Abstract
The human gut microbiome constantly converts natural products derived from the host and diet into numerous bioactive metabolites1,2,3. Dietary fats are essential micronutrients that undergo lipolysis to release free fatty acids (FAs) for absorption in the small intestine4. Gut commensal bacteria modify some unsaturated FAs—for example, linoleic acid (LA)—into various intestinal FA isomers that regulate host metabolism and have anticarcinogenic properties5. However, little is known about how this diet–microorganism FA isomerization network affects the mucosal immune system of the host. Here we report that both dietary factors and microbial factors influence the level of gut LA isomers (conjugated LAs (CLAs)) and that CLAs in turn modulate a distinct population of CD4+ intraepithelial lymphocytes (IELs) that express CD8αα in the small intestine. Genetic abolition of FA isomerization pathways in individual gut symbionts significantly decreases the number of CD4+CD8αα+ IELs in gnotobiotic mice. Restoration of CLAs increases CD4+CD8αα+ IEL levels in the presence of the transcription factor hepatocyte nuclear factor 4γ (HNF4γ). Mechanistically, HNF4γ facilitates CD4+CD8αα+ IEL development by modulating interleukin-18 signalling. In mice, specific deletion of HNF4γ in T cells leads to early mortality from infection by intestinal pathogens. Our data reveal a new role for bacterial FA metabolic pathways in the control of host intraepithelial immunological homeostasis by modulating the relative number of CD4+ T cells that were CD4+CD8αα+.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary information files. The RNA sequencing and 16S rRNA sequencing data are available in the NCBI database under BioProject identifier PRJNA815975 and PRJNA910679, respectively. Source data are provided with this paper.
References
Donia, M. S. & Fischbach, M. A. Small molecules from the human microbiota. Science 349, 1254766 (2015).
Alexander, M. & Turnbaugh, P. J. Deconstructing mechanisms of diet–microbiome–immune interactions. Immunity 53, 264–276 (2020).
Krautkramer, K. A., Fan, J. & Backhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 19, 77–94 (2021).
Ko, C. W., Qu, J., Black, D. D. & Tso, P. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 17, 169–183 (2020).
Salsinha, A. S., Pimentel, L. L., Fontes, A. L., Gomes, A. M. & Rodriguez-Alcala, L. M. Microbial production of conjugated linoleic acid and conjugated linolenic acid relies on a multienzymatic system. Microbiol. Mol. Biol. Rev. https://doi.org/10.1128/MMBR.00019-18 (2018).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Song, X. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020).
Campbell, C. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479 (2020).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Sano, T. et al. An IL-23R/IL-22 circuit regulates epithelial serum amyloid A to promote local effector Th17 responses. Cell 163, 381–393 (2015).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 (2011).
Mucida, D. et al. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 14, 281–289 (2013).
Sujino, T. et al. Tissue adaptation of regulatory and intraepithelial CD4+ T cells controls gut inflammation. Science 352, 1581–1586 (2016).
Bilate, A. M. et al. Tissue-specific emergence of regulatory and intraepithelial T cells from a clonal T cell precursor. Sci. Immunol. 1, eaaf7471 (2016).
Cervantes-Barragan, L. et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 357, 806–810 (2017).
Bilate, A. M. et al. T cell receptor is required for differentiation, but not maintenance, of intestinal CD4+ intraepithelial lymphocytes. Immunity 53, 1001–1014.e20 (2020).
Bousbaine, D. et al. A conserved Bacteroidetes antigen induces anti-inflammatory intestinal T lymphocytes. Science 377, 660–666 (2022).
Lebreton, F. et al. Tracing the enterococci from Paleozoic origins to the hospital. Cell 169, 849–861 e813 (2017).
Dale, J. L. et al. Comprehensive functional analysis of the Enterococcus faecalis core genome using an ordered, sequence-defined collection of insertional mutations in strain OG1RF. mSystems https://doi.org/10.1128/mSystems.00062-18 (2018).
Thurlow, L. R., Thomas, V. C. & Hancock, L. E. Capsular polysaccharide production in Enterococcus faecalis and contribution of CpsF to capsule serospecificity. J. Bacteriol. 191, 6203–6210 (2009).
Chen, L. et al. A reinforcing HNF4–SMAD4 feed-forward module stabilizes enterocyte identity. Nat. Genet. 51, 777–785 (2019).
Chen, L. et al. HNF4 regulates fatty acid oxidation and is required for renewal of intestinal stem cells in mice. Gastroenterology 158, 985–999.e9 (2020).
Lei, X. et al. Epithelial HNF4A shapes the intraepithelial lymphocyte compartment via direct regulation of immune signaling molecules. J. Exp. Med. https://doi.org/10.1084/jem.20212563 (2022).
Hertz, R., Magenheim, J., Berman, I. & Bar-Tana, J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4α. Nature 392, 512–516 (1998).
Wisely, G. B. et al. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure 10, 1225–1234 (2002).
Palanker, L., Tennessen, J. M., Lam, G. & Thummel, C. S. Drosophila HNF4 regulates lipid mobilization and β-oxidation. Cell Metab. 9, 228–239 (2009).
Xie, G. et al. A metabolite array technology for precision medicine. Anal. Chem. 93, 5709–5717 (2021).
Matyash, V., Liebisch, G., Kurzchalia, T. V., Shevchenko, A. & Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49, 1137–1146 (2008).
Acknowledgements
We thank T. Sherpa, J. Ramos and H. Ahmed for their help with GF mice; D. Ischiu Gutierrez for FACS experiments; E. Choi for lipid extraction; and L. Yang for bioinformatics. This work was supported in part by Department of Defense grants W81XWH1910625 and HT9425-23-0226 and Sponsored Research Agreements with Quark Ventures and Evelo Biosciences to D.L.K.; National Key R&D Program of China 2022YFA0807300, NSF of China 32270945, and STCSM 22ZR1468700 and 22140902400 to X. Song; China Postdoctoral Science Foundation 2022M723139 to H.Z.; NIH R01-AT010268 and Department of Defense W81XWH1910626 to S.F.O.; and NIH-NICHD T32:5T32HD55148-10 and Quark Ventures A31696 to F.S.G.; contributions of M.S.G. and S.S.M. were supported by the Harvard-wide Program on Antibiotic Resistance (PO1 AI1083214).
Author information
Authors and Affiliations
Contributions
D.L.K. and X. Song designed the research and wrote the manuscript. X. Song, H.Z., Y.Z., B.G., B.B., S.S.M., X. Sun, W.Z., F.S.G., M.W. and Q.Y. conducted or helped with the experiments. X. Song, H.Z., Y.Z. and F.Q. analysed the data. M.S.G. and S.F.O. contributed to relevant discussions, and M.S.G. assisted with manuscript editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Kenya Honda, Rodney Newberry and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Intraepithelial CD4+CD8αα+ cells are dominant in the small intestine and their levels are controlled by the gut microbiota.
(a) Representative plots and frequencies of CD8αα+ in the TCRβ+CD4+CD8β− IEL population in different tissues from Jackson, Taconic, or Charles River-derived SPF C57BL/6 mice. mLN, mesenteric lymph nodes. (b) Representative plots and frequencies of small intestinal (duodenum, jejunum, and ileum) CD8αα+ in the TCRβ+CD4+CD8β− IEL population from SPF or GF C57BL/6 mice. Data are representative of three independent experiments. n represents biologically independent animals. Bars indicate mean ± SEM values. Statistical analysis was performed using a two-tailed Student’s t-test in b.
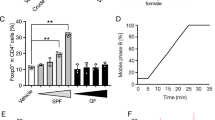
Extended Data Fig. 2 Bile acid signalling is not required for maintaining the levels of ileal intraepithelial CD4+CD8αα+ cells.
(a) Volcano plot comparing dietary metabolites from the rich and minimal diet (n = 3). Differentially decreased metabolites (left upper quadrant) or increased metabolites (right upper quadrant) in the minimal diet are shown. Different types of metabolites are highlighted by the indicated colors. (b) Frequencies of ileal CD8αα+ in the TCRβ+CD4+CD8β− IEL population from SPF mice fed with either a rich diet or a 5% cholestyramine-supplemented rich diet. (c) Frequencies of ileal CD8αα+ in the TCRβ+CD4+CD8β− IEL population from mice deficient in bile acid receptors (Nr1h4–/–, Vdr–/–, and Gpbar1–/–) and their littermate controls. Data are representative of two independent experiments in a. Data are pooled from three independent experiments in b and c. n represents biologically independent animals. Bars indicate mean ± SEM values. Statistical analysis was performed using a two-tailed Student’s t-test in a and b.
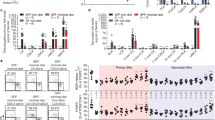
Extended Data Fig. 3 Gut microbial profiling and CLA quantification of rich-diet versus minimal-diet mice.
(a–e) Three-week-old SPF mice were fed a nutrient-rich diet or a minimal diet, and the microbial compositions in the ileal and colonic lumen were analyzed after 4 weeks by 16S rRNA sequencing. PCoA analysis on ileal (a) or colonic (b) luminal content, Rank abundance curve (c), and relative abundance of bacteria at the phylum (d) and family levels (e) are shown. (f) GC/MS quantitation of different CLAs in the luminal contents from the duodenum, jejunum, and ileum from SPF mice fed a nutrient-rich diet or a minimal diet. (g) LC/MS quantitation of CLAs in the luminal contents from the duodenum, jejunum, and ileum from SPF or GF mice. (h) LC/MS quantitation of CLAs produced by the indicated gut commensal strains cultured with linoleic acid (60 μM) for 24 h. Data are pooled from two independent experiments in a–e. Data are representative of two independent experiments in f–h. n represents biologically independent animals in a–c and f–g, and biologically independent samples in h. Bars indicate mean ± SEM values. Statistical analysis was performed using a two-tailed Student’s t-test in f and g.
Extended Data Fig. 4 Deficiency of linoleic acid isomerase in E. faecalis abolishes their ability to produce CLAs.
(a,b) Luminal or mucus layer bacterial quantitation (measured as CFU/g of ileal contents) in gnotobiotic mice monocolonized with E. faecalis MMH594 (a) and E. faecalis OG1RF (b) LAI mutants and their wild-type (WT) control strains. (c–f) LC/MS analysis of CLAs production from E. faecalis MMH594 (c,d) and E. faecalis OG1RF (e,f), LAI mutants and their wild-type (WT) control strains cultured with linoleic acid (30 μM) for 8 or 24 h. (g) Luminal or mucus layer bacterial quantitation (measured as CFU/g of ileal contents) of E. faecalis V583 LAI knockout and its wild-type (WT) control strain monocolonized in gnotobiotic mice. (h,i) LC/MS analysis of CLAs production from E. faecalis V583 LAI knockout and its wild-type (WT) control strain cultured with linoleic acid (30 μM) for 8 or 24 h. Data are representative of two independent experiments. n represents biologically independent animals in a,b, and g. Bars indicate mean ± SEM values.
Extended Data Fig. 5 Transcriptomes of ileal CD8αβ+, CD8αα+, and CD4+CD8αα+ cells in TCRβ+ IELs from SPF mice.
(a) Normalized expression values of Gzma, Gzmb, Prf1, and Ctam of ileal CD8αβ+, CD8αα+, and CD4+CD8αα+ cells in TCRβ+ IELs from SPF mice. (b) Volcano plot comparing transcriptomes of ileal CD4+CD8αα+ (n = 4) with CD8αβ+ (n = 3) IELs from SPF mice. Differentially down-regulated genes (highlighted in blue) or up-regulated genes (highlighted in red) in ileal CD4+CD8αα+ IELs are shown. (c) Volcano plot comparing transcriptomes of ileal CD4+CD8αα+ (n = 4) with CD8αα+ (n = 3) IELs from SPF mice. Differentially down-regulated genes (highlighted in blue) or up-regulated genes (highlighted in red) in ileal CD4+CD8αα+ IELs are shown. Data are pooled from three independent experiments. n represents biologically independent samples. Bars indicate mean ± SEM values. Two-tailed Student’s t-test was used for b and c.
Extended Data Fig. 6 HNF4α and γ are highly expressed in the small intestine.
Quantitative mRNA expression of HNF4α and HNF4γ in different tissues of SPF mice. mLN, mesenteric lymph nodes. Data are representative of three independent experiments. n represents biologically independent animals. Bars indicate mean ± SEM values.
Extended Data Fig. 7 Ileal TCRβ+ IEL profiling of mice with cell type-specific abolition of HNF4α in gut epithelial cells or CD4+ T cells.
(a–d) Frequencies of ileal CD4+ in the TCRβ+ IEL population (a), CD8αα+ in the TCRβ+CD4+CD8β− IEL population (b), CD8αβ+ in the TCRβ+ IEL population (c), or CD8αα+ in the TCRβ+CD4−CD8β− IEL population (d) from Hnf4αflox/floxVil1Cre mice and their littermate controls. (e–h) Frequencies of ileal CD4+ in the TCRβ+ IEL population (e), ThPOK+ in the TCRβ+CD4+CD8β− IEL population (f), CD8αβ+ in the TCRβ+ IEL population (g), or CD8αα+ in the TCRβ+CD4−CD8β− IEL population (h) from Hnf4αflox/floxCD4Cre mice and their littermate controls. Data are pooled from two independent experiments. n represents biologically independent animals. Bars indicate mean ± SEM values. Statistical analysis was performed using a two-tailed Student’s t-test in d.
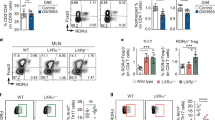
Extended Data Fig. 8 Gut immune profiling of Hnf4γ-deficient mice.
Representative plots or frequencies of various cell populations in Hnf4γ–/– mice and their littermate controls. (a) ileal ThPOK+ in the TCRβ+CD4+CD8β− IELs, (b) ileal CD4+ in the TCRβ+ IELs, (c) ileal CD8αβ+ in the TCRβ+ IELs, (d) ileal CD8αα+ in the TCRβ+CD4−CD8β− IELs, (e) splenic CD4+ in the TCRβ+ population, (f) splenic CD8αβ+ in the TCRβ+ population, (g) splenic CD8αα+ in the TCRβ+CD4−CD8β− population, (h) ileal Foxp3+ Tregs in the CD4+TCRβ+ LPLs, (i) ileal RORγ+Helios– Tregs in the Foxp3+CD4+TCRβ+ LPLs, (j) ileal RORγ+ Th17 in the Foxp3−CD4+TCRβ+ LPLs, (k) colonic Foxp3+ Tregs in the CD4+TCRβ+ LPLs, (l) colonic RORγ+Helios– Tregs in the Foxp3+CD4+TCRβ+ LPLs, and (m) colonic RORγ+ Th17 in the Foxp3−CD4+TCRβ+ LPL population are shown. (n) Quantitative mRNA expression of IEL developmental genes in ileal epithelial cells isolated from Hnf4γ–/– mice and their littermate controls. Data are pooled from two or three independent experiments. n represents biologically independent animals. Bars indicate mean ± SEM values. Statistical analysis was performed using a two-tailed Student’s t-test in a.
Extended Data Fig. 9 CLAs do not activate HNF4α.
(a) Protein-lipid overlay assay of in vitro translated HNF4α protein with CLAs or LA. (b–d) Dual-luciferase reporter assay of empty vector control (b), HNF4α (c), or HNF4γ (d) activation in HEK293T cells treated with various concentrations of CLAs. Data are representative of two independent experiments. n represents biologically independent replicates in b–d. Bars indicate mean ± SEM values. Statistical analysis was performed using a one-way analysis of variance followed by the Bonferroni post hoc test in d.
Extended Data Fig. 10 Ileal TCRβ+ IEL profiling of mice with IL-18 signalling abolition.
Representative plots or frequencies of cell populations from Il18–/– mice and their littermate controls, (a) ileal ThPOK+ in the TCRβ+CD4+CD8β− IELs, (b) ileal CD4+ in the TCRβ+ IELs, (c) ileal CD8αβ+ in the TCRβ+ IELs, (d) ileal CD8αα+ in the TCRβ+CD4−CD8β− IELs are shown. Representative plots or frequencies of cell populations from Il18r1–/– mice and their littermate controls, (e) ileal ThPOK+ in the TCRβ+CD4+CD8β− IELs, (f) ileal CD4+ in the TCRβ+ IELs, (g) ileal CD8αβ+ in the TCRβ+ IELs, (h) ileal CD8αα+ in the TCRβ+CD4−CD8β− IELs are shown. Data are pooled from three independent experiments. n represents biologically independent animals. Bars indicate mean ± SEM values. Statistical analysis was performed using a two-tailed Student’s t-test in a and e.
Supplementary information
Supplementary Figures
This file contains Supplementary Fig. 1, the flow cytometry gating strategy, and Supplementary Fig. 2, the uncropped blots.
Supplementary Table 1
Metabolomics for the nutrient-rich dient and the minimal diet.
Supplementary Table 2
Complete list of bacteria used in this study.
Supplementary Table 3
Complete list of real-time qPCR primers used in this study.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, X., Zhang, H., Zhang, Y. et al. Gut microbial fatty acid isomerization modulates intraepithelial T cells. Nature 619, 837–843 (2023). https://doi.org/10.1038/s41586-023-06265-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06265-4
This article is cited by
-
Leveraging circulating microbiome signatures to predict tumor immune microenvironment and prognosis of patients with non-small cell lung cancer
Journal of Translational Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.