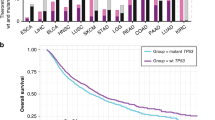
Abstract
Sex exerts a profound impact on cancer incidence, spectrum and outcomes, yet the molecular and genetic bases of such sex differences are ill-defined and presumptively ascribed to X-chromosome genes and sex hormones1. Such sex differences are particularly prominent in colorectal cancer (CRC) in which men experience higher metastases and mortality. A murine CRC model, engineered with an inducible transgene encoding oncogenic mutant KRASG12D and conditional null alleles of Apc and Trp53 tumour suppressors (designated iKAP)2, revealed higher metastases and worse outcomes specifically in males with oncogenic mutant KRAS (KRAS*) CRC. Integrated cross-species molecular and transcriptomic analyses identified Y-chromosome gene histone demethylase KDM5D as a transcriptionally upregulated gene driven by KRAS*-mediated activation of the STAT4 transcription factor. KDM5D-dependent chromatin mark and transcriptome changes showed repression of regulators of the epithelial cell tight junction and major histocompatibility complex class I complex components. Deletion of Kdm5d in iKAP cancer cells increased tight junction integrity, decreased cell invasiveness and enhanced cancer cell killing by CD8+ T cells. Conversely, iAP mice engineered with a Kdm5d transgene to provide constitutive Kdm5d expression specifically in iAP cancer cells showed an increased propensity for more invasive tumours in vivo. Thus, KRAS*-STAT4-mediated upregulation of Y chromosome KDM5D contributes substantially to the sex differences in KRAS* CRC by means of its disruption of cancer cell adhesion properties and tumour immunity, providing an actionable therapeutic strategy for metastasis risk reduction for men afflicted with KRAS* CRC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The sequencing data generated in this study were deposited in the National Center for Biotechnology Information (NCBI) Bioproject database under accession number PRJNA773740 and Gene Expression Omnibus (GEO) database under accession number GSE232459. TCGA data from patients with CRC, including RNA-seq, mutation and survival data, were downloaded from cBioPortal Colorectal Adenocarcinoma Firehose Legacy (n = 640) and the Human Protein Atlas (https://www.proteinatlas.org/). Integrated CRC patient data sets by CRCSC14 were downloaded from https://www.synapse.org (Synapse ID syn2623706), including information about patients such as sex, survival, mutation status and TNM (tumour, node, metastasis) stage. RNA-seq for iKAP mouse tumours (primary, metastatic, dox-on and -off) and cell lines (dox-on and -off) were previously performed and deposited in NCBI Sequence Read Archive (SRA) SRP097890. The Tsuji Colorectal Cancer RNA microarray data from primary and metastatic tumours were downloaded from Oncomine56 (https://www.oncomine.org, also deposited in GEO under GSE28702). CRC cell line RNA-seq and mutation data from CCLE were downloaded from Depmap portal (https://depmap.org/portal/ccle/). Peptide identification in immunoprecipitation-MS used the human protein database UniProt (https://www.uniprot.org/). Source data are provided with this paper.
References
Haupt, S., Caramia, F., Klein, S. L., Rubin, J. B. & Haupt, Y. Sex disparities matter in cancer development and therapy. Nat. Rev. Cancer 21, 393–407 (2021).
Boutin, A. T. et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 31, 370–382 (2017).
Rubin, J. B. et al. Sex differences in cancer mechanisms. Biol. Sex Differ. 11, 17 (2020).
Mervic, L. Time course and pattern of metastasis of cutaneous melanoma differ between men and women. PLoS ONE 7, e32955 (2012).
Kim, S. E. et al. Sex- and gender-specific disparities in colorectal cancer risk. World J. Gastroenterol. 21, 5167–5175 (2015).
Li, J., Ma, X., Chakravarti, D., Shalapour, S. & DePinho, R. A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 35, 787–820 (2021).
Wood, L. D. et al. The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113 (2007).
Liao, W. et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 35, 559–572.e7 (2019).
Sakai, E. et al. Combined mutation of Apc, Kras, and Tgfbr2 effectively drives metastasis of intestinal cancer. Cancer Res. 78, 1334–1346 (2018).
Blair, L. P., Cao, J., Zou, M. R., Sayegh, J. & Yan, Q. Epigenetic regulation by lysine demethylase 5 (KDM5) enzymes in cancer. Cancers 3, 1383–1404 (2011).
Li, H. T., Lu, Y. Y., An, Y. X., Wang, X. & Zhao, Q. C. KRAS, BRAF and PIK3CA mutations in human colorectal cancer: relationship with metastatic colorectal cancer. Oncol. Rep. 25, 1691–1697 (2011).
Modest, D. P. et al. Clinical characterization of patients with metastatic colorectal cancer depending on the KRAS status. Anticancer Drugs 22, 913–918 (2011).
Mannan, A. & Hahn-Stromberg, V. K-ras mutations are correlated to lymph node metastasis and tumor stage, but not to the growth pattern of colon carcinoma. Acata Pathol. Microbiol. Immunol. Scand. 120, 459–468 (2012).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394, 1467–1480 (2019).
Banerjee, S. et al. Comparative analysis of clonal evolution among patients with right- and left-sided colon and rectal cancer. iScience 24, 102718 (2021).
Hugen, N., van de Velde, C. J. H., de Wilt, J. H. W. & Nagtegaal, I. D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 25, 651–657 (2014).
Fornes, O. et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 48, D87–D92 (2020).
Visconti, R. et al. Importance of the MKK6/p38 pathway for interleukin-12-induced STAT4 serine phosphorylation and transcriptional activity. Blood 96, 1844–1852 (2000).
Mehra-Chaudhary, R., Matsui, H. & Raghow, R. Msx3 protein recruits histone deacetylase to down-regulate the Msx1 promoter. Biochem. J. 353, 13–22 (2001).
Gao, H. et al. VentX, a novel lymphoid-enhancing factor/T-cell factor-associated transcription repressor, is a putative tumor suppressor. Cancer Res. 70, 202–211 (2010).
Cobaleda, C., Perez-Caro, M., Vicente-Duenas, C. & Sanchez-Garcia, I. Function of the zinc-finger transcription factor SNAI2 in cancer and development. Annu. Rev. Genet. 41, 41–61 (2007).
Harrison, D. A. The Jak/STAT pathway. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a011205 (2012).
Palmer, D. C. & Restifo, N. P. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 30, 592–602 (2009).
Yi, C. et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell 19, 527–540 (2011).
Dusek, R. L. & Attardi, L. D. Desmosomes: new perpetrators in tumour suppression. Nat. Rev. Cancer 11, 317–323 (2011).
Bratt, A. et al. Angiomotin regulates endothelial cell-cell junctions and cell motility. J. Biol. Chem. 280, 34859–34869 (2005).
Zihni, C., Mills, C., Matter, K. & Balda, M. S. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 17, 564–580 (2016).
McNeil, E., Capaldo, C. T. & Macara, I. G. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 17, 1922–1932 (2006).
Tsukita, S., Yamazaki, Y., Katsuno, T., Tamura, A. & Tsukita, S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 27, 6930–6938 (2008).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Loven, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Mumbach, M. R. et al. HiChIP: efficient and sensitive analysis of protein-directed genome architecture. Nat. Methods 13, 919–922 (2016).
Morrison, B. J., Steel, J. C. & Morris, J. C. Reduction of MHC-I expression limits T-lymphocyte-mediated killing of cancer-initiating cells. BMC Cancer 18, 469 (2018).
Seliger, B., Maeurer, M. J. & Ferrone, S. Antigen-processing machinery breakdown and tumor growth. Immunol. Today 21, 455–464 (2000).
Lankat-Buttgereit, B. & Tampe, R. The transporter associated with antigen processing: function and implications in human diseases. Physiol. Rev. 82, 187–204 (2002).
Kaklamanis, L. et al. Loss of major histocompatibility complex-encoded transporter associated with antigen presentation (TAP) in colorectal cancer. Am. J. Pathol. 145, 505–509 (1994).
Barbosa, A. M. et al. Increased CD3(+), CD8(+), or FoxP3(+) T lymphocyte infiltrations are associated with the pathogenesis of colorectal cancer but not with the overall survival of patients. Biology 10, 808 (2021).
Mlecnik, B. et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl Med. 8, 327ra326 (2016).
Li, N. et al. ZMYND8 reads the dual histone mark H3K4me1-H3K14ac to antagonize the expression of metastasis-linked genes. Mol. Cell 63, 470–484 (2016).
Zhang, Y., Iratni, R., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell 89, 357–364 (1997).
Alland, L. et al. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387, 49–55 (1997).
Laherty, C. D. et al. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell 89, 349–356 (1997).
Martin, T. A. The role of tight junctions in cancer metastasis. Semin. Cell Dev. Biol. 36, 224–231 (2014).
Cromme, F. V. et al. Differences in MHC and TAP-1 expression in cervical cancer lymph node metastases as compared with the primary tumours. Br. J. Cancer 69, 1176–1181 (1994).
Garcia-Lora, A., Martinez, M., Algarra, I., Gaforio, J. J. & Garrido, F. MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components. Int. J. Cancer 106, 521–527 (2003).
Garrido, F., Aptsiauri, N., Doorduijn, E. M., Garcia Lora, A. M. & van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 39, 44–51 (2016).
Setiadi, A. F. et al. Epigenetic enhancement of antigen processing and presentation promotes immune recognition of tumors. Cancer Res. 68, 9601–9607 (2008).
Klose, R. J., Kallin, E. M. & Zhang, Y. JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 7, 715–727 (2006).
Li, N. et al. JARID1D is a suppressor and prognostic marker of prostate cancer invasion and metastasis. Cancer Res. 76, 831–843 (2016).
Heijmans, J. et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 3, 1128–1139 (2013).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Terranova, C. et al. An integrated platform for genome-wide mapping of chromatin states using high-throughput ChIP–sequencing in tumor tissues. J. Vis. Exp. https://doi.org/10.3791/56972 (2018).
Servant, N. et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 16, 259 (2015).
Budczies, J. et al. Cutoff Finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS ONE 7, e51862 (2012).
Rhodes, D. R. et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6, 1–6 (2004).
Acknowledgements
We thank L. Marasigan for assistance with maintenance of the mouse colonies and Y. Qi for supplying organoid culture medium. We thank D. Maru for help with tumour staging. We thank K. Rai, M. Curran, S. Kopetz, G. Lozano, J. Hu, Y. Ge, D. Yu and M. Barton for insightful suggestions and criticisms. We thank P. Chen, P. Hou, Y. Xia, P. Deng, K.-C. Chen and all members of the DePinho laboratory for helpful discussions and support. We thank D. Aten in the Department of Strategic Communications at The University of Texas MD Anderson Cancer Center, for the preparation of Extended Data Fig. 10. This study made use of MD Anderson’s Research Histology, Pathology and Imaging Core, the High-Resolution Electron Microscopy Facility and the Advanced Cytometry & Sorting Core Facility supported by the NIH Cancer Center Support grant no. P30 CA016672, the Advanced Technology Genomics Core supported by both grant nos. NIH P30 CA016672 and NIH 1S10OD024977-01, and the Advanced Microscopy Core supported by grant no. NIH S10 RR029552. We thank the University of Texas Southwestern Medical Center (UTSW) Proteomics Core Facility for conducting the immunoprecipitation-MS. J. Li was supported by the Cancer Prevention and Research Institute of Texas (CPRIT) Research Training Program (grant no. RP210028). W.L. was supported by the National Natural Science Foundation of China (grant nos. 81872401 and 82173289). K.A.L. was supported by the National Center for Advancing Translational Sciences of the NIH under award nos. TL1TR003169 and UL1TR003167 and the CPRIT Research Training Program (grant no. RP210028). W.-H.H. was supported by the CPRIT Research Training Program (grant no. RP210028) and NIH F99/K00 (grant no. CA274661-01). R.L. was supported by NIH T32 (grant no. 5T32CA186892-04). D.C. was supported by the Triumph postdoctoral training programme at MD Anderson supported by CPRIT (grant no. RP170067) and by pilot funding through the Digestive Disease Center-National Institute of Diabetes and Digestive and Kidney Diseases award (grant no. P30CA16672). Work in R.A.D.’s laboratory was supported by NIH R01 CA231360, CPRIT (grant no. RP220364), the MD Anderson SPORE in Gastrointestinal Cancer-DRP Award, and the Harry Graves Burkhart III Distinguished University Chair in Cancer Research.
Author information
Authors and Affiliations
Contributions
J. Li, Y.A.W. and R.A.D. designed the project and analysed the data. Z.L. established the iKAP primary and metastatic cell lines, conducted the in vivo study in nude mice and helped with the survival analysis in patients with CRC. W.L. helped with tumour staging. J.W.H. and X.X. generated the iAP-KDM5D mouse model. J. Liu helped with MHC-I detection, antigen presentation assay and T cell killing assay. Y.Y. helped with immunofluorescence, confocal imaging, paracellular permeability assay and the quantification of the tight junction integrity. S.J. maintained, monitored and recorded the health condition of the genetically engineered mice. H.S.S. helped with qPCR of FFPE tumour tissue, immunoprecipitation-MS and co-IP experiment. M.S. helped with IHC. K.A.L. helped with organoid culture and engineering. C.-J.W. analysed the RNA-seq data and performed GSEA. K.D. performed the transmission electron microscopy and helped with the quantification of the tight junction integrity. W.-H.H. established the iKAP tumour organoid lines and helped with animal surgeries. R.L. helped with the luciferase reporter assay. I.K. helped with the tumour staging. C.T. and K.A. helped with the HiChIP experiment and analysis. D.C. helped with organoid culture. X.S. helped with mouse genotyping. J.L. and R.A.D. wrote the manuscript. D.J.S. edited the manuscript and reviewed data.
Corresponding author
Ethics declarations
Competing interests
R.A.D. holds equity as a former advisor and/or director of Tvardi Therapeutics, Asylia Therapeutics, Stellanova Therapeutics and Sporos Bioventures. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Toshiro Sato and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 CRC sex differences in survival and metastasis are KRAS*-specific.
a, Stages of female and male iAP and iKAP tumours (two-tailed Chi-square test). b,c, Kaplan-Meier OS analysis for patients in CRCSC data set. d,e, Kaplan-Meier disease-free survival (DFS) analysis for patients in CRCSC data set. For survival analysis (b–e), log-rank (Mantel-Cox) test was used.
Extended Data Fig. 2 Comparisons of proximal and distal CRC in humans and mice.
a, Kaplan-Meier OS analysis for patients in CRCSC data set. b, Frequency of KRAS mutation in patients in CRCSC data set (two-tailed Binomial test). c, Subtypes of KRAS mutations in patients in CRCSC data set. d, Kaplan-Meier DFS analysis for patients with stage 1 or stage 2 cancers in CRCSC data set. e, Percentage of mutation combinations of KRAS (‘K’), APC (‘A’) and TP53 (‘P’) in TCGA CRC patients. f, Kaplan-Meier OS analysis for patients with indicated mutations in TCGA CRC data set. g, Stages of iKAP proximal tumours induced by injection of 4-OHT at proximal colon (two-tailed Chi-square test). For survival analysis (a,d,f), log-rank (Mantel-Cox) test was used.
Extended Data Fig. 3 KDM5D is upregulated in KRAS* driven metastasis.
a, qPCR of Kdm5d in male normal colonoids isolated from proximal (‘pro’) or distal (‘dis’) colon of healthy mice. Relative expression was calculated by normalizing to the ‘WT’ condition of each pair (n = 3 wells per group). b, Western blot confirming successful overexpression of KRASG12D and activation of KRAS signalling and STAT4 signalling in colonoids used in a. Phospho-STAT4 and total STAT4 levels were quantified by normalizing to Actin and ‘WT’ condition. The samples were derived from the same experiment and gel/blots were processed in parallel. c, Two-tailed Pearson correlation analysis of KRAS signalling score and KDM5D expression in TCGA male CRC patients. KRAS signalling score was calculated with GSVA using expression of all genes in TCGA RNA-seq data and KRAS signalling activation signature which was derived from differentially expressed genes in RNA-seq of iKAP tumours with dox on versus off (SRP097890) (n = 58 proximal CRC patients; n = 38 distal CRC patients). d, qPCR of Kdm5d in male normal colonoids with indicated genotypes (n = 3 wells per group). e, Western blot of colonoids with indicated genotypes in d. The samples were derived from the same experiment and gel/blots were processed in parallel. f, qPCR of Kdm5d in male normal colonoids. Relative expression was calculated by normalizing to ‘WT’ (n = 3 wells per group). g, qPCR of Kdm5d in 3346 and matched liver metastatic cell line L2-1 (independent of L2-3). Relative expression was calculated by normalizing to 3346 (n = 3 wells per group). h, qPCR of Kdm5d in organoids derived from male iKAP primary (‘Pri’) and matched spontaneous liver metastatic (‘Mets’) tumours. Relative expression was calculated by normalizing to ‘Pri’ (n = 3 wells per group). i, Representative images of in vitro invasion assays of organoids used in h to validate the increased mobility of metastatic cells compared to primary cells. j, Quantitation of i (n = 3 wells per group). k, Expression of KDM5D in human male CRC cell lines with KRAS mutations in CCLE database. l, Kaplan-Meier OS analysis for TCGA male CRC patients with high or low KDM5D expression (log-rank (Mantel-Cox) test). The optimal cutoff value of KDM5D expression was determined with Cutoff Finder application. m, Kdm5c expression (z score) in iKAP tumour RNA-seq data (SRP097890). n, KDM5C expression in TCGA CRC patient RNA-seq data. o, KDM5C expression in CRC patients in Tsuji Colorectal Cancer RNA microarray data set downloaded from Oncomine database. For box plots in m–o, centre lines denote medians; box limits denote 25th–75th percentile (Q1-Q3); whiskers are drawn up to the smallest or largest observed value that was still within 1.5 times the interquartile range below the first quartile or above the third quartile, respectively; all other observed points are plotted as outliers). All bar plots show mean value ± s.d.; except for correlation analysis (c) and survival analysis (l), P was derived with two-tailed unpaired t test; no adjustment for multiple comparisons.
Extended Data Fig. 4 Kdm5d deletion suppresses tumour cell metastasis.
a, qPCR of Kdm5d in L2-3 and two independent Kdm5d knockout cell lines (‘KO1’ and ‘KO2’) derived from L2-3. Relative expression was calculated by normalizing to ‘L2-3’ (n = 3 wells per group). b, Proliferation assay of 3346, L2-3 and KO1 (n = 3 wells per group; error bars indicate s.d.; two-tailed Mann-Whitney test; no adjustment for multiple comparisons). c, Representative images of migration and invasion assay. d, Quantitation of c (n = 3 wells per group). e, Growth curves of subcutaneous tumours in male nude mice (two-tailed Mann-Whitney test; no adjustment for multiple comparisons). f, Liver metastases detected by luminescence in male nude mice with intrasplenically injected male iKAP cells with a luciferase reporter (n = 5 mice per group). g, Quantitation of luminescence signals in f (n = 5 mice per group). h, Kaplan-Meier OS analysis of mice in f (n = 5 mice per group; log-rank (Mantel-Cox) test). i, Representative liver tissues with metastatic tumours from an independent experimental repeat of f–h (n = 3 livers per group). j, Number of gross surface metastasis in livers in i. Cells injected into spleen metastasize to liver through portal vein. Surrounding the blood vessel, the cells form initial liver metastasis, followed by whole liver spread. The formation of liver surface metastasis indicates higher metastatic potential of the cells and more aggressive disease. k, Percentage of histologically metastatic lesion area in all liver lobes in each mouse. l, Representative histology of liver metastasis (representing three tissues per group). m, qPCR of KDM5D in 2806, a cell line derived from female iKAP distal primary CRC, and 2806 with overexpression of WT or enzymatic-dead (‘ED’) human KDM5D. Relative expression was calculated by normalizing to ‘hKDM5DWT OE’ (n = 3 wells per group). n, Representative images of migration and invasion assays of cell lines in m. o, Quantitation of n (n = 3 wells per group). p, Liver metastases detected by luminescence in female nude mice with intrasplenically injected female iKAP cells with a luciferase reporter (n = 3 mice per group). q, Quantitation of luminescence signals in p (n = 3 mice per group, except for n = 2 mice for hKDM5DWT OE group at 27 days). r, Liver metastatic tumours from mice in p. For all bar/dot plots (a,d,g,j,k,m,o,q), data are shown as mean value ± s.d.; P was derived with two-tailed unpaired t test; no adjustment for multiple comparisons.
Extended Data Fig. 5 STAT4 activation correlates with KRAS signalling activation and KDM5D expression.
a, STAT4 motifs in mouse and human in indicated databases. b, Representative images of IHC of iKAP primary tumours (representing three individual tumours per group). c, Representative images of IHC of tissue microarrays (TMA) of male CRC patients’ primary tumours (representing results from 24 patients, as detailed in d). d, Pearson correlation analysis of H-scores of phospho-STAT4 and phospho-ERK in nucleus of epithelial cells in TMA of male CRC patients’ primary tumours (n = 24 patients, 9 with proximal CRC, 15 with distal CRC). e, ChIP-seq tracks of STAT4 IP and input around promoters of Socs1, Socs2, and Socs3 in iKAP cells cultured with dox. f, qPCR of L2-3 with WT or phosphomimetic STAT4 overexpression. Relative expression was calculated by normalizing to ‘L2-3’ (n = 3 wells per group; two-tailed unpaired t test; mean value ± s.d.; no adjustment for multiple comparisons). g, Pearson correlation analysis of STAT4, SOCS1, SOCS2, SOCS3 and KDM5D expression in TCGA male CRC patients. X- and Y-axis units are mRNA expression (RNA-seq V2 RSEM) (n = 229, 228, 230, 229 patients). h,i, Pearson correlation analysis of STAT4, SOCS2 and KDM5D expression in TCGA male CRC patients with proximal or distal primary tumours. X- and Y-axis units are mRNA expression (RNA-seq V2 RSEM) (for h, n = 101, 102 patients; for i, n = 115, 115 patients).
Extended Data Fig. 6 AMOT is downregulated in metastasis and negatively regulated by KDM5D.
a, Metaplot of H3K4me1 in iKAP cells. Average signal in the window of 5 kb up-/downstream of TSSs was used for two-tailed Mann-Whitney test. 3346 versus L2-3, P = 0.3899, L2-3 versus KO, P = 0.0269, 3346 versus KO, P = 0.0013. b, qPCR of 3346, L2-1 and L2-1 with Kdm5d knockout (L2-1 KO). Relative expression was calculated by normalizing to ‘3346’ (n = 3 wells per group). c, qPCR of human male metastatic CRC cells LoVo and two independent KDM5D knockout clones (‘KO1’ and ‘KO2’). Relative expression was calculated by normalizing to ‘LoVo’ (n = 3 wells per group). LoVo cell line has KRASG13D, APC loss of function mutations (nonsense mutation and frameshift deletion) and missense mutation, and wild-type TP53. d, qPCR of organoids from iKAP primary (‘Pri’) and matched spontaneous metastatic (‘Mets’) tumours, which were validated in Extended Data Fig. 3h–j. Relative expression was calculated by normalizing to ‘Pri’ (n = 3 wells per group). e, RNA microarray data of CRC primary and metastatic tumours from patients in Tsuji Colorectal Cancer data set in Oncomine (box plots, center lines denote medians; box limits denote 25th–75th percentile [Q1-Q3]; whiskers are drawn up to the smallest or largest observed value that was still within 1.5 times the interquartile range below the first quartile or above the third quartile, respectively; all other observed points are plotted as outliers). f, ChIP-seq tracks of H3K4me2/3 IP and input around Amot promoter in iKAP cells. g, Pearson correlation analysis of AMOT and KDM5D expression in TCGA CRC male patients. X- and Y-axis units are mRNA expression (RNA-seq V2 RSEM) (n = 228 patients). h, qPCR of female colonoids with indicated genotypes (‘WT’, wild type; ‘K’, KRASG12D overexpression in WT colonoids) and corresponding colonoids with mouse Kdm5d overexpression. Kdm5d relative expression was calculated by normalizing to ‘KDM5DOE’; Amot relative expression was calculated by normalizing to ‘Ctrl’ (n = 3 wells per group). i, Growth curves of subcutaneous tumours from L2-3 cell line and L2-3 with Amot overexpression in male nude mice (two-tailed Mann-Whitney test). j, Representative liver metastatic tumours in male nude mice with intrasplenically injected male iKAP cells. k, Number of gross surface metastasis in livers in j. l, Percentage of histologically metastatic lesion area in all liver lobes in each mouse in j. m, Representative histology of liver metastasis (representing five livers per group). n, Kaplan-Meier OS analysis of mice from an independent experimental repeat of j–m (log-rank (Mantel-Cox) test). o, Western blot of fractionated iKAP cell cytoplasmic membrane. NaK ATPase was used as the membrane-specific loading control. p,q, Paracellular permeability assay of iKAP cells. FITC-conjugated dextran with molecular weight of 2000 kDa was used (for p, n = 2 wells per group; for q, n = 4 wells per group). r, GSEA of iKAP cell RNA-seq. s, Expression of AMOT in human CRC cell lines with KRAS mutations in CCLE (box plots, center lines denote medians; box limits denote 25th–75th percentile [Q1-Q3]; whiskers are drawn up to the smallest or largest observed value that was still within 1.5 times the interquartile range below the first quartile or above the third quartile, respectively; all other observed points are plotted as outliers). All bar/dot plots (b–d,h,k,l,p,q) show mean value ± s.d. For b–e,h,k,l,p,q,s, P was derived with two-tailed unpaired t test; no adjustment for multiple comparisons.
Extended Data Fig. 7 KDM5D regulates H3K27ac and super-enhancer activity.
a,b, Heatmaps (a) and metaplots (b) of H3K27ac in iKAP cells. Average signal in the window of 5 kb up-/downstream TSSs was used to focus on the gene enhancers, and two-tailed Mann-Whitney test was performed. 3346 versus L2-3, P < 0.0001, L2-3 versus KO, P < 0.0001, 3346 versus KO, P < 0.0001. c, Putative super-enhancers ranked by H3K27ac signal in iKAP cells. ‘Super’ indicates super-enhancer, ‘Typical’ indicates typical enhancer. The super-enhancer predicted to regulate the MHC gene cluster was pointed out. d, The percentage of long and short-range interactions in iKAP cells detected by HiChIP analysis. e, Interaction map between and within chromosomes in iKAP cells detected by HiChIP. f, Top: ChIP-seq tracks of enhancer markers H3K27ac and H3K4me1 in the MHC gene cluster, super-enhancer region was highlighted in yellow; bottom: the interaction map generated by HiChIP analysis in iKAP cells. For e and f, red to white color range indicates more to less interaction.
Extended Data Fig. 8 TAP1/2 are regulated by KDM5D and are correlated with CRC metastasis and shorter survival.
a, qPCR of iKAP cells. Relative expression was calculated by normalizing to ‘3346’ (n = 3 wells per group). Fold changes between L2-3 and KO of all three genes were < 1.5, indicating no expression correlation between genes tested and Kdm5d. b, qPCR of iKAP cells. Relative expression was calculated by normalizing to ‘3346’ (n = 3 wells per group). c, qPCR of human male metastatic CRC cells LoVo and two independent KDM5D knockout clones (‘KO1’ and ‘KO2’). Relative expression was calculated by normalizing to ‘LoVo’ (n = 3 wells per group). d, qPCR of organoids from male iKAP primary (‘Pri’) and metastatic (‘Mets’) tumours. Relative expression was calculated by normalizing to ‘Pri organoids’ (n = 3 wells per group). e, qPCR of female colonoids of indicated genotypes and corresponding colonoids with mouse Kdm5d overexpression. Relative expression was calculated by normalizing to ‘Ctrl’ (n = 3 wells per group). f,g, RNA microarray data of TAP1 (f) and TAP2 (g) in CRC primary and metastatic tumours from patients in Tsuji Colorectal Cancer data set in Oncomine (box plots, center lines denote medians; box limits denote 25th–75th percentile [Q1-Q3]; whiskers are drawn up to the smallest or largest observed value that was still within 1.5 times the interquartile range below the first quartile or above the third quartile, respectively; all other observed points are plotted as outliers). h,i, Kaplan-Meier OS analysis of TCGA CRC patients with low and high expression of TAP1 (h) or TAP2 (i) (log-rank (Mantel-Cox) test). The optimal cutoff values of TAP1 and TAP2 expression were determined with Cutoff Finder application. All bar plots (a–e) show mean value ± s.d. For a–g, P was derived with two-tailed unpaired t test; no adjustment for multiple comparisons.
Extended Data Fig. 9 KDM5D interacts with Sin3-HDAC complex.
a,b, Western blot for co-IP experiment in HEK293T cells. ‘∅’ indicates control vectors. WT, wild type, ED, enzymatic dead. c, qPCR of 2806 cell line and 2806 with overexpression of WT or ED form of mouse Kdm5d. Kdm5d relative expression was calculated by normalizing to ‘2806 mKDM5DWT OE’; Amot, Tap1 and Tap2 relative expression was calculated by normalizing to ‘2806’ (n = 3 wells per group; two-tailed unpaired t test; mean value ± s.d.; no adjustment for multiple comparisons).
Supplementary information
Supplementary Figures
Supplementary Fig. 1 contains western blot raw data. Supplementary Fig. 2 contains flow cytometry gating strategies.
Supplementary Table 1
List of most dynamic putative super-enhancer regions.
Supplementary Table 2
Flag-KDM5D immunoprecipitation-MS targets.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Lan, Z., Liao, W. et al. Histone demethylase KDM5D upregulation drives sex differences in colon cancer. Nature 619, 632–639 (2023). https://doi.org/10.1038/s41586-023-06254-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06254-7
This article is cited by
-
High Oestrogen receptor alpha expression correlates with adverse prognosis and promotes metastasis in colorectal cancer
Cell Communication and Signaling (2024)
-
Survival outcome and prognostic factors for early-onset and late-onset metastatic colorectal cancer: a population based study from SEER database
Scientific Reports (2024)
-
Androgen drives melanoma invasiveness and metastatic spread by inducing tumorigenic fucosylation
Nature Communications (2024)
-
Deubiquitinase USP7 stabilizes KDM5B and promotes tumor progression and cisplatin resistance in nasopharyngeal carcinoma through the ZBTB16/TOP2A axis
Cell Death & Differentiation (2024)
-
Explaining the male bias in cancers
Nature Reviews Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.