Abstract
A complete understanding of how exposure to environmental substances promotes cancer formation is lacking. More than 70 years ago, tumorigenesis was proposed to occur in a two-step process: an initiating step that induces mutations in healthy cells, followed by a promoter step that triggers cancer development1. Here we propose that environmental particulate matter measuring ≤2.5 μm (PM2.5), known to be associated with lung cancer risk, promotes lung cancer by acting on cells that harbour pre-existing oncogenic mutations in healthy lung tissue. Focusing on EGFR-driven lung cancer, which is more common in never-smokers or light smokers, we found a significant association between PM2.5 levels and the incidence of lung cancer for 32,957 EGFR-driven lung cancer cases in four within-country cohorts. Functional mouse models revealed that air pollutants cause an influx of macrophages into the lung and release of interleukin-1β. This process results in a progenitor-like cell state within EGFR mutant lung alveolar type II epithelial cells that fuels tumorigenesis. Ultradeep mutational profiling of histologically normal lung tissue from 295 individuals across 3 clinical cohorts revealed oncogenic EGFR and KRAS driver mutations in 18% and 53% of healthy tissue samples, respectively. These findings collectively support a tumour-promoting role for PM2.5 air pollutants and provide impetus for public health policy initiatives to address air pollution to reduce disease burden.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Duplex-seq data for the PEACE and BDRE cohorts are available at the European Genome–Phenome Archive (EGA) with the identifier EGAS00001006951. Duplex-seq data generated from PEACE study samples during this study are not publicly available and restrictions apply to the availability of these data. Such Duplex-seq data are available through the Cancer Research UK and University College London Cancer Trials Centre (ctc.peace@ucl.ac.uk) for academic, non-commercial research purposes upon reasonable request and subject to review of a project proposal that will be evaluated by a PEACE data access committee, entering into an appropriate data access agreement and subject to any applicable ethical approvals. Duplex-seq data generated from the BDRE study are available through J. DeGregori (James.Degregori@cuanschutz.edu) for academic, non-commercial research purposes upon reasonable request, entering into an appropriate data access agreement and subject to any applicable ethical approvals. The Duplex-seq data for the BDRE and PEACE studies were generated using a larger panel of probes that covered approximately 50 kb of the genome, spanning hotspots frequently mutated in cancers. This full dataset has been provided for the 17 never-smoker individuals from the PEACE study. For all other samples, only data for the EGFR and KRAS regions queried are included in this manuscript. The RNA-seq data for the COPA study are available at the EGA with the identifier EGAS00001006966. De-identified participant data are available upon reasonable request to C.C. (christopher.carlsten@ubc.ca) for academic, non-commercial research purposes. Data availability is subject to a data access agreement and applicable ethical approvals. Mouse WGS data are available at the European Nucleotide Archive (ENA) with the identifier PRJEB58221 (ERP143287). Mouse RNA-seq data are available at the ENA with the identifier PRJEB59269 (ERP144330). Source data are provided with this paper.
Code availability
Code for analysis of epidemiology, RNA-seq and WGS data and processing of healthy lung tissue are available at Zenodo (https://doi.org/10.5281/zenodo.7705022).
References
Berenblum, I. & Shubik, P. A new, quantitative, approach to the study of the stages of chemical carcinogenesis in the mouse’s skin. Br. J. Cancer 1, 383–391 (1947).
Cogliano, V. J. et al. Preventable exposures associated with human cancers. J. Natl Cancer Inst. 103, 1827–1839 (2011).
Sun, S., Schiller, J. H. & Gazdar, A. F. Lung cancer in never smokers—a different disease. Nat. Rev. Cancer 7, 778–790 (2007).
Midha, A., Dearden, S. & McCormack, R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 5, 2892–2911 (2015).
Carrot-Zhang, J. et al. Genetic ancestry contributes to somatic mutations in lung cancers from admixed Latin American populations. Cancer Discov. 11, 591–598 (2021).
Myers, R. et al. High ambient air pollution exposure among never smokers versus ever smokers with lung cancer. J. Thorac. Oncol. 16, 1858–1858 (2021).
WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide (World Health Organization, 2021).
Kucab, J. E. et al. A compendium of mutational signatures of environmental agents. Cell 177, 821–836.e16 (2019).
Riva, L. et al. The mutational signature profile of known and suspected human carcinogens in mice. Nat. Genet. 52, 1189–1197 (2020).
Moody, S. et al. Mutational signatures in esophageal squamous cell carcinoma from eight countries with varying incidence. Nat. Genet. 53, 1553–1563 (2021).
Zhang, T. et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat. Genet. 53, 1348–1359 (2021).
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 376, 2109–2121 (2017).
Chen, Y.-J. et al. Proteogenomics of non-smoking lung cancer in East Asia delineates molecular signatures of pathogenesis and progression. Cell 182, 226–244.e17 (2020).
Frankell, A. M. et al. The evolution of lung cancer and impact of subclonal selection in TRACERx. Nature https://doi.org/10.1038/s41586-023-05783-5 (2023).
Takahashi, H., Ogata, H., Nishigaki, R., Broide, D. H. & Karin, M. Tobacco smoke promotes lung tumorigenesis by triggering IKKβ- and JNK1-dependent inflammation. Cancer Cell 17, 89–97 (2010).
Im, H-B. et al. South Korea. Encyclopaedia Britannica. https://www.britannica.com/place/South-Korea (accessed 9 March 2023).
The Ethnic Group. Executive Yuan. https://www.ey.gov.tw/state/99B2E89521FC31E1/2820610c-e97f-4d33-aa1e-e7b15222e45a (accessed 9 March 2023).
Huang, Y. et al. Air pollution, genetic factors, and the risk of lung cancer: a prospective study in the UK Biobank. Am. J. Respir. Crit. Care Med. 204, 817–825 (2021).
McDaniel Mims, B. & Grisham, M. B. Humanizing the mouse immune system to study splanchnic organ inflammation. J. Physiol. 596, 3915–3927 (2018).
Hogg, J. C. & Van Eeden, S. Pulmonary and systemic response to atmospheric pollution. Respirology 14, 336–346 (2009).
Sutherland, K. D. et al. Multiple cells-of-origin of mutant K-Ras-induced mouse lung adenocarcinoma. Proc. Natl Acad. Sci. USA 111, 4952–4957 (2014).
Choi, J. et al. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell 27, 366–382.e7 (2020).
Strunz, M. et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 11, 3559 (2020).
Ryu, M. H. et al. Impact of exposure to diesel exhaust on inflammation markers and proteases in former smokers with chronic obstructive pulmonary disease: a randomized, double-blinded, crossover study. Am. J. Respir. Crit. Care Med. 205, 1046–1052 (2022).
Ryu, M. H. Effects of Traffic-Related Air Pollution Exposure on Older Adults with and without Chronic Obstructive Pulmonary Disease. PhD thesis, Univ. of British Colombia (2021).
Nolan, E. et al. Radiation exposure elicits a neutrophil-driven response in healthy lung tissue that enhances metastatic colonization. Nat. Cancer 3, 173–187 (2022).
Dost, A. F. M. et al. Organoids model transcriptional hallmarks of oncogenic KRAS activation in lung epithelial progenitor cells. Cell Stem Cell 27, 663–678.e8 (2020).
Hiraiwa, K. & van Eeden, S. F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm. 2013, 619523 (2013).
Klughammer, B. et al. Examining treatment outcomes with erlotinib in patients with advanced non-small cell lung cancer whose tumors harbor uncommon EGFR mutations. J. Thorac. Oncol. 11, 545–555 (2016).
Takano, A. P. C. et al. Pleural anthracosis as an indicator of lifetime exposure to urban air pollution: an autopsy-based study in Sao Paulo. Environ. Res. 173, 23–32 (2019).
Mirsadraee, M. Anthracosis of the lungs: etiology, clinical manifestations and diagnosis: a review. Tanaffos 13, 1–13 (2014).
Kunzke, T. et al. Patterns of carbon-bound exogenous compounds in patients with lung cancer and association with disease pathophysiology. Cancer Res. 81, 5862–5875 (2021).
Deprez, M. et al. A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med. 202, 1636–1645 (2020).
Sikkema, L. et al. An integrated cell atlas of the human lung in health and disease. Preprint at bioRxiv https://doi.org/10.1101/2022.03.10.483747 (2022).
Tate, J. G. et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 47, D941–D947 (2019).
Su, F. et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 366, 207–215 (2012).
Yoshida, K. et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 578, 266–272 (2020).
Yokoyama, A. et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature 565, 312–317 (2019).
IARC Working Group on the & Evaluation of Carcinogenic Risks to Humans. IARC Monograph Volume105. Diesel And Gasoline Engine Exhausts And Some Nitroarenes (World Health Organization, 2014).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monograph Volume 109. Outdoor Air Pollution (World Health Organization, 2016).
Turner, M. C. et al. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J. Clin. 70, 460–479 (2020).
Chung, K. M. et al. Endocrine–exocrine signaling drives obesity-associated pancreatic ductal adenocarcinoma. Cell 181, 832–847.e18 (2020).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Crapo, J. D., Barry, B. E., Gehr, P., Bachofen, M. & Weibel, E. R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 126, 332–327 (1982).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung. Preliminary report. 1950. Bull. World Health Organ. 77, 84–93 (1999).
Kennedy, S. R. et al. Detecting ultralow-frequency mutations by duplex sequencing. Nat. Protoc. 9, 2586–2606 (2014).
Stoler, N. & Nekrutenko, A. Sequencing error profiles of Illumina sequencing instruments. NAR Genom. Bioinform. 3, lqab019 (2021).
Valentine, C. C. III et al. Direct quantification of in vivo mutagenesis and carcinogenesis using duplex sequencing. Proc. Natl Acad. Sci. USA 117, 33414–33425 (2020).
Eeftens, M. et al. Development of land use regression models for PM2.5, PM2.5 absorbance, PM10 and PMcoarse in 20 European study areas; results of the ESCAPE project. Environ. Sci. Technol. 46, 11195–11205 (2012).
Van Buuren, S. & Groothuis-Oudshoorn, K. mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Department for Environment Food and Rural Affairs. Modelled Background Pollution Data https://uk-air.defra.gov.uk/data/pcm-data#population_weighted_annual_mean_pm25_data (2021).
British Geological Survey. Radon Data: Indicative Atlas of Radon https://www.bgs.ac.uk/datasets/radon-data-indicative-atlas-of-radon/ (2023).
ONS Postcode Directory (Latest) Centroids (Office for National Statistics; 2021); https://geoportal.statistics.gov.uk/datasets/ons-postcode-directory-november-2022/about (accessed 9 March 2023).
(Air Korea; 2021); https://www.airkorea.or.kr/web (accessed 9 March 2023).
Cancer Registry Statistical Data (National Cancer Center; 2021); https://www.ncc.re.kr/main.ncc?uri=english/sub04_Statistics (accessed 13 March 2023).
Taiwan Air Quality Monitoring Network (Environmental Protection Administration; 2022); https://airtw.epa.gov.tw/ENG/Default.aspx (accessed 23 March 2023).
Politi, K. et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 20, 1496–1510 (2006).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 (2001).
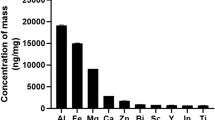
Schantz, M. M. et al. Development of two fine particulate matter standard reference materials (<4 μm and <10 μm) for the determination of organic and inorganic constituents. Anal. Bioanal. Chem. 408, 4257–4266 (2016).
Chan, Y. L. et al. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 317, L424–L430 (2019).
Major, J. et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 369, 712–717 (2020).
Pedregosa, F. et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Desai, T. J., Brownfield, D. G. & Krasnow, M. A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194 (2014).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Rosenthal, R., McGranahan, N., Herrero, J., Taylor, B. S. & Swanton, C. deconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol. 17, 31 (2016).
Ewels, P. A. et al. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 38, 276–278 (2020).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Young, M. D. et al. Single cell derived mRNA signals across human kidney tumors. Nat. Commun. 12, 3896 (2021).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Dekkers, J. F. et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat. Protoc. 16, 1936–1965 (2021).
Muiños, F., Martínez-Jiménez, F., Pich, O., Gonzalez-Perez, A. & Lopez-Bigas, N. In silico saturation mutagenesis of cancer genes. Nature 596, 428–432 (2021).
Acknowledgements
This research was conducted using the UK Biobank Resource under application number 82693. This work was supported by the Mark Foundation ASPIRE I Award (grant 21-029-ASP), the Lung Cancer Research Foundation Grant on Disparities in Lung Cancer, Advanced Grant (PROTEUS, grant agreement no. 835297), CRUK EDD (EDDPMA-Nov21\100034) and a Rosetrees Out-of-round Award (OoR2020\100009). W.H. is funded by an ERC Advanced Grant (PROTEUS, grant agreement no. 835297), CRUK EDD (EDDPMA-Nov21\100034), The Mark Foundation (grant 21-029-ASP) and has been supported by Rosetrees. E.L.L. receives funding from the NovoNordisk Foundation (ID 16584), The Mark Foundation (grant 21-029-ASP) and has been supported by Rosetrees. C.E.W. is supported by a RESPIRE4 fellowship from the European Respiratory Society and Marie-Sklodowska-Curie Actions. C.L. is supported by the Agency for Science, Technology & Research, Singapore and the Cancer Research UK City of London Centre and the City of London Centre Clinical Academic Training Programme. M.A. is supported by the City of London Centre Clinical Academic Training Programme (Year 3, SEBSTF-2021\100007). K.C. is supported by the Research Unit of Intelligence Diagnosis and Treatment in Early Non-small Cell Lung Cancer, the Chinese Academy of Medical Sciences (2021RU002), the National Natural Science Foundation of China (no. 82072566) and Peking University People’s Hospital Research and Development Funds (RS2019-01). T.K. receives grant support from JSPS Overseas Research Fellowships Program (202060447). S.-H.L. is supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2020R1A2C3006535), the National Cancer Center Grant (NCC1911269-3) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HR20C0025). L.H.S. receives grant support from the Berta Kamprad Foundation, the Swedish Cancer Society and the Swedish Research Council. R.M. and S.L. acknowledge funding from the Terry Fox Research Institute. N.M. is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (grant number 211179/Z/18/Z) and receives funding from Cancer Research UK, the Rosetrees and the NIHR BRC at University College London Hospitals and the CRUK University College London Experimental Cancer Medicine Centre. J. DeGregori, M.G., Y.E.M., D.T.M. and R.L.K. receive funding from the American Association for Cancer Research/Johnson&Johnson (18-90-52-DEGR), and J. DeGregori is supported by the Courtenay C. and Lucy Patten Davis Endowed Chair in Lung Cancer Research and a Merit Award from the Veteran’s Administration (1 I01 BX004495). M.G., Y.E.M., D.T.M. and R.L.K. were supported by the National Cancer Institute (NCI) RO1 CA219893. E.J.E.J. was supported by a NCI Ruth L. Kirschstein National Research Service Award T32-CA190216 and the Blumenthal Fellowship from the Linda Crnic Institute for Down Syndrome. C.I.T. acknowledges funding from UC Anschutz (LHNC T32CA174648). The work at the University of Colorado was also supported by NCI Cancer Center Support Grant P30CA046934. K. Litchfield is funded by the UK Medical Research Council (MR/P014712/1 and MR/V033077/1), the Rosetrees Trust and the Cotswold Trust (A2437) and Cancer Research UK (C69256/A30194). M.J.-H. is a CRUK Career Establishment Awardee has received funding from Cancer Research UK, IASLC International Lung Cancer Foundation, the National Institute for Health Research, the Rosetrees Trust, UKI NETs and the NIHR University College London Hospitals Biomedical Research Centre. C.S. is a Royal Society Napier Research Professor (RSRP\R\210001). His work is supported by the Francis Crick Institute that receives its core funding from Cancer Research UK (CC2041), the UK Medical Research Council (CC2041), and the Wellcome Trust (CC2041). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. C.S. is funded by Cancer Research UK (TRACERx (C11496/A17786), PEACE (C416/A21999) and CRUK Cancer Immunotherapy Catalyst Network); Cancer Research UK Lung Cancer Centre of Excellence (C11496/A30025); the Rosetrees Trust, Butterfield and Stoneygate Trusts; NovoNordisk Foundation (ID16584); Royal Society Professorship Enhancement Award (RP/EA/180007); National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre; the Cancer Research UK-University College London Centre; Experimental Cancer Medicine Centre; the Breast Cancer Research Foundation (US) (BCRF-22-157); Cancer Research UK Early Detection an Diagnosis Primer Award (grant EDDPMA-Nov21/100034); and The Mark Foundation for Cancer Research Aspire Award (grant 21-029-ASP). This work was supported by a Stand Up To Cancer‐LUNGevity-American Lung Association Lung Cancer Interception Dream Team Translational Research Grant (grant number: SU2C-AACR-DT23-17 to S.M. Dubinett and A.E. Spira). Stand Up To Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C. C.S. is in receipt of an ERC Advanced Grant (PROTEUS) from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 835297). We acknowledge the PEACE Consortium (PEACE Consortium members are named below) for their expertise and support in putting together the healthy tissue sample cohorts. We thank the clinical and administrative team of the PEACE study for their assistance in data curation (S. Shepherd, Z. Tippu, B. Shum, C. Lewis, M. O’Flaherty, A. Lucanas, E. Carlyle, L. Holt, F. Williams); nursing and biospecimen coordinators for their assistance in sample curation (K. Edmonds, L. Grostate, K. Lingard, D. Kelly, J. Korteweg, L. Terry, J. Biano, A. Murra, K. Kelly, K. Peat, N. Hunter); A. H. -K. Cheung for assistance in pathology review; J. Asklin and C. Forsberg for logistical and technical assistance; staff at the Chang Gung Memorial Hospital for providing Chang Gung Research Database (CGRD) data; staff who provided support at the Flow Cytometry Unit, the Experimental Histopathology Unit, the Advanced Light Microscopy Facility, the Advanced Sequencing Facility and the Biological Resources Unit, especially N. Chisholm and Jay O’Brien, at the Francis Crick Institute; A. Yuen, A. Azhar, K. Lau, C. Schwartz, A. Lee and C. Rider for their logistical support for the human exposure study; and staff at the Centre d’expertise et de services Génome Québec for their sequencing services and support. Data for this study are based on patient-level information collected by the NHS, as part of the care and support of cancer patients. The data are collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of NHS England (NHSE). We extend our thanks to the skilled Cancer Registration Officers (CROs) within the National Disease Registration Service, who abstracted and registered the English tumour and molecular testing data. For the purpose of Open Access, the author has applied for a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. PEACE Consortium members: A. Flanagan, A. Hackshaw, A. Jayaram, A. M. M. Hasan, A. Toncheva, A. Wingate, A. Bunkum, A. Sharp, A. Tookman, A. Murra, A. Magness, A. Coulton, A. M. Frankell, A. Cluroe, A. Kerr, A. Ortega-Franco, A. Lucanas, A. M. Schmitt, A. Furness, A. Rowan, A. Tutt, A. Green, A. Paterson, A.-L. Cattin, A. Fendler, A. Latifoltojar, A. Huebner, A. Thomas, B. T. Alba, B. Chain, B. Naidu, B. Shum, B. Olisemeke, B. Hampton, B. Hanley, B. Tanchel, G. Langman, C. Naceur-Lombardelli, C. Gerard, C. Caldas, C. Martínez-Ruiz, C. Dive, C. Stirling, C. Swanton, C. Ferris, C. Lewis, C. Milner-Watts, C. Spencer, C.-w. Lok, C. Bailey, C. Messiou, C. Wilson, C. Puttick, C. Lee, C. P. Marin, C. Alifrangis, C. Richard, C. T. Hiley, D. Hochhauser, D. Wetterskog, D. A. Moore, D. Deng, D. Marrone, D. Enting, D. Josephs, D. Kelly, D. A. Fennell, D. Biswas, D. Papadatos-Pastos, E. Carlyle, E. Provenzano, E. (L.) Karapanagiotou, E. Pintus, E. L. Lim, E. Beddowes, E. Colliver, E. Nye, F. Gishen, F. Gomes, F. H. Blackhall, F. Byrne, F. Athanasopoulou, G. Attard, G. Stamp, G. Middleton, G. D. Stewart, H. Feng, G. Pulman, G. Leone, H. Bridger, H. Shaw, H. Yan, H. Mudhar, H. Bancroft, H. Pallikonda, I. McNeish, I. Proctor, I. Tomlinson, I. Noorani, I. Lobon, J. Bridgewater, J. L. Reading, J. Black, J. Brenton, J. Larkin, J. Spicer, J. K. Rane, J. Biano, J. Nicod, J. Webb, J. L. Quesne, J. A. Shaw, J. Korteweg, K.-K. Shiu, K. Pearce, K. Young, K. S. S. Enfield, K. Kelly, K. Peat, K. Thol, K. G. Blyth, K. Litchfield, K. Allinson, K. Edmonds, K. Chan, K. Dijkstra, K. Grigoriadis, L. Farrelly, L. Grostate, L. Terry, L. Spain, L. Au, L. Pickering, L. Holt, L. Del Rosario, M. Jamal-Hanjani, M. Linch, M. Sivakumar, M. MacKenzie, M. Al Bakir, M. Collard, M. Forster, M. Falzon, M. Mangwende, M. Carter, M. G. Krebs, M. Emmerich, M. Akay, M. Jimenez-Linan, M. Dietzen, M. Angelova, M. Mitchison, M. O’Flaherty, N. Kanu, N. Magno, N. Yousaf, N. Wright, N. McGranahan, N. Hunter, O. Vainauskas, O. Curtis, O. Lucas, O. Pich, O. Al-Sawaf, P. Prymas, P. Roxburgh, P. Campbell, P. Oliveira, P. Cockcroft, P. Colloby, P. Ellery, P. Hill, P. Parker, P. Van Loo, P. Pawlik, P. Stone, S. Veeriah, R. Vendramin, R. Leslie, R. Fitzgerald, R. Zaidi, R. E. Hynds, R. Salgado, R. Wilson, R. Sinclair, R. Stewart, R. Mitu, S. Beck, S. K. Bola, S. Brandner, S. M. Janes, S. Lise, S. A. Quezada, S. Kuong A. Ung, S. Kadiri, S. Holden, S. Turajlic, S. Gamble, S. Jogai, S. Popat, S. Aitken, S. Benafif, J. Dransfield, S. Howlett, S. Shepherd, S. Ghosh, S. Irshad, S. Phillips-Boyd, S. Baijal, S. Zaccaria, S. Fraser, S. M. Lee, S. Hessey, Y. Ning S. Wong, S. Ward, S. Hazell, T. Enver, T. Karasaki, T. Fernandes, T. Ahmad, T. Sahwangarrom, T. Marafioti, T. B. K. Watkins, T. Maughan, U. Mahadeva, U. McGovern, V. Barbe, W. Drake, W. Hill, W. K. Liu, Y. Summers, Z. Tippu, Z. Rhodes, A. Stewart, A. Alzetani, A. J. Patel, A. Kirk, A. Kerr, A. J. Procter, A. Clipson, A. Rice, A. Bajaj, A. Devaraj, A. Grapa, A. G. Nicholson, A. Robinson, A. Leek, A. Montero, A. Karamani, A. Chaturvedi, A. Nakas, A. Nair, A. Ahmed, A. Osman, A. Sodha-Ramdeen, B. B. Campbell, C. Pilotti, C. Castignani, C. Veiga, C -A. Collins-Fekete, C. Proli, C. Lacson, C. Abbosh, C. E. Weeden, C. R. Lindsay, C. Dick, D. Kaniu, D. Chuter, D. Lawrence, D. R. Pearce, D. Patrini, D. Karagianni, D. Levi, D. G. Rothwell, E. Boleti, E. Borg, E. Kilgour, E. Smith, E. Hoxha, E. L. Cadieux, E. M. Hoogenboom, E. Lim, E. Fontaine, E. Gronroos, F. Granato, F. Galvez-Cancino, F. Monk, F. Fraioli, F. Gimeno-Valiente, G. A. Wilson, G. Royle, G. Kassiotis, G. Stavrou, G. Mastrokalos, G. Price, G. Matharu, H. Zhang, H. Zhai, H. K. Dhanda, H. Bhayani, H. Cheyne, H. Doran, H. L. Lowe, H. Shackleford, H. Chavan, H. Raubenheimer, H. J. W. L. Aerts, I. G. Matos, J. D. Hodgkinson, J. Goldman, J. R. M. Black, J. W. Holding, J. Wilson, J. F. Lester, J. Herrero, J. Richards, J. M. Lam, J. Riley, J. A. Hartley, J. Demeulemeester, J. Tugwood, J. Kisistok, J. Cave, J. Novasio, J. Choudhary, K. S. Peggs, K. Brown, K. Selvaraju, K. Gilbert, K. M. Kerr, K. Rammohan, K. Ang, K. G. Blyth, K. W. Ng, K. Chen, K. AbdulJabbar, K. Thakkar, K. Bhakhri, L. Ensell, L. Joseph, L. Robinson, L. Primrose, L. Scarlett, L. Ambrose, L. Priest, M. Djearaman, M. Hewish, M. N. Taylor, M. Shah, M. Scarci, M. V. Duran, M. Litovchenko, M. W. Sunderland, M. S. Hill, M. D. Forster, M. Hayward, M. Sokac, M. Carter, M. Thomas, M. J. Shackcloth, M. Leung, M. Escudero, M. Diossy, M. Taniƒá, M. Asif, M. Tufail, M. F. Chowdhry, M. Khalil, M. Scotland, M. Malima, N. Fernandes, N. Navani, N. Totten, N. J. Birkbak, N. Gower, N. Panagiotopoulos, N. Kostoulas, O. Ansorge, O. Chervova, P. Ingram, P. Gorman, P. Ashford, P. Bishop, P. De Sousa, P. Russell, P. Crosbie, P. Hobson, P. Shah, R. Rosenthal, R. Shah, R. Boyles, R. Khiroya, R. K. Stone, R. M. Thakrar, R. Bentham, R. C. M. Stephens, R. Bilancia, R. F. Schwarz, S. Saghafinia, S. Lopez, S. Booth, S. Danson, S. Rudman, S. Dulloo, S. Smith, S. Chee, S. Vanloo, S. Richardson, S. Austin, S. I. Buderi, S. Jordan, S. Begum, S. Rathinam, S. Boeing, S. Bandula, S. Moss, S. K. Bola, T. Denner, T. P. Mourikis, T. L. Kaufmann, T. Cruickshank, T. Clark, V. Spanswick, V. Joshi, W.-T. Lu, X. Pan, Y. Wu, Y. Yuan, Y. Naito, Z. Ramsden, Z. Szallasi, A. Dwornik, G. Langman, H. Shackleford, A. Osman, B. Naidu, K. Bowles, C. Grieco, M. L. Le, P. D. D’Arienzo and E. Turay. E.G was supported by an ERC Advanced grant (PROTEUS, grant agreement no 835297).
Author information
Authors and Affiliations
Consortia
Contributions
W.H. and E.L.L jointly conceptualized the project, designed the project, performed the experiments analyses and wrote the manuscript. W.H. led and performed the mouse experiments, and E.L.L. led and performed the bioinformatics and epidemiology analyses. C.E.W. performed the mouse experiments, helped write the manuscript and curated the mutation literature. C.L. performed the human RNA-seq analyses and curated the pollution data. M.A. performed the UKBB analyses. K.C. assembled and analysed the TRACERx cohort dataset. F.-C.K. and M.-H.L. performed the Taiwan epidemiological analyses. F. Marongiu, E.J.E.J., C.I.T., M.G., Y.E.M., D.T.M. and R.L.K. generated and analysed the Duplex-seq data. O.P. wrote the Duplex-seq bioinformatics pipeline and performed the mutational signature analyses. H.C. and S.-H.L. performed the Korea epidemiological analyses. F.v.M., J.B., A.M. and D.R.C. were involved in mouse data acquisition. F.S.R. was involved with organoid experiments. S.V., A.R. and C.N.-L. curated and performed DNA extractions on TRACERx and PEACE samples. T.K. helped analyse patient clinical characteristics. D.M., S.D. and M.S. performed pathological assessments of human tissue samples. A.N., B.B., J.R.M.B. and C.M.-R. performed mouse RNA-seq analyses. M.H.R., R.D.H. and S.L. designed and generated data for the human crossover study. A.S.-B. And S.L.P. were involved in mouse pathology analyses. M.L., K. Lavelle, J.P., S.H. and F.E.M. curated England’s National Disease Registration Service data et. R.M. curated the Canadian cohort. M.A.B. and C.B. wrote and ran the MiSeq pipeline. C.A., L.H.S., Y.C. and A.M.G. performed the ddPCR experiments. I.M., J. Downward, T.J., N.K. and E.G. provided supervision for the mouse experiments. M.-J.F., M.H., P.A. and N.M. provided guidance and supervision for the bioinformatics analyses. S.L., P.S., R.H., C.T., C.D.B., A.H. and K. Litchfield provided supervision for epidemiological analyses. C.C. provided supervision for the human crossover study. J. DeGregori designed the BDRE study and supervised the healthy tissue profiling work. M.J.-H. designed the PEACE and TRACERx study protocols, and E.L.L. and M.J.-H. jointly supervised the study and collaborations. C.S. supervised the work, provided strategic oversight and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.A.B. has consulted for Achilles Therapeutics. L.H.S., Y.C. and A.M.G. have ownership interest in SAGA Diagnostics. S.V. is a co-inventor to a patent to detecting molecules in a sample (US patent 10578620). D.A.M. reports speaker fees from AstraZeneca, Eli Lilly and Takeda, consultancy fees from AstraZeneca, Thermo Fisher, Takeda, Amgen, Janssen, MIM Software, Bristol-Myers Squibb and Eli Lilly, and has received educational support from Takeda and Amgen. C.A. has received speaking honoraria or expenses from Novartis, Roche, AstraZeneca and Bristol-Myers Squibb and reports employment at AstraZeneca. C.A. is an inventor on a European patent application relating to assay technology to detect tumour recurrence (PCT/GB2017/053289). The patent has been licensed to commercial entities and under their terms of employment, C.A is due a revenue share of any revenue generated from such licence(s). C.A. declares a patent application (PCT/US2017/028013) for methods to detect lung cancer. C.A. is a named inventor on a patent application to determine methods and systems for tumour monitoring (PCT/EP2022/077987). T.J. is a member of the Board of Directors of Amgen and Thermo Fisher Scientific, and a co-Founder of Dragonfly Therapeutics and T2 Biosystems. T.J. serves on the Scientific Advisory Board (SAB) of Dragonfly Therapeutics, SQZ Biotech and Skyhawk Therapeutics. T.J. is also President of Break Through Cancer. K. Litchfield has a patent on indel burden and CPI response pending and speaker fees from Roche tissue diagnostics, research funding from CRUK TDL–Ono–LifeArc alliance, Genesis Therapeutics, and consulting roles with Ellipses Pharma, Monopteros and Kynos Therapeutics. N.M. has received consultancy fees and has stock options in Achilles Therapeutics. N.M. holds European patents relating to targeting neoantigens (PCT/EP2016/ 059401), identifying patient response to immune checkpoint blockade (PCT/EP2016/071471), determining HLA LOH (PCT/GB2018/052004), and predicting survival rates of patients with cancer (PCT/GB2020/050221). C.T. has received honoraria for educational activities and advisory boards from AstraZeneca and Roche (all proceeds donated to registered charity 11511580). C.D.B. has consultantships with GRAIL, LLC, NHS Galleri Trial, IDMC, Mercy BioAnalytics, Lucid DX and Medial EarlySign. J. Downward has acted as a consultant for AstraZeneca, Jubilant, Theras, Roche and Vividion and has funded research agreements with Bristol-Myers Squibb, Revolution Medicines and AstraZeneca. A.H. has received fees for being a member of Independent Data Monitoring Committees for Roche-sponsored clinical trials, and academic projects co-ordinated by Roche. M.J.-H. is a CRUK Career Establishment Awardee and has received funding from CRUK, NIH National Cancer Institute, IASLC International Lung Cancer Foundation, Lung Cancer Research Foundation, Rosetrees Trust, UKI NETs, NIHR, NIHR UCLH Biomedical Research Centre. M.J.-H. has consulted for, and is a member of, the Achilles Therapeutics Scientific Advisory Board and Steering Committee, has received speaker honoraria from Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster, Bristol Myers Squibb, and is co-inventor on a European patent application relating to methods to detect lung cancer PCT/US2017/028013).M.G., Y.E.M., R.L.K. and D.T.M. acknowledge grant support from Bristol-Myers Squibb. C.S. acknowledges grant support from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Pfizer, Roche-Ventana, Invitae (previously Archer Dx Inc–collaboration in minimal residual disease sequencing technologies), and Ono Pharmaceutical. C.S. is an AstraZeneca Advisory Board member and Chief Investigator for the AZ MeRmaiD 1 and 2 clinical trials and is also Co-Chief Investigator of the NHS Galleri trial funded by GRAIL and a paid member of GRAIL’s SAB. He receives consultant fees from Achilles Therapeutics (also SAB member), Bicycle Therapeutics (also a SAB member), Genentech, Medicxi, Roche Innovation Centre–Shanghai, Metabomed (until July 2022), and the Sarah Cannon Research Institute. He had stock options in Apogen Biotechnologies and GRAIL until June 2021, and currently has stock options in Epic Bioscience, Bicycle Therapeutics, and has stock options and is co-founder of Achilles Therapeutics. C.S. is an inventor on a European patent application relating to assay technology to detect tumour recurrence (PCT/GB2017/053289), the patent has been licensed to commercial entities, and under his terms of employment, C.S. is due a revenue share of any revenue generated from such licence(s). C.S. holds patents relating to targeting neoantigens (PCT/EP2016/059401), identifying patient response to immune checkpoint blockade (PCT/EP2016/071471), determining HLA LOH (PCT/GB2018/052004), predicting survival rates of patients with cancer (PCT/GB2020/050221), identifying patients who respond to cancer treatment (PCT/GB2018/051912), a US patent relating to detecting tumour mutations (PCT/US2017/28013), methods for lung cancer detection (US20190106751A1) and both a European and US patent related to identifying insertion/deletion mutation targets (PCT/GB2018/051892) and is co-inventor to a patent application to determine methods and systems for tumour monitoring (PCT/EP2022/077987). C.S has received honoraria from Amgen, AstraZeneca, Pfizer, Novartis, GlaxoSmithKline, MSD, Bristol Myers Squibb, Illumina, and Roche-Ventana.
Peer review
Peer review information
Nature thanks Aaron Cohen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Study design, DNA analysis & epidemiology.
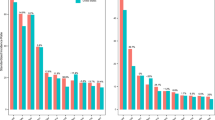
A) Study design schematic featuring the 3 aspects of the paper. LEFT: Epidemiological analysis of cancer incidence and PM2.5. MIDDLE: Pollution exposure in mouse models. RIGHT: Normal lung tissue analysis. B) TX421 Tumours from Smokers. Barplots indicating proportion of SNVs in each tumour attributed to each SBS mutational signature. The barplots (Top: Lung adenocarcinoma (LUAD), Bottom: Lung sqaumous cell carcinoma (LUSC)) reflect the probability that clonal driver mutations in patients, where smoking-related signatures have been detected, are caused by different mutational processes (SBS4 and SBS92 smoking, SBS2 and SBS13 APOBEC, SBS1 and SBS5 ageing). Each observed driver mutation in each patient is given a mutational-signature-causing probability based on the trinucleotide context and the signatures exposure of the patient (see Methods) and then these probabilities are aggregated. Asterisks represent patients where the smoking-related aggregated probabilities are below 0.5. C) Correlation between PM2.5 levels and EGFR mutant (EGFRm) adenocarcinoma lung cancer incidence in England. The blue line: robust linear regression line; grey shading: 95% confidence interval. D-E) The Canadian Lung Cancer Cohort. D) Distribution of 3 year and 20 year cumulative PM2.5 exposure levels for all patients in the Canadian cohort. Red lines mark the thresholds that were used to determine Low, Intermediate and High groups that are used in (D). These are the 1st (6.77 ug/m3) and 5th quintiles (7.27 ug/m3) of the distribution. The full distribution is displayed in the top plot, while the bottom plot displays a narrower range of 4–10 ug/m3 (for clarity). E) Counts and frequencies of EGFRm in the Canadian Cohort, where 3 year and 20 year cumulative PM2.5 exposure levels were available. Patients are grouped into high, intermediate and low groups based on thresholds established as described in (D). These groups are defined based on 3 year cumulative PM2.5 exposure data (left) and based on 20 year cumulative PM2.5 exposure data (right). The bar plots display the counts and frequency of EGFRm amongst patients within each group. The map was created using DEFRA data in R. The illustrations in A were created using BioRender (https://biorender.com).
Extended Data Fig. 2 Effect of PM in multiple mouse models of lung cancer.
A) Schematic of PM exposure and representative huEGFRL858R IHC of ET mice induced with AT2-specific Ad5-SPC-Cre exposed to PM or PBS control and quantification of neoplastic lesions (n = 14 PBS, n = 11 PM). Mann-Whitney test. B) Schematic of PM exposure followed by induction of EGFRL858R and quantification of precancerous lesions/mm2 of lung tissue (n = 9 PBS; n = 7 5 μg; n = 11 50 μg PM). One-way ANOVA. C) Schematic of PM exposure and representative H&E of a lung adenocarcinoma in a 50 μg PM exposed, doxycycline treated CCSP-rtTa; TetO-EGFRL858R mice; quantification of number of adenocarcinomas per mouse below (n = 9 per group). One-way ANOVA. D) Schematic of PM exposure and representative IHC for red fluorescent protein (RFP, marks tdTomato+ cells) in Rosa26LSL-tdTomato/+;KrasLSL-G12D/+ mouse model in control or 50 μg PM exposed conditions; quantification of number of hyperplastic lesions per mouse (n = 9 control, n = 9 5 μg and n = 12 50 μg). One-way ANOVA. Scale bar 50 μm (C main), 20 μm (C insert), 100 μm A & D.
Extended Data Fig. 3 Whole genome sequencing analysis of mouse tumours.
WGS analysis of tumours from ET mice exposed to air pollution (n = 5) and those exposed to PBS controls (n = 5). Each mouse tumour is compared vs the corresponding germline from the same mouse. A) Mutational profiles for each tumour sample according to the mutation trinucleotide context. LEFT: PBS Controls, RIGHT: 50 μg PM. B) Barplots indicate the counts of mutations in each sample, where bars are colored based on the base change. C) Boxplot comparing the counts of mutations between tumours from pollution exposed mice (50 μg PM) and tumours from PBS exposed mice (PBS Control). All mutations are summarised in one plot on the left, and are then further divided based on the base change of the mutation (n = 5 mice per group). Two-sided t-test comparing numbers of mutations between PBS and air pollution p-values are displayed. The boxplot line represents the median, the hinges of the box represent the 1st and 3rd quartiles and the limits of the whiskers represent the 1.5 interquartile range. D) Attribution of mutations in each tumour sample to each single base substitution (SBS) mutation signature. The shading indicates the weight of the signature within each sample. Majority of the weights have been assigned to ageing related signatures (SBS40, SBS5, SBS1) Komogolomov-Smirnoff test p-value = 0.26–0.68.
Extended Data Fig. 4 Immune cell profiling in response to PM.
A) Immune cell frequencies in the lungs determined by flow cytometry 24 h post-exposure from induced T and ET mice after 50 μg PM (red) or PBS control (blue) (n = 8 mice per group). Data are presented as the frequency among live CD45+ immune cells. One-way ANOVA. B) Representative immunofluorescent images of CD68+ macrophages (cyan) and tdTomato+ EGFR mutant cells (red) within ET lungs exposed to control or 50 μg PM. Quantification of CD68+ cells per mm2 of lung tissue (n = 4 mice per group). One-way ANOVA. C) Representative immunofluorescent images of CD68 (red), CD11b (green) and merged images from induced ET mice after 3 weeks of exposure to PBS (top) or 50 μg PM (bottom). Quantification of alveolar macrophages (AMΦ, CD68+CD11b−) and interstitial macrophages (IMΦ, CD68+CD11b+) per mm2 of lung tissue, selecting 10 x random 500 μm2 fields of view per mouse (n = 3 mice per group). One-way ANOVA. D) Representative immunofluorescent images of CD68+ macrophages (cyan) within CCSP-rtTA; TetO-EGFRL858R lungs treated with PBS (top) or 50 μg PM (bottom) 10 weeks post oncogene induction; quantification of CD68+ cells per mm2 of lung tissue, selecting 20 x random 500 μm2 fields of view per mouse (n = 3 mice per group). Unpaired t-test. E) Representative immunofluorescent images of CD68+ macrophages (cyan) and tdTomato+ KrasG12D mutant cells (red) within KT lungs treated with PBS (top panel) or 50 μg PM (bottom) 10 weeks post oncogene induction; quantification of CD68+ cells per mm2 of lung tissue, selecting 20 x 500 μm2 fields of view containing RFP+ cells per mouse (n = 3 mice per group). Unpaired t-test. Scale bar 50 µm B & D, 150 µm C & E. Gating strategies for flow cytometry analysis provided in Extended Data Fig. 6.
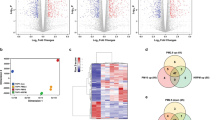
Extended Data Fig. 5 PM-mediated transcriptional changes, effects on AT2 cells and characterising IL-1β.
A-B) Significantly enriched GSEA pathways upregulated in T-PM lung epithelial cells compared to T control mice (A), in ET-PM lung epithelial cells compared to ET control mice (B). For each comparison, barplots indicate the -log10(FDR) of the Komogolomov-Smirnoff test p-value for each pathway. C) AT2 activated progenitor score derived from scRNAseq of bleomycin treated mouse lung used to deconvolute bulk RNA-seq of T and ET mice exposed to 50 μg PM or PBS, (n = 5 mice per group). Welch’s t-test between control and PM. Line represents mean of data. D) Schematic displaying experimental set-up of clinical exposure study in never-smoker volunteers, crossover design with (i) and (ii) in random order separated by 4-week washout. E) Fold change (FC) of significantly upregulated genes (identified in mouse) compared to the fold change of genes changed in the clinical exposure study. Common directionality across species indicated by colour (negative: blue background; positive: red background). F) Schematic of AT2 culture from T or ET mice exposed to 50 μg PM or PBS, with induction of tdTomato or oncogene ex vivo. G) Representative fluorescent images of tdTomato+ AT2 organoids at day 14 from ET mice exposed to PBS or 50 μg PM in vivo. Scale bar 100 μm. H) Quantification of tdTom+ AT2 organoid forming efficiency, data represents averages from 2 technical replicates/mouse; n = 4 mice from T control and PM; n = 5 mice for ET control and PM. One-way ANOVA. I) Representative fluorescent imaging of tdTomato (yellow), Keratin 8 (magenta), SPC (blue) on a wholemount AT2 organoid from an ET mouse treated with 50 μg PM. Scale bar is 20 μm. J) LEFT: Representative IL-1β RNAscope performed on lungs from ET mice treated with PBS or 50 μg PM after 3 weeks of exposure. Scale bar 20 µm. RIGHT: Quantification of IL-1β+ cells per mm2 of lung tissue from 30 random fields of view (control, n = 3 mice) and 28 fields of view (50 μg PM, n = 3 mice). Mann-Whitney test p-value is displayed. K) LEFT: Representative image of IL-1β RNAscope (green) in CD68 positive (red) macrophages in an ET mouse exposed to 50 μg PM, arrows indicate positive macrophages. n = 3 mice exposed to 50 μg PM. Scale bar 50 μm. RIGHT: Quantification of IL-1β positive CD68+ cells compared to CD68− cells at 3 weeks post induction in ET mice following exposure to PM. Mann-Whitney test. L) LEFT: Representative fluorescent images of EGFRL858R naive (non-PM exposed) AT2 organoids from ET mice treated with control or IL-1β in vitro. tdTomato (yellow) organoids stained with SPC (blue) and Keratin 8 (magenta). Scale bar 50 μm. RIGHT: Quantification of organoid size with each dot representing an organoid at day 14 of control (blue) or IL-1β treated (orange). Organoids derived from n = 2 mice per group. Mann-Whitney test. M) Schematic of anti-IL-1β treatment treatment (black triangles) during PM exposure (black lines) and harvest (red triangle). The illustrations in d and f were created using BioRender (https://biorender.com).
Extended Data Fig. 6 Flow cytometry Gating strategy used to identify epithelial and immune cells.
A, B) Example of flow gating strategy to determine frequency of lung (A) alveolar macrophages, interstitial macrophages, neutrophils, dendritic cells and (B) epithelial cells both tdTomato positive and negative. All samples were first gated to exclude debris and doublets, followed by live cell discrimination. C) Representative picture from a tdTomato mouse treated with control PBS for 3 weeks using sort strategy to enrich for for AT2 cells defined in Major et al. 61 and both alveolar and interstitial macrophages defined in Choi et al. 22.
Extended Data Fig. 7 CONSORT Diagrams for the normal lung tissue profiling cohorts.
TOP: TRACERx study, MIDDLE: PEACE study, BOTTOM: BDRE study.
Extended Data Fig. 8 Normal tissue study design and ddPCR results.
A) Schematic indicating normal lung tissue cohorts analysed by ddPCR and Duplex-seq. B) TRACERx and PEACE Cohort for ddPCR of 5 EGFR mutations. (i) Clinical information for each patient, (ii) Tumour EGFR mutation status, (iii) Normal EGFR mutation status. C) Representative H & E images from anthracotic pigment identification in TRACERx normal tissue. D) Comparing area of normal tissue harbouring anthracotic pigment in never smokers (n = 43) and smokers (n = 138). Each dot represents the ratio of pigmented area respective to total tissue in each anthracosis positive normal lung tissue sample. Two-sided Wilcox test p-value is reported. E) Regression analysis of characteristics influences EGFR mutant (EGFRm) presence in normal lung tissue for ddPCR-TRACERx cohort (n = 195). The illustrations in a were created using BioRender (https://biorender.com).
Extended Data Fig. 9 Normal tissue Duplex-seq results.
A) Top: EGFR Mutations detected using Duplex-seq across EGFR exons 18–21 on normal lung samples from the BDRE Study. Bottom: VAFs of each EGFR mutation are displayed. B) Top: KRAS Mutations detected using Duplex-seq across KRAS exons 2-3 on normal lung samples from the BDRE Study. Bottom: VAFs of each KRAS mutation are displayed. A-B) Only cancer-related mutations annotated in the cancer gene census are displayed. Mutations with strong evidence of being a lung cancer driver mutation are indicated in red, while mutations with some evidence of being a lung cancer driver mutation are indicated in pink, all other drivers annotated in COSMIC are indicated in blue. C) VAFs of KRAS mutations across samples of different cancer types. The one patient who received BRAF inhibitor treatment is indicated in purple. D) Comparing VAFs of high confidence (var count >=2, strong evidence) driver mutations in EGFR and KRAS. TOP: Boxplots summarise VAFs across samples. The boxplot line represents the median, the hinges of the box represent the 1st and 3rd quartiles and the limits of the whiskers represent the 1.5 interquartile range. Mutations are grouped according to the gene harbouring the mutation and smoking status of the patient. Two-sided Wilcox test p-values are reported. BOTTOM: dot plots show VAFs of mutations in each sample. Where a sample has 2 mutations (n = 4), they are both indicated. Dots are coloured by the gene harbouring the mutation (EGFR or KRAS). A paired t-test was performed between the VAFs of EGFR and KRAS mutations in these 4 cases. (Paired t-test p = 0.015) (Details of driver mutations can be found in Supplementary Table S8).
Supplementary information
Supplementary Table 1
Patient Characteristics of the England Cohort. Summary of clinical characteristics from the England lung cancer cohort. The ‘EGFRwt vs EGFRm’ sheet compares patients with and without EGFR mutations in their tumours, whereas the ‘EGFRm Tested vs Non Tested’ sheet compares patients who were tested and untested for EGFR mutations. Chi-squared test P values are reported.
Supplementary Table 2
Patient characteristics of the Korea cohort. Summary of clinical characteristics from the South Korea lung cancer cohort. The ‘EGFRwt vs EGFRm’ sheet compares patients with and without EGFR mutations in their tumours. Chi-squared test P values are reported.
Supplementary Table 3
Patient characteristics of the Taiwan cohort. Summary of clinical characteristics from the Taiwan lung cancer cohort. The ‘EGFRwt vs EGFRm’ sheet compares patients with and without EGFR mutations in their tumours, whereas the ‘EGFRm Tested vs Non Tested’ compares patients who were tested and untested for EGFR mutations. Chi-squared test P values are reported.
Supplementary Table 4
UK Biobank interaction tests and cancer type definitions. Results of multivariable Cox regressions investigating PM2.5 and cancer incidence in the UKBB. The ‘Lung (main)’ sheet shows the results for all covariates for the analysis on the full cohort. The ‘PanCancer’ sheet features the results for PM2.5 for all cancer types analysed. The ‘Lung (adenocarcinoma only)’ and ‘Lung (migration)’ sheets contain the results for all covariates when analysing only LUAD and those who remained at their baseline address for at least 3 years before baseline, respectively. Cox-regression P values are reported. The ‘ICD10codes_cancerTypes’ sheet contains the ICD tenth version codes used to define each analysed cancer type.
Supplementary Table 5
Mouse RNA-seq. Results of the differential expression analysis of RNA-seq libraries from reporter tdTomato mice exposed to PBS (T), or particulate matter (T+PM); or tdTomato;EGFRL858R mice exposed to PBS (ET+PBS) or particulate matter (ET+PM). The ‘Mouse DGE Analysis’ sheet features for each gene, metrics output from DESeq2 and the top two PCs from the PC analysis. The ‘Mouse and Human DGE Analysis Comparison’ sheet features for each gene where human and mouse orthologues can be mapped, the differential expression analysis metrics between air pollution exposed libraries (Mouse: T+PM; Human: Diesel Exhaust (DE)) vs control (Mouse: T; Human: Filtered Air (FA)) libraries.
Supplementary Table 6
Healthy tissue Datasets analysed for EGFR mutations. Published datasets of DNA sequencing of healthy human tissues (skin, lung, oesophagus, colorectal, small intestine, liver, uterus and bladder) describing patient cohorts, sampling, sequencing technology and mutation calling. The presence of any EGFR mutation and EGFRL858R mutation with associated VAFs are reported.
Supplementary Table 7
Cohort clinical characteristics of the TRACERx, PEACE and BDRE studies. Clinical characteristics of each patient included in the healthy lung tissue profiling work. There is one sheet dedicated to each cohort: ‘ddPCR TRACERx’, ‘ddPCR PEACE’, ‘Duplex-seq PEACE’ and ‘Duplex-seq BDRE’.
Supplementary Table 8
Evidence of EGFR or KRAS cancer driver mutation status. Non-silent EGFR and KRAS mutations, detected by Duplex-Seq within PEACE and BDRE cohorts, were researched for published evidence of cancer driver status. For EGFR, literature reports of >1 patient with mutation (not including compound mutations) achieving stable disease (SD), partial response (PR) or complete response (CR) to a clinical EGFR inhibitor combined with supporting evidence (defined from in vitro studies, mouse model data or protein modelling analyses) were classified as ‘strong evidence’ drivers. Reports of 1 patient with SD, PR or CR to clinical EGFR inhibition or the presence of other supporting evidence were defined as ‘some evidence’ drivers. No reported patient sensitivity to EGFR inhibition or little supporting reports were defined as ‘weak evidence’ drivers. For KRAS, literature reports of frequent occurrence in cancer (above or equal to 2% of KRAS mutant cancers), in vitro studies and mouse model data were classified as ‘strong evidence’ drivers; protein modelling analyses were classified as ‘some evidence’ drivers.
Supplementary Table 9
Frequency of EGFR mutations and KRAS mutations in healthy lung tissue. Summaries of the proportion of patients with healthy lung tissues harbouring EGFR or KRAS mutations. Patients are stratified according to diagnosis, sex and smoking status. Proportion test P values are reported.
Supplementary Table 10
List of antibodies. Antibodies used for flow cytometry, immunohistochemical and immunofluorescence analyses of mouse tissue.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hill, W., Lim, E.L., Weeden, C.E. et al. Lung adenocarcinoma promotion by air pollutants. Nature 616, 159–167 (2023). https://doi.org/10.1038/s41586-023-05874-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05874-3
This article is cited by
-
Distribution and characteristics of malignant tumours by lung lobe
BMC Pulmonary Medicine (2024)
-
Enhanced recovery after surgery program focusing on chest tube management improves surgical recovery after video-assisted thoracoscopic surgery
Journal of Cardiothoracic Surgery (2024)
-
Can aerosol optical depth unlock the future of air quality monitoring and lung cancer prevention?
Environmental Sciences Europe (2024)
-
Climate change: why oncologists need to get involved
BJC Reports (2024)
-
Lung cancer in patients who have never smoked — an emerging disease
Nature Reviews Clinical Oncology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.