Abstract
RNA viruses have evolved elaborate strategies to protect their genomes, including 5′ capping. However, until now no RNA 5′ cap has been identified for hepatitis C virus1,2 (HCV), which causes chronic infection, liver cirrhosis and cancer3. Here we demonstrate that the cellular metabolite flavin adenine dinucleotide (FAD) is used as a non-canonical initiating nucleotide by the viral RNA-dependent RNA polymerase, resulting in a 5′-FAD cap on the HCV RNA. The HCV FAD-capping frequency is around 75%, which is the highest observed for any RNA metabolite cap across all kingdoms of life4,5,6,7,8. FAD capping is conserved among HCV isolates for the replication-intermediate negative strand and partially for the positive strand. It is also observed in vivo on HCV RNA isolated from patient samples and from the liver and serum of a human liver chimeric mouse model. Furthermore, we show that 5′-FAD capping protects RNA from RIG-I mediated innate immune recognition but does not stabilize the HCV RNA. These results establish capping with cellular metabolites as a novel viral RNA-capping strategy, which could be used by other viruses and affect anti-viral treatment outcomes and persistence of infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The sequencing data and the count files obtained for the different experiments are available at NCBI Gene Expression Omnibus with the accession GSE180956. Fasta files used for mapping are available at https://doi.org/10.17894/ucph.c8675313-45c6-4a90-b4ab-c65abffd3f38. Data used for plotting of Figs. 1, 2 and 4 are available as Source Data 1–3. Source data are provided with this paper.
Code availability
Analysis of the CapZyme-seq data is based on existing tools and is described in detail at https://github.com/jeppevinther/CapZyme. Additional information about the code used for the analyses described in this paper is available from the corresponding author upon request.
References
Li, Y., Yamane, D., Masaki, T. & Lemon, S. M. The yin and yang of hepatitis C: synthesis and decay of hepatitis C virus RNA. Nat. Rev. Microbiol. 13, 544–558 (2015).
Li, Y., Masaki, T., Yamane, D., McGivern, D. R. & Lemon, S. M. Competing and noncompeting activities of miR-122 and the 5′ exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc. Natl Acad. Sci. USA 110, 1881–1886 (2013).
Bukh, J. The history of hepatitis C virus (HCV): basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J. Hepatol. 65, S2–S21 (2016).
Cahova, H., Winz, M. L., Hofer, K., Nubel, G. & Jaschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 519, 374–377 (2015).
Jiao, X. et al. 5′ End nicotinamide adenine dinucleotide cap in human cells promotes RNA decay through DXO-mediated deNADding. Cell 168, 1015–1027.e1010 (2017).
Walters, R. W. et al. Identification of NAD+ capped mRNAs in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 114, 480–485 (2017).
Bird, J. G. et al. Highly efficient 5′ capping of mitochondrial RNA with NAD+ and NADH by yeast and human mitochondrial RNA polymerase. eLife 7, e42179 (2018).
Wang, Y. et al. NAD+-capped RNAs are widespread in the Arabidopsis transcriptome and can probably be translated. Proc. Natl Acad. Sci. USA 116, 12094–12102 (2019).
Luo, G. et al. De novo initiation of RNA synthesis by the RNA-dependent RNA polymerase (NS5B) of hepatitis C virus. J. Virol. 74, 851–863 (2000).
Zhong, W., Uss, A. S., Ferrari, E., Lau, J. Y. & Hong, Z. De novo initiation of RNA synthesis by hepatitis C virus nonstructural protein 5B polymerase. J. Virol. 74, 2017–2022 (2000).
Cai, Z., Liang, T. J. & Luo, G. Effects of mutations of the initiation nucleotides on hepatitis C virus RNA replication in the cell. J. Virol. 78, 3633–3643 (2004).
Yi, M. & Lemon, S. M. Structure-function analysis of the 3′ stem-loop of hepatitis C virus genomic RNA and its role in viral RNA replication. RNA 9, 331–345 (2003).
Ramirez, S. & Bukh, J. Current status and future development of infectious cell-culture models for the major genotypes of hepatitis C virus: essential tools in testing of antivirals and emerging vaccine strategies. Antiviral Res. 158, 264–287 (2018).
Li, Y. P., Ramirez, S., Gottwein, J. M. & Bukh, J. Non-genotype-specific role of the hepatitis C virus 5′ untranslated region in virus production and in inhibition by interferon. Virology 421, 222–234 (2011).
Li, Y. P., Gottwein, J. M., Scheel, T. K., Jensen, T. B. & Bukh, J. MicroRNA-122 antagonism against hepatitis C virus genotypes 1–6 and reduced efficacy by host RNA insertion or mutations in the HCV 5′ UTR. Proc. Natl Acad. Sci. USA 108, 4991–4996 (2011).
Saito, T., Owen, D. M., Jiang, F., Marcotrigiano, J. & Gale, M. Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 (2008).
Sedano, C. D. & Sarnow, P. Hepatitis C virus subverts liver-specific miR-122 to protect the viral genome from exoribonuclease Xrn2. Cell Host Microbe 16, 257–264 (2014).
Amador-Canizares, Y., Bernier, A., Wilson, J. A. & Sagan, S. M. miR-122 does not impact recognition of the HCV genome by innate sensors of RNA but rather protects the 5′ end from the cellular pyrophosphatases, DOM3Z and DUSP11. Nucleic Acids Res. 46, 5139–5158 (2018).
Jopling, C. L., Yi, M., Lancaster, A. M., Lemon, S. M. & Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309, 1577–1581 (2005).
Gebert, L. F. R., Law, M. & MacRae, I. J. A structured RNA motif locks Argonaute2:miR-122 onto the 5′ end of the HCV genome. Nat. Commun. 12, 6836 (2021).
Chen, Y. G., Kowtoniuk, W. E., Agarwal, I., Shen, Y. & Liu, D. R. LC/MS analysis of cellular RNA reveals NAD-linked RNA. Nat. Chem. Biol. 5, 879–881 (2009).
Kowtoniuk, W. E., Shen, Y., Heemstra, J. M., Agarwal, I. & Liu, D. R. A chemical screen for biological small molecule-RNA conjugates reveals CoA-linked RNA. Proc. Natl Acad. Sci. USA 106, 7768–7773 (2009).
Frindert, J. et al. Identification, biosynthesis, and decapping of NAD-capped RNAs in B. subtilis. Cell Rep. 24, 1890–1901 e1898 (2018).
Julius, C. & Yuzenkova, Y. Bacterial RNA polymerase caps RNA with various cofactors and cell wall precursors. Nucleic Acids Res. 45, 8282–8290 (2017).
Vvedenskaya, I. O. et al. CapZyme-seq comprehensively defines promoter-sequence determinants for RNA 5′ capping with NAD. Molecular Cell 70, 553–564 e559 (2018).
Wang, J. et al. Quantifying the RNA cap epitranscriptome reveals novel caps in cellular and viral RNA. Nucleic Acids Res. 47, e130 (2019).
Doamekpor, S. K. et al. DXO/Rai1 enzymes remove 5′-end FAD and dephospho-CoA caps on RNAs. Nucleic Acids Res. 48, 6136–6148 (2020).
Marceau, C. D. et al. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 (2016).
Maruta, T. et al. An Arabidopsis FAD pyrophosphohydrolase, AtNUDX23, is involved in flavin homeostasis. Plant Cell Physiol. 53, 1106–1116 (2012).
Catanese, M. T. et al. Different requirements for scavenger receptor class B type I in hepatitis C virus cell-free versus cell-to-cell transmission. J. Virol. 87, 8282–8293 (2013).
Mercer, D. F. et al. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 7, 927–933 (2001).
Pham, L. V. et al. HCV genome-wide analysis for development of efficient culture systems and unravelling of antiviral resistance in genotype 4. Gut 71, 627–642 (2022).
Howe, A. Y. et al. Molecular mechanism of hepatitis C virus replicon variants with reduced susceptibility to a benzofuran inhibitor, HCV-796. Antimicrob. Agents Chemother. 52, 3327–3338 (2008).
Schwerk, J., Negash, A., Savan, R. & Gale, M. Jr. Innate immunity in hepatitis C virus infection. Cold Spring Harb. Perspect. Med. 11, a036988 (2021).
Vasou, A. et al. Modular cell-based platform for high throughput identification of compounds that inhibit a viral interferon antagonist of choice. Antiviral Res. 150, 79–92 (2018).
Chen, S. et al. Heterocellular induction of interferon by negative-sense RNA viruses. Virology 407, 247–255 (2010).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Li, X. D., Sun, L., Seth, R. B., Pineda, G. & Chen, Z. J. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl Acad. Sci. USA 102, 17717–17722 (2005).
Loo, Y. M. et al. Viral and therapeutic control of IFN-β promoter stimulator 1 during hepatitis C virus infection. Proc. Natl Acad. Sci. USA 103, 6001–6006 (2006).
Jones, C. T. et al. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat. Biotechnol. 28, 167–171 (2010).
Narbus, C. M. et al. HepG2 cells expressing microRNA miR-122 support the entire hepatitis C virus life cycle. J. Virol. 85, 12087–12092 (2011).
Yang, W. et al. Correlation of the tight junction-like distribution of claudin-1 to the cellular tropism of hepatitis C virus. J. Biol. Chem. 283, 8643–8653 (2008).
Park, J. O. et al. Metabolite concentrations, fluxes and free energies imply efficient enzyme usage. Nat. Chem. Biol. 12, 482–489 (2016).
Yamane, D. et al. Regulation of the hepatitis C virus RNA replicase by endogenous lipid peroxidation. Nat. Med. 20, 927–935 (2014).
Sumpter, R. Jr. et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79, 2689–2699 (2005).
Kincaid, R. P., Lam, V. L., Chirayil, R. P., Randall, G. & Sullivan, C. S. RNA triphosphatase DUSP11 enables exonuclease XRN-mediated restriction of hepatitis C virus. Proc. Natl Acad. Sci. USA 115, 8197–8202 (2018).
Sharma, S. et al. Identification of a novel deFADding activity in human, yeast and bacterial 5′ to 3′ exoribonucleases. Nucleic Acids Res. 50, 8807–8817 (2022).
Tilgner, M. & Shi, P. Y. Structure and function of the 3′ terminal six nucleotides of the west nile virus genome in viral replication. J. Virol. 78, 8159–8171 (2004).
Teramoto, T. et al. Genome 3′-end repair in dengue virus type 2. RNA 14, 2645–2656 (2008).
Lescar, J. & Canard, B. RNA-dependent RNA polymerases from flaviviruses and Picornaviridae. Curr. Opin. Struct. Biol. 19, 759–767 (2009).
Blight, K. J., McKeating, J. A. & Rice, C. M. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76, 13001–13014 (2002).
Li, Y. P. et al. Highly efficient full-length hepatitis C virus genotype 1 (strain TN) infectious culture system. Proc. Natl Acad. Sci. USA 109, 19757–19762 (2012).
Li, Y. P., Ramirez, S., Mikkelsen, L. & Bukh, J. Efficient infectious cell culture systems of the hepatitis C virus (HCV) prototype strains HCV-1 and H77. J. Virol. 89, 811–823 (2015).
Ramirez, S. et al. Cell culture studies of the efficacy and barrier to resistance of sofosbuvir-velpatasvir and glecaprevir-pibrentasvir against hepatitis C virus genotypes 2a, 2b, and 2c. Antimicrob. Agents Chemother. 64, e01888-19 (2020).
Ramirez, S. et al. Highly efficient infectious cell culture of three hepatitis C virus genotype 2b strains and sensitivity to lead protease, nonstructural protein 5A, and polymerase inhibitors. Hepatology 59, 395–407 (2014).
Ramirez, S., Mikkelsen, L. S., Gottwein, J. M. & Bukh, J. Robust HCV genotype 3a infectious cell culture system permits identification of escape variants with resistance to sofosbuvir. Gastroenterology 151, 973–985.e972 (2016).
Humes, D. et al. Recombinant hepatitis C virus genotype 5a infectious cell culture systems expressing minimal JFH1 NS5B sequences permit polymerase inhibitor studies. Virology 522, 177–192 (2018).
Pham, L. V. et al. HCV genotype 6a escape from and resistance to velpatasvir, pibrentasvir, and sofosbuvir in robust infectious cell culture models. Gastroenterology 154, 2194–2208.e2112 (2018).
Lindenbach, B. D. et al. Complete replication of hepatitis C virus in cell culture. Science 309, 623–626 (2005).
Mandl, C. W., Ecker, M., Holzmann, H., Kunz, C. & Heinz, F. X. Infectious cDNA clones of tick-borne encephalitis virus European subtype prototypic strain Neudoerfl and high virulence strain Hypr. J. Gen. Virol. 78, 1049–1057 (1997).
Tsetsarkin, K. et al. Infectious clones of chikungunya virus (La Reunion isolate) for vector competence studies. Vector Borne Zoonotic Dis. 6, 325–337 (2006).
Mendez, E., Ruggli, N., Collett, M. S. & Rice, C. M. Infectious bovine viral diarrhea virus (strain NADL) RNA from stable cDNA clones: a cellular insert determines NS3 production and viral cytopathogenicity. J. Virol. 72, 4737–4745 (1998).
Scheel, T. K. et al. A broad RNA virus survey reveals both miRNA dependence and functional sequestration. Cell Host Microbe 19, 409–423 (2016).
Prentoe, J. et al. HVR1-mediated antibody evasion of highly infectious in vivo adapted HCV in humanised mice. Gut 65, 1988–1997 (2016).
Engle, R. E., Russell, R. S., Purcell, R. H. & Bukh, J. Development of a TaqMan assay for the six major genotypes of hepatitis C virus: comparison with commercial assays. J. Med. Virol. 80, 72–79 (2008).
Lothert, K. et al. Development of a downstream process for the production of an inactivated whole hepatitis C virus vaccine. Sci. Rep. 10, 16261 (2020).
Mathiesen, C. K. et al. Adaptive mutations enhance assembly and cell-to-cell transmission of a high-titer hepatitis C virus genotype 5a core-NS2 JFH1-based recombinant. J. Virol. 89, 7758–7775 (2015).
Sølund, C. et al. Direct acting antiviral treatment of chronic hepatitis C in Denmark: factors associated with and barriers to treatment initiation. Scand. J. Gastroenterol. 53, 849–856 (2018).
Weis, N. & Thomsen, R. W. [The Danish Database for Hepatitis B and C]. Ugeskr. Laeger. 174, 2521 (2012).
Ogawa, T. et al. Molecular characterization of organelle-type Nudix hydrolases in Arabidopsis. Plant Physiol. 148, 1412–1424 (2008).
Singh, Y. & Bird, J. G. A gel electrophoresis-based assay for measuring enzymatic RNA decapping activity. Methods Enzymol. 675, 323–350 (2022).
Poulsen, L. D. & Vinther, J. RNA-seq for bacterial gene expression. Curr. Protoc. Nucleic Acid Chem. 73, e55 (2018).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17, 10–12 (2011).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–U354 (2012).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, giab008 (2021).
R Core Team. R: A Language and Environment for Statistical Computing version 4.0.2. http://www.R-project.org/ (R Foundation for Statistical Computing, 2020).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Saeed, M. et al. Efficient replication of genotype 3a and 4a hepatitis C virus replicons in human hepatoma cells. Antimicrob. Agents Chemother. 56, 5365–5373 (2012).
Coleman, T. M., Wang, G. & Huang, F. Superior 5′ homogeneity of RNA from ATP-initiated transcription under the T7 phi 2.5 promoter. Nucleic Acids Res. 32, e14 (2004).
Trivedi, S. et al. Viral persistence, liver disease, and host response in a hepatitis C-like virus rat model. Hepatology 68, 435–448 (2018).
Ward, J. C. et al. Insights into the unique characteristics of hepatitis C virus genotype 3 revealed by development of a robust sub-genomic DBN3a replicon. J. Gen. Virol. 101, 1182–1190 (2020).
Voitenleitner, C., Bechtel, J., Arfsten, A. & Hamatake, R. Hepatitis C genotype 1a replicon improved through introduction of fitness mutations. Biotechniques 52, 273–275 (2012).
Wose Kinge, C. N. et al. Hepatitis C virus genotype 5a subgenomic replicons for evaluation of direct-acting antiviral agents. Antimicrob. Agents Chemother. 58, 5386–5394 (2014).
Blight, K. J., McKeating, J. A., Marcotrigiano, J. & Rice, C. M. Efficient replication of hepatitis C virus genotype 1a RNAs in cell culture. J. Virol. 77, 3181–3190 (2003).
Gottwein, J. M. et al. Novel infectious cDNA clones of hepatitis C virus genotype 3a (strain S52) and 4a (strain ED43): genetic analyses and in vivo pathogenesis studies. J. Virol. 84, 5277–5293 (2010).
Bick, M. J. et al. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J. Virol. 77, 11555–11562 (2003).
Grosdidier, A., Zoete, V. & Michielin, O. Fast docking using the CHARMM force field with EADock DSS. J. Comput. Chem. 32, 2149–2159 (2011).
Macke, T. J. & Case, D. A. in Molecular Modeling of Nucleic Acids Vol. 682 (eds Leontis, N. B. & SantaLucia Jr, J.) 379–393 (American Chemical Society, 1997).
Appleby, T. C. et al. Viral replication. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 347, 771–775 (2015).
Schrödinger, L. The PyMOL Molecular Graphics System, Version 2.4.2 (2022).
Kent, W. J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Acknowledgements
The authors thank C. M. Rice and J. Christiansen for critical comments; members of the authors’ laboratories for helpful discussions; K. Kokkonos, L. Mikkelsen and N. Brinkmann for assistance with reagents; M. Evans for HepG2-HFL cells; C. Adamson for A549-pr(IFNβ)-GFP cells; P. Meuleman for help with establishing the human liver chimeric mouse model; and the BIO-UCPH sequencing and biocomputing core facilities for access to necessary infrastructure. Mass spectrometry analyses were performed by the Proteomics and Modomics Experimental Core (PROMEC), Norwegian University of Science and Technology, which is a member of the National Network of Advanced Proteomics Infrastructure (NAPI), Norway. This work was supported by Independent Research Fund Denmark grant DFF-6110-00350 (J.B. and J.V.) and DFF-9039-00380B (S.R. and J.B.); European Research Council (ERC) Starting Grant 802899 (TKHS); Novo Nordisk Foundation Distinguished Investigator grant NNF19OC0054518 (J.B.), Novo Nordisk Foundation Project Grants in Bioscience and Basic Biomedicine NNF18OC0052354 (J.V.), NNF19OC0055462 (J.B.), and NNF19OC0058443 (T.K.H.S.); Carlsberg Foundation Young Researcher Fellowship (T.K.H.S.), Research Infrastructure grant CF18-1075; Innovation Fund Denmark grant 1046-00020B (J.M.G.); Danish Agency for Science and Higher Education grant 0237-00005B (S.R. and J.B.); Candys Foundation grant 2017-248 (A.O., J.M.G. and J.B.); PROMEC is funded by The Central Norway Regional Health Authority and the Research Council of Norway INFRASTRUKTUR programme (295910) (C.B.V.).
Author information
Authors and Affiliations
Contributions
Conceptualization: J.B., T.K.H.S. and J.V. (lead). Funding acquisition: C.B.V., J.M.G., S.R., J.B., T.K.H.S. and J.V. Experiments: CapZyme-seq (A.V.S., H.S.L., K.M.A. and A.A.-C.), LC–MS/MS (C.B.V. and A.K.), protein expression (A.V.S., G.I., A.P.-C., J.E.R.G. and N.W.L.), human liver chimeric mouse infection (K.H.), patient samples (C.S. and N.W.), virus infection and cell assays (L.R.R.-R., H.S.L., R.C., E.J., L.V.P., L.N. and N.F.), NS5B polymerase expression and in vitro replication experiments (A.V.S., L.A.R., C.F.-A. and G.I.), NS5B modelling (S.B.), RT–qPCR reduction assay (L.R.R.-R. and H.S.L.), HCV RNA half-life (L.R.R.-R. and L.V.P.), innate immune response assays (L.R.R.-R. and E.J.), and high-titre viral supernatant (A.O.). Project administration: A.V.S., J.B., T.K.H.S. and J.V. (lead). Supervision: J.M.G., S.R., J.B., T.K.H.S. and J.V. Visualization: J.V. Writing original draft: A.V.S., L.R.R.-R., J.B., T.K.H.S. and J.V. (lead). All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Michael Gale Jr and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
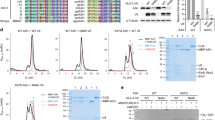
Extended Data Fig. 1 Specificity of enzymes used for CapZyme-seq enrichment.
(a) Schematic representation of the AtNUDX23 and AtNUDX23-E169Q proteins used in this study. For both, the N-terminal 75 residues were truncated to remove the chloroplast localisation signal present in the WT protein. (b) AMP production in the presence of increasing concentrations of ATP, FAD and NAD+ for the indicated enzymes and the no enzyme control (n = 4, independent replicates). Data are presented as mean +/– SD. (c) Strategy for the RT-qPCR reduction assay. (d) RT-qPCR reduction assay testing activity of AtNUDX23 and Rpp against in vitro transcribed FAD-capped RNA (left) and showing the lack of activity of the AtNUDX23-E169Q protein (right). (e) RT-qPCR reduction assay testing activity of AtNUDX23 and mRNA decapping enzyme (MDE) against in vitro transcribed m7G-capped RNA. (f) RT-qPCR reduction assay testing activity of AtNUDX23, Rpp and NudC against in vitro transcribed NAD+-capped RNA. For (d-f), data are presented as mean +/– SD, n = 3 independent replicates.
Extended Data Fig. 2 CapZyme-seq reads map to 5’ termini of RNAs.
Normalized sequencing depth plots for CapZyme-seq libraries: no enzyme control, AtNUDX23 and Rpp enrichment. Reads were mapped to human noncoding RNAs plus the most highly expressed mRNAs (> 2 TPM) in Huh7.5 cells. The normalized sequencing depth is shown for (a) all RNAs combined, (b) SSU rRNA, (c) J6/JFH1-c2 HCV(+) RNA, (d) J6/JFH1-c2 HCV(–) RNA, (e) 5S rRNA, (f) BCYRN1 RNA and (g) U6-ATAC RNA. (h) Schematic representation of tRNA processing: RNase P mediates processing of 5’ppp termini of primary tRNAs to 5’p mature tRNAs. (i) Normalized CapZyme-seq sequencing depth for tRNA-Leu-TAG-3-1 is shown for no enzyme control, AtNUDX23- and Rpp enrichment (top panels). Base wise conservation (phyloP score) for 100 vertebrate species and gene annotation (both obtained from UCSC genome browser) are shown in the bottom panels. Conserved positions have positive phyloP scores (blue). For browsing of the CapZyme-seq data at other human genomic loci, the following UCSC genome session can be used: http://www.genome.ucsc.edu/s/vinther/hg19_CapZyme%2Dseq_tRNA%2DLeu%2DTAG.
Extended Data Fig. 3 CapZyme-seq analysis of TBEV, BVDV and CHIKV infected cells.
Mean-difference plots showing fold changes (log2) as a function of average abundance (log) for reads in 5’ termini of individual RNA molecules. (a) CapZyme-seq analysis of RNA isolated from SH-SY5Y neuroblastoma cells infected with TBEV using Rpp, AtNUDX23, NudC and CapClip for enrichment. NudC has broad specificity, including cleavage of FAD, NAD+ and m7GpppN; Cap-Clip Acid Pyrophosphatase hydrolyzes m7GpppN (n = 3 biological replicates). (b) CapZyme-seq analysis of RNA isolated from MDBK bovine kidney cells infected with BVDV using Rpp and AtNUDX23 for enrichment (n = 3 biological replicates). (c) CapZyme-seq analysis of RNA isolated from TIG3 fibroblast cells infected with CHIKV using Rpp and AtNUDX23 for enrichment (n = 2 biological replicates).
Extended Data Fig. 4 Time course analysis of 5’ capping for JFH1-SGR-Feo replicon and LC-MS/MS detection of RNA 5’FAD caps.
(a) Enrichment of 5’ terminal reads for JFH1-SGR-Feo (–) at the indicated time points. DESeq2 log2(fold change) values were calculated by comparison of enzyme treatment (AtNUDX23 in dark blue and Rpp in light blue) to no enzyme control libraries. The 4 and 12 hrs time points for JFH1-SGR-Feo (–) are associated with uncertainty, because of low counts. (n = 2 biological replicates; mean is represented by the line). (b) As in (a) but for JFH1-SGR-Feo (+). (c) Analysis of viral replication at different time points. Relative luciferase units (RLU) show HCV IRES mediated translation, and replication levels can be deduced by comparing JFH1-SGR-Feo to JFH1-SGR-Feo-GNN (catalytically dead replicon control) at the indicated time points after transfection (n = 3 biological replicates). (d) Sample preparation for FAD detection using LC-MS/MS. (e) FAD product ion mass spectrum. Insert shows the structure of FAD with the detected ions indicated. (f) LC-MS/MS chromatogram showing the detection of the FAD control. (g) LC-MS/MS chromatogram showing the detection of FAD in a control Huh7.5 mock-infected sample (light blue), intracellular RNA from HCV infected Huh7.5 cells (dark blue) and in HCV particles concentrated from supernatant (yellow). (h) LC-MS/MS FAD quantification. FAD concentration based on internal stable isotope-labelled standards for FAD and normalization to ribonucleotide content. RNA was isolated from J6/JFH1 infected or control Huh7.5 cells (mock) and treated with or without Nuclease P1. Due to different lot activities, lower concentration of Nuclease P1 (0.006 U/µg total RNA) was used here, compared to the experiment presented in Fig. 1g (0.12 U/µg total RNA), resulting in lower FAD/rNs ratios. The p-values are calculated using one-sided Welch’s unequal variances t-test. Data are presented as mean +/– SD (from left to right n = 9, 3, 9 and 6 biological replicates). (i) Intracellular FAD levels in Huh7.5 cells with and without riboflavin and HCV infection or after lumiflavin treatment. Cells were cultured with or without supplementation of riboflavin (0.4 mg/L) or FAD (10 µM) or were treated with increasing lumiflavin concentrations. HCV indicates infection with J6/JFH1. Fluorescence measurements were used for FAD quantification; shown values for FAD pmol/cell were calculated using a standard curve. Data are presented as mean +/– SD (n = 3 biological replicates).
Extended Data Fig. 5 Identification of 5’ capping for different HCV strains.
CapZyme-seq analysis of RNA isolated from Huh7.5 cells infected with the indicated HCV genotypes/strains. Mean-difference plots show log2 (fold changes) as a function of log2 (average abundance) for reads mapping to the 5’ termini of individual RNA molecules. Enrichment with Rpp (a) and AtNUDX23 (b) was based on comparison of enzyme-treated libraries to no enzyme control libraries. The log2 (fold values) observed for HCV(+), HCV(–) and 5S rRNA (5’ppp) for the different genotypes/strains are summarized in (c) for Rpp and (d) for AtNUDX23. Data is derived from a single biological replicate. Main Fig. 2c shows replicate experiments for selected strains.
Extended Data Fig. 6 The effect of riboflavin depletion and lumiflavin treatment on cellular viability and HCV genotype-specific dependency on FAD.
(a) Cell viability after lumiflavin treatment or riboflavin depletion. (b) Measurement of translational activity at 4 hrs after transfection of JFH1-SGR-Feo RNA with and without 0.4 mg/L riboflavin and 10µM FAD. Transfection at several time points after riboflavin depletion was included to exclude that differences in cell viability affected HCV IRES-mediated translation. (c) Measurement of Sindbis virus (SINV) replication after transfection of in vitro transcribed RNA from infectious SINV Toto-1101/Luc reporter virus with and without 0.4 mg/L riboflavin and 10 µM FAD. Transfection at several time points after riboflavin depletion was included to exclude that differences in cell viability affected SINV replication. (d) Replication of DBN3a-SGR(5’G) and GNN (non-replicating mutant) in Huh7.5 cells grown in riboflavin-depleted media. DBN3a-SGR-Fluc(5’G) had partially reverted to 5’A at the 72 hrs time point. Conditions with riboflavin (0.4 mg/L) and FAD (10 µM) included are indicated. Replication was measured in relative luciferase units (RLU) at the indicated time points and quantified relative to 4 hrs. (e) Inhibition of HCV infection by lumiflavin. Infection was measured in focus forming units relative to the untreated control obtained after infection with HCV TNcc (1a), J6/JFH1 (2a) or DBN3acc (3a) strains. For all panels, data are presented as mean +/– SD, n = 3 biological replicates.
Extended Data Fig. 7 FAD dependent replication initiation by HCV NS5B.
(a) Coomassie stained SDS-PAGE of the HCV NS5B WT, GNN, C316F, C316A, S365L and C366A recombinant proteins. (b) RNA templates used for in vitro replication reactions. (c) Time-dependent formation of the initiation product. HCV NS5B polymerase was incubated with HCV3END10A RNA in the presence of FAD and CTP, complemented with α 32P-labeled CTP for visualization. Reactions were terminated at the indicated time points. Products were resolved with 18 % denaturing PAGE together with 5’ radioactively labelled markers. (d) The replication extension products contain FAD. NS5B polymerase was incubated with HCV3END10A RNA in the presence of FAD, ATP, CTP, UTP (complemented with α-32P-labeled UTP) and either GTP or 3’dGTP. After 1 hr incubation, the reactions were treated with AtNUDX23 or AtNUDX23-E169Q. The products were resolved with 18 % denaturing 0.2 % boronate PAGE. The replication reactions subjected to AtNUDX23 or AtNUDX23-E169Q treatment are identical to the α-32P-labeled UTP reactions separated on a regular PAGE gel in main Fig. 3c. The presence of boronate retards FAD-capped RNAs compared to uncapped RNAs due to the relatively stable diol complexes formed between the gel derived boronyl groups and naturally present diols of the RNA 3’ end and of FAD. Accordingly, both 5’ FAD capping and 3’ deoxy termination of extension products will affect migration in the boronate gel. Products were annotated by comparison between the 3’dGTP/GTP conditions, AtNUDX23/AtNUDX23-E169Q conditions and the migration observed on the regular PAGE gel (main Fig. 3c). (e) FAD promotes replication initiation on different RNA templates. Indicated RNA templates were incubated with HCV NS5B polymerase. ATP, FAD or control was added as initiating nucleotide. Elongating UTP or CTP nucleotides were supplemented with α32P-labeled UTP or CTP, respectively. The resulting products were resolved with 18 % denaturing PAGE. (f) Lack of HCV NS5B mediated post-initiation FAD capping. To exclude that NS5B mediated 5’FAD capping could happen post-initiation on a 5’ppp initiated RNA, 5’ppp RNA was incubated with FMN in the presence of NS5B polymerase. RNA was resolved with 18 % PAGE and visualised by nucleic acid staining (left) and with fluorescent signal indicative of the 5’FAD cap (right). Increasing concentrations of 5’FAD capped RNA generated with T7 polymerase were used as markers on the same gel. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 8 Prediction model of FAD – HCV NS5B interaction.
(a) In silico docking analysis of FAD into HCV NS5B using SwissDock. Full fitness scores for the top 36 positions obtained by docking of lumiflavin into HCV NS5B are shown. (b) Close-up view of the palm II binding pocket demonstrating the overlap between the nesbuvir (green) binding site and the putative binding site of the lumiflavin moiety of FAD (blue). The docking was based on (PDB: 3FQL) with the nesbuvir molecule removed. (c) Predicted contacts between NS5B and lumiflavin when docked in the palm II site of NS5B. (d) Predicted lumiflavin binding site modelled into the structure of the NS5B primed initiation complex (PDB:4WTM) colored by subdomains: fingers (pink), palm (light blue), thumb (pale green) and beta-loop (yellow). The HCV(+) 3’ end sequence was modeled into the position of the template (red). An adenosine (blue) and CMP (green) were modeled into the position of the priming and incoming nucleotides, respectively. The close-up view shows the active site with two localized Mn2+ ions in violet. The direct distance between the lumiflavin N10 methyl and the ribose C5 is indicated. (e) Observed FAD N10 methyl-C5 distances from 4,928 crystal structures of proteins containing a FAD cofactor. (f–h) Triplicate analysis (independent replicates) of de novo initiation with FAD for the indicated NS5B mutants using the HCV3END10A template with the FAD extension and initiation signal quantified below the gels. The p-values are calculated using two-sided Welch’s unequal variances t-test. N.S. equals p-val > 0.05. For gel source data, see Supplementary Fig. 1.
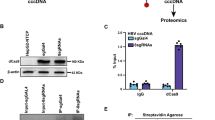
Extended Data Fig. 9 RNA stimulation of innate immune responses.
(a) Analysis of the in vitro transcribed RNAs used in cell-intrinsic innate immune recognition experiments (main Fig. 4c–e and g, and Extended Data Fig. 10a,b). RNA was resolved with 18 % PAGE and visualised by nucleic acid staining (left) or with fluorescent signal from the 5’FAD cap (right). (b) Gating strategy for flow cytometry experiments. Mock- and poly (I:C) transfected A549/pr(IFNβ).GFP reporter cells were used to set gates. Live cells were SSC-A:FSC-A gated, followed by FSC-H:FSC-A gating to select single cells. Single cells were then GFP(–) or GFP(+) gated. (c) Immunoblot for RIG-I after control or RIGI siRNA mediated knock-down in A549/pr(IFNβ).GFP reporter cells. The RIG-I specific band is indicated. (d) Immunoblot for MDA5 after control or MDA5 siRNA mediated knock-down in A549/pr(IFNβ).GFP reporter cells and induction of MDA5 expression by transfection with 5´ppp JFH1-SGR-Feo-GNN RNA. 50 IU/mL IFN-a 2a, is shown for comparison; this concentration was too low to induce MDA5 expression at the shown exposure level. (e) Analysis of innate immune activation upon transfection of JFH1-SGR-Feo-GNN replicon RNA into A549/pr(IFNβ).GFP cells after knock-down of RIG-I or MDA5. Data are presented as mean +/– SD, n = 3 biological replicates. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 10 RNA stimulation of innate immune responses.
(a) Immunoblot for phosphorylated NF-kB subunit p65 in HepG2-HFL cells 4 hrs after stimulation with 5’ppp or 5’FAD as indicated. (b) Immunoblot for phosphorylated IRF3 in A549/pr(IFNβ).GFP cells 12 hrs after stimulation with 5’ppp or 5’FAD as indicated. The upper band, consistent with the size of p-IRF3 appears only after stimulation. Note the different loading order compared to panel (a). (c) mRNA expression levels for IFNB1, IFNL1, IFNL2/3 and IFIT1 18 hrs post infection of HepG2-HFL cells with equal RNA titers of indicated HCV strains shown relative to polyI:C induced levels. Data represent mean +/– SD (n = 3 biological replicates). ND: Not detected. (d) Immunoblot for RIG-I after control or RIGI siRNA mediated knock-down in HepG2-HFL cells and induction of RIG-I expression by transfection of 100 ng in vitro transcribed 5’ppp J6/JFH1 RNA, 100 ng Poly I:C or treatment with 500 U/mL IFN-a 2a. (e) mRNA expression levels for IFNB1, IFNL1, IFNL2/3 and IFIT1 18 hrs post infection of HepG2-HFL cells with indicated HCV strains shown relative to levels obtained for Mock infected control siRNA treated cells. Data represent mean +/– SD (n = 3 biological replicates). Prior to infection, cells were transfected with control siRNA or RIGI targeting siRNA. The p-values in (c) and (e) are calculated using one-sided Welch’s unequal variances t-test by comparing to the mock infection samples. For gel source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Fig. 1
Raw data images of uncropped gels.
Supplementary Table 1
Oligonucleotides used in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sherwood, A.V., Rivera-Rangel, L.R., Ryberg, L.A. et al. Hepatitis C virus RNA is 5′-capped with flavin adenine dinucleotide. Nature 619, 811–818 (2023). https://doi.org/10.1038/s41586-023-06301-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06301-3
This article is cited by
-
Toll/interleukin-1 receptor (TIR) domain-containing proteins have NAD-RNA decapping activity
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.