fig. 6 Possible Lipid binding sites and phosphorylation sites.
a, Gβγ subunits was modeled into the NTSR1–GRK2 structure by structural alignment of GRK2 from NTSR1–GRK2 structure and GRK2–Gαq–Gβγ structure complex (PDB code: 2BCJ). GRK2 was shown in blue and GRK5 (PDB code: 6PJX) was shown in green. The possible lipid binding sites from GRK2 are highlighted in orange and GRK5 are highlighted in dark red. NLBD, the N-terminal lipid binding site. CLBD, the C-terminal lipid binding site. b, WT and mutated GRK2 recruitment to NTSR1 induced by NTS (left panel), relative expression level of WT and mutated GRK2 (right panel). NLBD refers to the combined mutations of N-terminal basic residues (R27A, K30A, K31A). CLBD means the combined mutations R535A, R539A, K540A, K541A, K543A, K545A. Combined mutation refers to all the mutations combined. Data were shown as mean ± S.D. from three independent experiments (n = 3), performed in duplicate. Statistical significance of differences between WT and mutants was determined by two-sided one-way ANOVA. *P < 0.05 and **P < 0.01 and ***P < 0.001 versus WT. The detailed information is provided in Extended Data Table 3. c, Phosphorylation sites of the active NTSR1. The extended loop of ICL3 or the elongated C-terminal tail of NTSR1 could reach the active cleft of GRK2, thus are available to be phosphorylated by GRK2. P represents phosphorylation modifications.
Extended Data Fig. 7 Sequence alignment of human GRKs.
The N-terminal helix (αN) is highlighted in red. The RHD is highlighted in green and KD is in light blue. The AST loop extended from the kinase domain is in light purple. The PHD of GRK2 and GRK3 are in pink. N-terminal lipid binding domain (NLBD) and C-terminal lipid binding domain (NLBD) according to GRK5 are highlighted in dark yellow and light green, respectively. Residues that may interact with membrane lipid are highlighted in dark blue. And residues from GRK2 that interact with NTSR1 are highlighted in yellow. α, α helix. β, β strand.
Extended Data Fig. 8 Structural comparison of NTSR1–GRK2–Gαq complex with NTSR-Gi complex and other GPCR-G protein complexes.
Superposition of the receptor TMD fragment from different GPCR complexes showed that the active GPCRs had very similar 3D architecture, and the location of α5 helix from different G proteins overlapped with the N-terminal helix of GRK2.
Extended Data Fig. 9 Gq protein and arrestin signaling of NTSR1 potentiated by NTS and SBI-553.
NTS is a balanced agonist, which binds to orthosteric site and promotes NTSR1 to mediate both G protein and arrestin signaling. SBI-553 is an arresin-biased allosteric modulator, which binds to the intracellular site and promotes GRK2 binding and arrestin signaling, but blocks Gq protein binding and signaling (red circled star). ++ marks indicate membrane binding.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Supplementary Tables 1–7.
Supplementary Video 1
3DVA of NTSR1–GRK2–Gαq complexes. 3DVA of the two cryo-EM maps also reveal the dynamic swing of GRK2 around NTSR1, especially the Gαq subunit and the relative positions between the RHD and kinase domain.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, J., Liu, H., Zhao, F. et al. GPCR activation and GRK2 assembly by a biased intracellular agonist. Nature 620, 676–681 (2023). https://doi.org/10.1038/s41586-023-06395-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06395-9
This article is cited by
-
G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery
Signal Transduction and Targeted Therapy (2024)
-
Cryo-electron microscopy for GPCR research and drug discovery in endocrinology and metabolism
Nature Reviews Endocrinology (2024)
-
Cryo-EM structures of adenosine receptor A3AR bound to selective agonists
Nature Communications (2024)
-
Molecular recognition and activation mechanism of short-chain fatty acid receptors FFAR2/3
Cell Research (2024)
-
Recent advances in chemical protein synthesis: method developments and biological applications
Science China Chemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.