Abstract
Chromothripsis, the shattering and imperfect reassembly of one (or a few) chromosome(s)1, is an ubiquitous2 mutational process generating localized and complex chromosomal rearrangements that drive genome evolution in cancer. Chromothripsis can be initiated by mis-segregation errors in mitosis3,4 or DNA metabolism5,6,7 that lead to entrapment of chromosomes within micronuclei and their subsequent fragmentation in the next interphase or following mitotic entry6,8,9,10. Here we use inducible degrons to demonstrate that chromothriptically produced pieces of a micronucleated chromosome are tethered together in mitosis by a protein complex consisting of mediator of DNA damage checkpoint 1 (MDC1), DNA topoisomerase II-binding protein 1 (TOPBP1) and cellular inhibitor of PP2A (CIP2A), thereby enabling en masse segregation to the same daughter cell. Such tethering is shown to be crucial for the viability of cells undergoing chromosome mis-segregation and shattering after transient inactivation of the spindle assembly checkpoint. Transient, degron-induced reduction in CIP2A following chromosome micronucleation-dependent chromosome shattering is shown to drive acquisition of segmental deletions and inversions. Analyses of pancancer tumour genomes showed that expression of CIP2A and TOPBP1 was increased overall in cancers with genomic rearrangements, including copy number-neutral chromothripsis with minimal deletions, but comparatively reduced in cancers with canonical chromothripsis in which deletions were frequent. Thus, chromatin-bound tethers maintain the proximity of fragments of a shattered chromosome enabling their re-encapsulation into, and religation within, a daughter cell nucleus to form heritable, chromothriptically rearranged chromosomes found in the majority of human cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Whole-genome sequencing data (FASTA files) from RPE1 cell lines generated for this study are available through SRA with accession no. PRJNA961206. Gene expression data from TCGA used in this study were downloaded from a publicly available repository (https://portal.gdc.cancer.gov). Processed copy number profiles from SNP6 arrays for TCGA were downloaded from https://github.com/VanLoo-lab/ascat/tree/master/ReleasedData/TCGA_SNP6_hg19. Chromothripsis was called from whole-genome-sequenced cancers using the ShatterSeek algorithm by the PCAWG consortium and downloaded from https://dcc.icgc.org/releases/PCAWG/evolution_and_heterogeneity/clustered_mut_processes. Source data are provided with this paper.
Code availability
No custom algorithms were developed for the bioinformatic analysis. Data analysis code is available on request. All source data for graphical representations are provided with this paper.
References
Stephens, P. J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Cortes-Ciriano, I. et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat. Genet. 52, 331–341 (2020).
Zhang, C. Z. et al. Chromothripsis from DNA damage in micronuclei. Nature 522, 179–184 (2015).
Ly, P. et al. Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nat. Genet. 51, 705–715 (2019).
Maciejowski, J. et al. APOBEC3-dependent kataegis and TREX1-driven chromothripsis during telomere crisis. Nat. Genet. 52, 884–890 (2020).
Maciejowski, J., Li, Y., Bosco, N., Campbell, P. J. & de Lange, T. Chromothripsis and kataegis induced by telomere crisis. Cell 163, 1641–1654 (2015).
Umbreit, N. T. et al. Mechanisms generating cancer genome complexity from a single cell division error. Science 368, eaba0712 (2020).
Ly, P. et al. Selective Y centromere inactivation triggers chromosome shattering in micronuclei and repair by non-homologous end joining. Nat. Cell Biol. 19, 68–75 (2017).
Crasta, K. et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature 482, 53–58 (2012).
Kato, H. & Sandberg, A. A. Chromosome pulverization in human cells with micronuclei. J. Natl Cancer Inst. 40, 165–179 (1968).
Bakhoum, S. F. & Cantley, L. C. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell 174, 1347–1360 (2018).
Shoshani, O. et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature Genet. 52, 331–341 (2020).
Kloosterman, W. P. et al. Chromothripsis is a common mechanism driving genomic rearrangements in primary and metastatic colorectal cancer. Genome Biol. 12, R103 (2011).
Molenaar, J. J. et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483, 589–593 (2012).
Teles Alves, I. et al. Gene fusions by chromothripsis of chromosome 5q in the VCaP prostate cancer cell line. Hum. Genet. 132, 709–713 (2013).
Ly, P. & Cleveland, D. W. Rebuilding chromosomes after catastrophe: emerging mechanisms of chromothripsis. Trends Cell. Biol. 27, 917–930 (2017).
Tang, S., Stokasimov, E., Cui, Y. & Pellman, D. Breakage of cytoplasmic chromosomes by pathological DNA base excision repair. Nature 606, 930–936 (2022).
Stucki, M. et al. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123, 1213–1226 (2005).
Clouaire, T. et al. Comprehensive mapping of histone modifications at DNA double-strand breaks deciphers repair pathway chromatin signatures. Mol. Cell 72, 250–262 (2018).
Iacovoni, J. S. et al. High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 29, 1446–1457 (2010).
Soto, M. et al. p53 Prohibits propagation of chromosome segregation errors that produce structural aneuploidies. Cell Rep. 19, 2423–2431 (2017).
Santaguida, S. et al. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev. Cell 41, 638–651 (2017).
Hatch, E. M. & Hetzer, M. W. Linking micronuclei to chromosome fragmentation. Cell 161, 1502–1504 (2015).
Minocherhomji, S. et al. Replication stress activates DNA repair synthesis in mitosis. Nature 528, 286–290 (2015).
Lobachev, K., Vitriol, E., Stemple, J., Resnick, M. A. & Bloom, K. Chromosome fragmentation after induction of a double-strand break is an active process prevented by the RMX repair complex. Curr. Biol. 14, 2107–2112 (2004).
Kaye, J. A. et al. DNA breaks promote genomic instability by impeding proper chromosome segregation. Curr. Biol. 14, 2096–2106 (2004).
Clay, D. E., Bretscher, H. S., Jezuit, E. A., Bush, K. B. & Fox, D. T. Persistent DNA damage signaling and DNA polymerase theta promote broken chromosome segregation. J. Cell Biol. 220, e202106116 (2021).
de Jager, M. et al. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol. Cell 8, 1129–1135 (2001).
De Marco Zompit, M. et al. The CIP2A-TOPBP1 complex safeguards chromosomal stability during mitosis. Nat. Commun. 13, 4143 (2022).
Leimbacher, P. A. et al. MDC1 interacts with TOPBP1 to maintain chromosomal stability during mitosis. Mol. Cell 74, 571–583 (2019).
Adam, S. et al. The CIP2A-TOPBP1 axis safeguards chromosome stability and is a synthetic lethal target for BRCA-mutated cancer. Nat. Cancer 2, 1357–1371 (2021).
Laine, A. et al. CIP2A interacts with TopBP1 and drives basal-like breast cancer tumorigenesis. Cancer Res. 81, 4319–4331 (2021).
Wardlaw, C. P., Carr, A. M. & Oliver, A. W. TopBP1: a BRCT-scaffold protein functioning in multiple cellular pathways. DNA Repair (Amst.) 22, 165–174 (2014).
Nabet, B. et al. The dTAG system for immediate and target-specific protein degradation. Nat. Chem. Biol. 14, 431–441 (2018).
Kim, J. E., McAvoy, S. A., Smith, D. I. & Chen, J. Human TopBP1 ensures genome integrity during normal S phase. Mol. Cell. Biol. 25, 10907–10915 (2005).
Bagge, J., Oestergaard, V. H. & Lisby, M. Functions of TopBP1 in preserving genome integrity during mitosis. Semin. Cell Dev. Biol. 113, 57–64 (2021).
Gallina, I., Christiansen, S. K., Pedersen, R. T., Lisby, M. & Oestergaard, V. H. TopBP1-mediated DNA processing during mitosis. Cell Cycle 15, 176–183 (2016).
Pedersen, R. T., Kruse, T., Nilsson, J., Oestergaard, V. H. & Lisby, M. TopBP1 is required at mitosis to reduce transmission of DNA damage to G1 daughter cells. J. Cell Biol. 210, 565–582 (2015).
Junttila, M. R. et al. CIP2A inhibits PP2A in human malignancies. Cell 130, 51–62 (2007).
Hoadley, K. A. et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173, 291–304 (2018).
Yang, J. et al. CTLPScanner: a web server for chromothripsis-like pattern detection. Nucleic Acids Res. 44, W252–W258 (2016).
ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 578, 82–93 (2020).
Steele, C. D. et al. Signatures of copy number alterations in human cancer. Nature 606, 984–991 (2022).
Groelly, F. J., Fawkes, M., Dagg, R. A., Blackford, A. N. & Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 23, 78–94 (2023).
Papathanasiou, S. et al. Transgenerational transcriptional heterogeneity from cytoplasmic chromatin. Preprint at bioRxivhttps://doi.org/10.1101/2022.01.12.475869 (2022).
Frattini, C. et al. TopBP1 assembles nuclear condensates to switch on ATR signaling. Mol. Cell 81, 1231–1245 (2021).
Kim, A. et al. Biochemical analysis of TOPBP1 oligomerization. DNA Repair (Amst.) 96, 102973 (2020).
Korbel, J. O. & Campbell, P. J. Criteria for inference of chromothripsis in cancer genomes. Cell 152, 1226–1236 (2013).
Khanna, A. & Pimanda, J. E. Clinical significance of cancerous inhibitor of protein phosphatase 2A in human cancers. Int. J. Cancer 138, 525–532 (2016).
Knijnenburg, T. A. et al. Genomic and molecular landscape of DNA damage repair deficiency across The Cancer Genome Atlas. Cell Rep. 23, 239–254 (2018).
Acknowledgements
This work was financially supported by grants from the US National Institutes of Health (nos. R35 GM122476 to D.W.C. and R01ES030993-01A1, R01ES032547 and R01CA269919 to L.B.A.). P.T. is supported by a postdoctoral fellowship from the Hope Funds for Cancer Research (no. HFCR 21-04-02). L.B.A. is supported by a Packard Fellowship for Science and Engineering. The computational analyses reported in this manuscript utilized the Triton Shared Computing Cluster at the San Diego Supercomputer Center of UC San Diego. We thank M. Stucki, D. Pellman and S. Jackson for sharing reagents.
Author information
Authors and Affiliations
Contributions
P.T. designed and carried out experiments. P.T. and D.W.C. analysed data. D.W.C. oversaw the study. C.D.S. carried out all bioinformatics and genomics analyses. L.B.A. oversaw bioinformatics and genomics analyses. F.K.C.A. carried out analysis in Extended Data Fig. 10e. P.T. and D.W.C. wrote the manuscript, with input from C.D.S. and L.B.A.
Corresponding author
Ethics declarations
Competing interests
L.B.A. is a compensated consultant and has equity interest in io9, LLC. His spouse is an employee of Biotheranostics, Inc. L.B.A. is an inventor of US Patent no. 10,776,718 and he also declares US provisional applications with serial nos. 63/289,601, 63/269,033, 63/366,392 and 63/367,846. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Daniel Durocher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
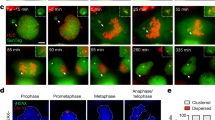
Extended Data Fig. 1 Fragmentation of Y-chromosome after micronucleation.
(A) Quantitation of chromosomal fragment tethering for RPE1 WT vs p53−/− cells (similar experimental setup as used in Fig. 1c for Mps1i condition) (n = 9 independent experiments, total 498 and 861 cells were analyzed for RPE1 WT and RPE1 p53−/− conditions, respectively). Two-tailed unpaired t-test was applied, P = 0.0009. (B) Experimental schematic for (C) and (D). (C) Representative images of Y-chromosome in chromosome spreads from experiment outlined in (B). Yellow arrows point to Y-chromosome fragments. (D) Quantitation of fragmented Y-chromosome from experiments outlined in (B) (n = 3 independent experiments, total 203 cells were analyzed). For (A) and (D), mean +/− SEM are shown.
Extended Data Fig. 2 Micronuclear envelopes do not persist in mitosis and PolD3 does not mediate clustering of broken chromosome fragments in mitosis.
Representative image showing (A) Lamin A/C or (B) Lamin B1 staining on damaged and clustered Y-chromosome in mitosis (for A(A) and B (B), 2 independent experiments were performed). (C) Schematic for experiment in (D), (E), and (F). (D) Immunoblots showing depletion of PolD3 and Lig4 from experiment described in (C) (uncropped blots in Supplementary Fig. 1). (E) Quantitation of damaged Y-chromosome for experiment shown in (C). (F) Quantitation of fragment dispersal of a damaged Y-chromosome for experiment shown in (C) (for (E) and (F), n = 3 independent experiments; total 651, 555, and 637 cells were analyzed for control, siPolD3, and siLig4 conditions, respectively). (One-way analysis of variance with Bonferroni’s multiple comparison test was applied, ** P < 0.001 and ns P > 0.05.) (G) Schematic of experimental setup for (H), (I), and (J). (H) Images of CEN-select colony formation assay from experiment outlines in (G). (I,J) Quantitation of clonogenic growth from the experiment outlined in (G) and shown in (H) (for (I) and (J), n = 3 independent experiments were analyzed; one-way analysis of variance with Bonferroni’s multiple comparison test was applied, ** P < 0.001 and ns P > 0.05). For (E), (F), (I), and (G) the graphs mean +/− SEM are shown.
Extended Data Fig. 3 Neither the MRN complex nor PolQ mediates clustering of broken chromosome fragments in mitosis and micronuclei are defective in recruitment of DNA repair proteins.
Representative images showing lack of recruitment to damaged micronuclei of (A) 53BP1 or (B) BRCA1 (for (A) and (B), 2 independent experiments were performed). (C) Schematic of experimental setup for (D-G). (D) Immunoblot showing depletion of Rad50 and Mre11 for the experiment described in (C) (uncropped blots in Supplementary Fig. 1). Quantitation of (E) dispersed Y-chromosome fragments or (F) damaged Y chromosomes from the experiments outlined in (C) after siRNA depletion of Mre11 or Rad50. (For (E) and (F), n = 3 independent experiments; total 726, 742, and 727 cells were analyzed for control, siMre11, and siRad50 conditions, respectively. One-way analysis of variance with Bonferroni’s multiple comparison test was applied, ns P > 0.05.) (G) Quantitation of damaged Y-chromosomes from the experiment outlined in (C) upon siRNA depletion of PolQ (n = 3 independent experiments; total 240 and 217 cells were analyzed for control and PolQ depletion, respectively). (Two-tailed unpaired t-test was applied for data shown in (G); ns P > 0.05.). For (E), (F), and (G) mean +/− SEM are shown.
Extended Data Fig. 4 Localization of MDC1, TOPBP1, and CIP2A to micronuclei.
Representative images showing localization of (A,D) MDC1, (B,E) TOPBP1, and (C,F) CIP2A in micronuclei of DLD1 (n = 3 independent experiments, total 482, 290, and 319 DLD1 cells were analyzed for MDC1, TOPBP1, and CIP2A, respectively) and RPE p53−/− cells (n = 3 independent experiments, total 315, 360, and 299 RPE p53−/− cells were analyzed for MDC1, TOPBP1, and CIP2A, respectively) (percentage of cells (mean +/− SEM) with γH2AX positive micronuclei showing the phenotype in the images are indicated on the images). Yellow arrows point to the micronuclei.
Extended Data Fig. 5 Localization of MDC1, TOPBP1, and CIP2A to a broken chromatin bridge and micronuclear envelope integrity or p53 presence is not a major determinant of localization of MDC1, TOPBP1, and CIP2A to micronuclei.
Representative images showing localization of Lamin B1 and (A)γH2AX, (D) MDC1, (C) TOPBP1, and (D) CIP2A in micronuclei of RPE1 p53−/−. (E) Quantitation of representative phenotypes shown in (A-D) (n = 3 independent experiments, total 287, 307, 309, and 256 cells were analyzed for γH2AX, MDC1, TOPBP1, and CIP2A, respectively) (mean +/− SEM are shown). One-way analysis of variance with Bonferroni’s multiple comparison test was applied, *** P < 0.0001. (F) Cropped frames of Sup. Videos 3 showing localization of TOPBP1Clover to the micronuclear chromosome which had nuclear envelope ruptured before entry into mitosis. From 2 independent experiments, a total of 60 mitotic chromosomes previously within micronuclei were identified. In all cases, TOPBP1Clover recruitment occurred at the onset of mitosis but did not have detectable 3XNLSmScarlet before mitosis entry. Representative images showing localization of (G) MDC1, (H) TOPBP1, and (I) CIP2A on broken micronuclear chromosome in mitosis in RPE1 WT and RPE1 p53−/− cells, same experimental setup as outline is Fig. 2c was used (for G, H, and I, 2 independent experiments were performed). Representative images showing localization of (J) MDC1, (K) TOPBP1, and (L) CIP2A on the remnant of a broken chromatin bridge induced by topoisomerase inhibition in RPE1 p53−/− cells (n = 3 independent experiments, a total of 161, 192, and 165 cells were measured for MDC1, TOPBP1, and CIP2A staining, respectively (percentage of cells (mean +/− SEM) with phenotype shown are indicated on the images). Yellow arrow points to the remnant of a broken bridge. (M) Cropped Frames of Sup. Videos 4 showing recruitment of TOPBP1Clover to the base of the chromatin bridge in interphase.
Extended Data Fig. 7 Role of MDC1, TOPBP1, and CIP2A in repair of a damaged micronuclear chromosome.
(A) Immunoblot showing depletion of TOPBP1, CIP2A, and MDC1 from the experiment shown in Fig. 3a–c (uncropped blots in Supplementary Fig. 1). (B) Quantitation of damaged Y-chromosomes (tethered or dispersed) for the experiment outlined in Fig. 3a (n = 3 independent experiments; number of cells analyzed is the same as in Fig. 3c). (C) Experimental outline for experiment shown in (D). (D) Quantitation of cells with dispersed micronuclear chromosome fragments in mitosis from the experiments outlined in (C). n = 3 independent experiments; total 447, 345, and 385 cells were analyzed for control, MDC1−/−, and CIP2A−/− conditions, respectively. For both (B) and (D) One-way analysis of variance with Bonferroni’s multiple comparison test was applied, ns P > 0.05, *** P < 0.0001. For (B) and (D) mean +/− SEM are shown.
Extended Data Fig 8 Role of TOPBP1 and CIP2A in tethering fragments of damaged chromosome resulting from micronuclei or chromatin bridge during mitosis.
Schematic of (A) TOPBP1dTag and (B) dTagCIP2A tagging at genomic loci and an agarose gel image showing successful bi-allelic tagging. (C) Immunoblot showing dynamics of TOPBP1dTag degradation upon addition of dTAG-13 (for (A), (B), and (C), the experiment was performed once). (D) Quantitation of TOPBP1dTag levels from (B). Data were fitted with single exponential decay curve, half-life 14 min. (E) Immunoblot showing depletion of TOPBP1dTag upon addition of dTAG-13 from the experiment outlined in Fig. 3d (two independent repeats were performed). (F) Experimental outline for (G) and (H). (G) Quantitation of cells with dispersed micronuclear Y-fragments in mitosis upon TOPBP1dTag degradation in G2. (H) Quantitation of cells with damaged Y-chromosome fragments (tethered or dispersed) in mitosis after degradation of TOPBP1dTag in G2 (for (G) and (H), n = 3 independent experiments; total 693 and 672 cells were analyzed for control and TOPBP1dTag degradation conditions, respectively). (I) Quantitation of cells with damaged Y-fragments (tethered or dispersed) in mitosis upon degradation of TOPBP1dTag in mitosis for the experiment in Fig. 3d (the number of cells analyzed is the same as in Fig. 3e). For (G-I), mean +/− SEM are shown and two-tailed unpaired t-test was applied; *** P < 0.0001 (for G P = 0.0011) and ns P > 0.05. (J) Immunoblot showing degradation of dTagCIP2A and TOPBP1dTag for the experiment outlined in Fig. 3f (immunoblot was performed for 2 of the 3 independent repeats). (for (C), (E), and (J), uncropped blots in Supplementary Fig. 1) (K) Experimental outline for (L) and (M). (L) Representative images of RPE p53−/− cells showing tethered and dispersed damaged micronuclear fragments in mitosis after induction of chromatin bridge by inhibition of Topoisomerase II. (M) Quantitation of cells with dispersed micronuclear fragments in mitosis following chromatin bridge induction upon degradation of TOPBP1dTag or dTagCIP2A (n = 3 independent experiments; total 420, 518, and 396 cells were analyzed for control, TOPBP1dTag degradation, and dTagCIP2A degradation conditions, respectively). (Mean +/− SEM are shown and one-way analysis of variance with Bonferroni’s multiple comparison test was applied, *** P < 0.0001.).
Extended Data Fig. 9 PP2A inhibition by CIP2A is not required for tethering damaged micronuclear chromosomal fragments during mitosis.
(A) Experimental outline for the experiment shown in (B) and (C). (B) Representative images of RPE p53−/− cells showing tethered and dispersed damaged micronuclear fragments in mitosis upon the indicated treatment(s). (C) Quantitation of cells with dispersed micronuclear fragments in mitosis following micronuclei induction upon degradation of dTagCIP2A (n = 3 independent experiments; total 361, 281, 351, and 364 cells were analyzed for control, okadaic acid treated, dTagCIP2A degradation, and dTagCIP2A degradation with okadaic acid treated conditions, respectively). (Mean +/− SEM are shown and one-way analysis of variance with Bonferroni’s multiple comparison test was applied, *** P < 0.0001 and ns P > 0.05).
Extended Data Fig. 10 A complex of MDC1-TOPBP1-CIP2A recruited at γH2AX containing chromatin is the major mediator of tethering of chromosomal fragments generated from micronuclei or chromatin bridge during mitosis.
(A) Immunoblot showing degradation of dTagCIP2A and TOPBP1dTag and expression of various TOPBP1 mutants (uncropped blots in Supplementary Fig. 1). Quantitation of cells with dispersed micronuclear fragments in mitosis following induction of (B) micronuclei or (C) chromatin bridge in indicated condition (n = 3 independent experiments; a total 559, 608, 516, 530, 558, and 559 cells were analyzed for control, TOPBP1dTag degradation, TOPBP1WT, TOPBP1Δ756–891, TOPBP1K155,250E, and TOPBP1K155,250E; Δ756–891 conditions, respectively for (B); a total 302, 458, 190, 435, 456, and 301 cells were analyzed for control, TOPBP1dTag degradation, TOPBP1WT, TOPBP1Δ756–891, TOPBP1K155,250E, and TOPBP1K155,250E; Δ756–891 conditions, respectively for (C)). (One-way analysis of variance with Bonferroni’s multiple comparison test was applied, ns P > 0.05 *** P < 0.0001.) Mean +/− SEM is shown on the graph. (D) Representative images showing localization of TOPBP1 on shattered chromosomal fragments in mitosis in RPE1 WT, MDC1−/−, and CIP2A−/− cells. (E) Graph showing TOPBP1 intensity normalized to γH2AX intensity in the indicated conditions (n = 27, 20, and 20 cells were analyzed for WT, MDC1−/−, and CIP2A−/− condition, respectively; a total of 113, 107, and 191 foci were measured for WT, MDC1−/−, and CIP2A−/− condition respectively). One-way analysis of variance with Bonferroni’s multiple comparison test was applied, ** P = 0.0035, *** P < 0.0001. (F) Representative images showing localization of TOPBP1 on shattered chromosomal fragments in mitosis in RPE1 WT and H2AXS139A/S139A cells. (G) Graph showing percentage of cells showing TOPBP1 positive chromosome in mitosis following micronucleation (for (G) and (J) n = 3 independent experiments, a total of 364 and 419 cells for WT and H2AXS139A/S139A condition, respectively were measured). Two-tailed unpaired t-test was applied for (G) P = 0.0039, (H), and (J) P = 0.0009, ** P > 0.001 *** P < 0.0001. (H) Graph showing TOPBP1 intensity in the indicated conditions (n = 20 cells were analyzed for WT and H2AXS139A/S139A condition, respectively; a total of 76, and 74 foci were measured for WT and H2AXS139A/S139A condition, respectively). (I) Representative images showing mitotic cell with presence or absence of dispersed chromosome fragments. (J) Graph showing percent mitotic cells with dispersed chromatin fragments in WT vs H2AXS139A/S139A cells. (We note that this is likely to be substantially underestimated since without γH2AX staining to mark chromosome fragmentation, the only available assay is observable DAPI-stained fragments found away from the bulk chromosome mass.) Mean +/− SEM is shown on the graph.
Extended Data Fig. 11 Asymmetric fragment segregation to daughter cells and fragment tethering requirement for cell viability after micronucleation.
(A) Graph showing percent distribution of fragments between daughter cells in indicated condition from experiment shown in Fig. 4b–d. Percent distribution of fragments between daughter cells was calculated by dividing the number of fragments from the daughter that inherited the maximum number of fragments with the total number of fragments in both daughters and the resulting ratio was multiplied by 100. (B) Graph showing total number of fragments in both daughter cells for the indicated condition from experiment shown in Fig. 4b–d (for (A) and (B) n = 105, 107, and 107 daughter pairs for Control, dTagCIP2A, and TOPBP1dTag degradation condition, respectively were analyzed from one of the repeats of Fig. 4d). For (A) and (B), Individual data points are shown in open red circles, median is shown as solid black line, and dashed black line shows 25th and 75th quartile. (C) Representative images of colony formed for indicated conditions. Same experimental setup as outlined in Fig. 4e was used. (D) Quantitation of cell growth for the experiment in (C) (n = 3 independent experiments), mean +/− SEM are shown. For (A), (B) and (D), One-way analysis of variance with Bonferroni’s multiple comparison test was applied, ns P > 0.05, *** P < 0.0001.
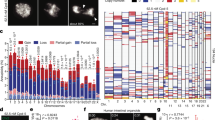
Extended Data Fig. 12 High expression of MDC1, TOPBP1, and CIP2A in tumors with a chromothriptically rearranged chromosome.
(A) Associations between gene expression (log(TPM+1)) of TOPBP1 (x-axis) and CIP2A (y-axis) as well as chromothriptic designation of samples (color) for 7,856 tumor samples from The Cancer Genome Atlas (TCGA). Main panel – scatter plot of TOPBP1 and CIP2A expression. Top panel – density of TOPBP1 expression in chromothriptic (orange) and non-chromothriptic samples (blue). Right panel – density of CIP2A expression in chromothriptic and non-chromothriptic samples. Difference in expression between chromothriptic and non-chromothriptic samples is tested with a two-sided Mann-Whitney test; ***p < 0.001 (for TOPBP1 p = 0, for CIP2A p = 2.5e-14). Chromothripsis is defined by CTLPscanner from SNP-array derived copy number profiles. (B) Associations between gene expression (log(TPM+1)) of TOPBP1 (x-axis) and MDC1 (y-axis) as well as chromothriptic designation of samples (color) for 7,856 tumor samples from The Cancer Genome Atlas (TCGA). Main panel – scatter plot of TOPBP1 and MDC1 expression. Top panel – density of TOPBP1 expression in chromothriptic (orange) and non-chromothriptic samples (blue). Right panel – density of MDC1 expression in chromothriptic and non-chromothriptic samples. Difference in expression between chromothriptic and non-chromothriptic samples is tested with a two-sided Mann-Whitney test; ***p < 0.001 (for TOPBP1 p = 9.3e-57, for MDC1 p = 1.1e-16). Chromothripsis is defined by CTLPscanner from SNP-array derived copy number profiles. (C) Associations between gene expression (log(TPM+1)) of MDC1 (x-axis) and CIP2A (y-axis) as well as chromothriptic designation of samples (color) for 7,856 tumor samples from The Cancer Genome Atlas (TCGA). Main panel – scatter plot of MDC1 and CIP2A expression. Top panel – density of MDC1 expression in chromothriptic (orange) and non-chromothriptic samples (blue). Right panel – density of CIP2A expression in chromothriptic and non-chromothriptic samples. Difference in expression between chromothriptic and non-chromothriptic samples is tested with a two-sided Mann-Whitney test; ***p < 0.001 (for MDC1 p = 2.0e-267, for CIP2A p = 2.5e-14). Chromothripsis is defined by CTLPscanner from SNP-array derived copy number profiles. (D) Associations between gene expression (log(TPM+1)) of TOPBP1 (x-axis) and CIP2A (y-axis) as well as chromothriptic designation of samples (color) for 667 tumor samples from The Cancer Genome Atlas (TCGA) that overlap with Pan-cancer analysis of Whole Genomes (PCAWG) samples. Main panel – scatter plot of TOPBP1 and CIP2A expression. Top panel – density of TOPBP1 expression in chromothriptic (orange) and non-chromothriptic samples (blue). Right panel – density of CIP2A expression in chromothriptic and non-chromothriptic samples. Difference in expression between chromothriptic and non-chromothriptic samples is tested with a two-sided Mann-Whitney test; *p < 0.05, ***p < 0.001 (for TOPBP1 p = 0.031, for CIP2A p = 3.0e-4). Chromothripsis is defined by shatterSeek from WGS-derived copy number profiles. Log refers to natural log unless specified. (E) Gene expression of TOPBP1 and CIP2A (log(TPM+1)) in 135 chromothriptic tumor samples from The Cancer Genome Atlas (TCGA) that overlap with Pan-cancer Analysis of Whole Genomes (PCAWG) samples. Samples are categorized as joint upper (red), middle (purple) or lower (blue) expression of both genes by bisecting the line of best fit with a decision boundary at the upper quartile or lower quartiles of each gene (green dotted lines). Light grey dotted lines denote median values of gene expression, black dotted line indicates the bisecting line of the median. TPM = transcripts per million. Chromothriptic samples were determined by shatterSeek. (F) Associations between gene expression groups defined in (E) (y-axis), and chromothripsis associated copy number signatures (x-axis; CN4:9), using two-sided Fisher’s exact tests. Correction for multiple testing was performed using the Benjamini-Hochberg method. Color denotes odds ratio, size of points indicates the significance of the test. ***p < 0.001. OR = odds ratio. CN7 is a signature associated with chromothripsis amplification. (G) Central coefficient estimates (circles) and 95% confidence intervals (horizontal lines) from linear regression models with the categorized number of segments and chromothriptic status (CTLPscanner) as predictor variables, and TOPBP1 (top), CIP2A (middle) and MDC1 (bottom) gene expression as response variables. Squares denote reference groups. X-axis = effect size of variables on gene expression. *** = p < 0.001, two-sided Wald test, n = 7,856. (H) Difference in gene expression (x-axis, log(TMP+1)) between chromothriptic and non-chromothriptic cancer samples from The Cancer Genome Atlas, as designated by CTLPscanner for 266 DNA repair genes (Knijnenburg et al., 2018). y-axis = significance (−log2(Q values), two-sided Mann-Whitney test). Genes labelled in red are genes of interest. Genes with >0.1 difference in mean expression and q < 0.05 are considered enriched in chromothriptic samples (orange area). Q values are corrected for multiple testing using the Benjamini-Hochberg method. (I) Pearson’s correlation coefficient between all pairwise comparisons of gene pairs from 266 DNA repair genes (Knijnenburg et al., 2018) calculated in chromothriptic (x-axis) and non-chromothriptic (y-axis) samples separately, as defined by CTLPscanner. Red = gene pair involving two of TOPBP1, CIP2A or MDC1. Grey: all other gene pairs. Black dotted line: x = y. Log refers to natural log unless specified.
Extended Data Fig. 13 Model for chromothriptic chromosome formation.
Chromosomes within micronuclei (that were formed as a result of errors in mitosis) are fragmented before or upon entry into mitosis. The chromosomal fragments (γH2AX positive or γH2AX negative) are tethered during mitosis by combined action of MDC1-TOPBP1-CIP2A or TOPBP1-CIP2A. Tethering of chromosomal fragments during mitosis ensures their transfer to the same daughter nucleus for subsequent ligation in random order, thereby resulting in reassembly of a heritable, highly rearranged chromosome.
Supplementary information
Supplementary Figure 1
Uncropped immunoblots.
Supplementary Video 1
MDC1 localization to micronuclear chromosome. DLD1 cell with micronucleus, induced by Y-centromere inactivation, expressing GFPMDC1 undergoing mitosis.
Supplementary Video 2
TOPBP1 localization to micronuclear chromosome. DLD1 cell with micronucleus, induced by Y-centromere inactivation, expressing TOPBP1Clover undergoing mitosis.
Supplementary Video 3
TOPBP1 localization to chromosome in ruptured micronucleus. RPE1 p53–/– cell expressing TOPBP1Clover and 3XNLSmScarlet with micronucleus, whose nuclear envelope has been ruptured, entering mitosis.
Supplementary Video 4
TOPBP1 localization to chromosome in chromatin bridge. RPE1 p53–/– cell expressing TOPBP1Clover with chromatin bridge induced by topoisomerase inhibition showing recruitment of TOPBP1Clover to broken chromatin bridge.
Supplementary Video 5
Encapsulation of fragmented micronuclear chromosome into a new micronucleus. DLD1 cell with micronucleus, induced by Y-centromere inactivation, expressing TOPBP1Clover undergoing mitosis. The damaged micronuclear chromosomal fragments remains tethered and form a new micronucleus in the daughter cell.
Supplementary Video 6
Breakage of initially clustered fragmented micronuclear chromosome in mitosis into smaller clusters. DLD1 cell with micronucleus, induced by Y-centromere inactivation, expressing GFPMDC1 undergoing mitosis. The damaged micronuclear chromosomal fragments initially form a cluster, which deforms and breaks into a smaller cluster during passage through mitosis.
Supplementary Video 7
Continued clustering of micronuclear chromosome fragments during prolonged mitosis in the presence of TOPBP1. A RPE p53–/– TOPBP1dTag cell, arrested in mitosis by Eg5 inhibitor, with damaged and clustered chromosome induced by Mps1 inhibition and marked with GFPMDC1. TOPBP1 degradation was not induced and chromosomal fragments remained clustered during mitosis.
Supplementary Video 8
Dispersal of micronuclear chromosome fragments in prolonged mitosis following TOPBP1 degradation. RPE p53–/– TOPBP1dTag cell, arrested in mitosis by Eg5 inhibitor, with damaged and clustered chromosome induced by Mps1 inhibition and marked with GFPMDC1. TOPBP1 degradation was induced (by addition of dTAG-13) at the start of the video; the chromosomal fragments are clustered initially but disperse as TOPBP1dTag is degraded.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trivedi, P., Steele, C.D., Au, F.K.C. et al. Mitotic tethering enables inheritance of shattered micronuclear chromosomes. Nature 618, 1049–1056 (2023). https://doi.org/10.1038/s41586-023-06216-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06216-z
This article is cited by
-
Scrambling the genome in cancer: causes and consequences of complex chromosome rearrangements
Nature Reviews Genetics (2024)
-
The two sides of chromosomal instability: drivers and brakes in cancer
Signal Transduction and Targeted Therapy (2024)
-
Extrachromosomal DNA in cancer
Nature Reviews Cancer (2024)
-
A mitotic glue for shattered chromosomes
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.