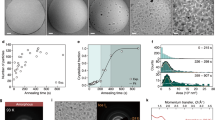
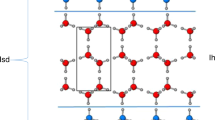
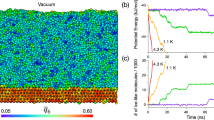
Abstract
Ice is present everywhere on Earth and has an essential role in several areas, such as cloud physics, climate change and cryopreservation. The role of ice is determined by its formation behaviour and associated structure. However, these are not fully understood1. In particular, there is a long-standing debate about whether water can freeze to form cubic ice—a currently undescribed phase in the phase space of ordinary hexagonal ice2,3,4,5,6. The mainstream view inferred from a collection of laboratory data attributes this divergence to the inability to discern cubic ice from stacking-disordered ice—a mixture of cubic and hexagonal sequences7,8,9,10,11. Using cryogenic transmission electron microscopy combined with low-dose imaging, we show here the preferential nucleation of cubic ice at low-temperature interfaces, resulting in two types of separate crystallization of cubic ice and hexagonal ice from water vapour deposition at 102 K. Moreover, we identify a series of cubic-ice defects, including two types of stacking disorder, revealing the structure evolution dynamics supported by molecular dynamics simulations. The realization of direct, real-space imaging of ice formation and its dynamic behaviour at the molecular level provides an opportunity for ice research at the molecular level using transmission electron microscopy, which may be extended to other hydrogen-bonding crystals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data generated and analysed in this study are included with the paper.
References
Bartels-Rausch, T. Ten things we need to know about ice and snow. Nature 494, 27–29 (2013).
König, H. Eine kubische Eismodifikation. Z. Kristallogr. Cryst. Mater. 105, 279–286 (1943).
Whalley, E. Scheiner’s halo: evidence for ice Ic in the atmosphere. Science 211, 389–390 (1981).
Kuhs, W., Bliss, D. & Finney, J. High-resolution neutron powder diffraction study of ice Ic. J. Phys. Colloq. 48, C1-631–C1-636 (1987).
Mayer, E. & Hallbrucker, A. Cubic ice from liquid water. Nature 325, 601–602 (1987).
Murray, B. J., Knopf, D. A. & Bertram, A. K. The formation of cubic ice under conditions relevant to Earth’s atmosphere. Nature 434, 202–205 (2005).
Moore, E. B. & Molinero, V. Is it cubic? Ice crystallization from deeply supercooled water. Phys. Chem. Chem. Phys. 13, 20008–20016 (2011).
Kuhs, W. F., Sippel, C., Falenty, A. & Hansen, T. C. Extent and relevance of stacking disorder in “ice Ic”. Proc. Natl Acad. Sci. USA 109, 21259–21264 (2012).
Malkin, T. L., Murray, B. J., Brukhno, A. V., Anwar, J. & Salzmann, C. G. Structure of ice crystallized from supercooled water. Proc. Natl Acad. Sci. USA 109, 1041–1045 (2012).
Lupi, L. et al. Role of stacking disorder in ice nucleation. Nature 551, 218–222 (2017).
Malkin, T. L. et al. Stacking disorder in ice I. Phys. Chem. Chem. Phys. 17, 60–76 (2015).
McMillan, J. A. & Los, S. C. Vitreous ice: irreversible transformations during warm-up. Nature 206, 806–807 (1965).
Morishige, K. & Uematsu, H. The proper structure of cubic ice confined in mesopores. J. Chem. Phys. 122, 044711 (2005).
Arnold, G. P., Finch, E. D., Rabideau, S. W. & Wenzel, R. G. Neutron‐diffraction study of ice polymorphs. III. Ice Ic. J. Chem. Phys. 49, 4365–4369 (1968).
Bertie, J. E., Calvert, L. D. & Whalley, E. Transformations of ice II, ice III, and ice V at atmospheric pressure. J. Chem. Phys. 38, 840–846 (1963).
Kuhs, W. F., Genov, G., Staykova, D. K. & Hansen, T. Ice perfection and onset of anomalous preservation of gas hydrates. Phys. Chem. Chem. Phys. 6, 4917–4920 (2004).
Falenty, A. & Kuhs, W. F. “Self-preservation” of CO2 gas hydrates—surface microstructure and ice perfection. J. Phys. Chem. B 113, 15975–15988 (2009).
Ghosh, J., Bhuin, R. G., Vishwakarma, G. & Pradeep, T. Formation of cubic ice via clathrate hydrate, prepared in ultrahigh vacuum under cryogenic conditions. J. Phys. Chem. Lett. 11, 26–32 (2020).
del Rosso, L. et al. Cubic ice Ic without stacking defects obtained from ice XVII. Nat. Mater. 19, 663–668 (2020).
Komatsu, K. et al. Ice Ic without stacking disorder by evacuating hydrogen from hydrogen hydrate. Nat. Commun. 11, 464 (2020).
Ma, R. et al. Atomic imaging of the edge structure and growth of a two-dimensional hexagonal ice. Nature 577, 60–63 (2020).
Kobayashi, K., Koshino, M. & Suenaga, K. Atomically resolved images of Ih ice single crystals in the solid phase. Phys. Rev. Lett. 106, 206101 (2011).
Mishima, O., Calvert, L. D. & Whalley, E. ‘Melting ice’ I at 77 K and 10 kbar: a new method of making amorphous solids. Nature 310, 393–395 (1984).
Pan, D., Wan, Q. & Galli, G. The refractive index and electronic gap of water and ice increase with increasing pressure. Nat. Commun. 5, 3919 (2014).
Tajima, Y., Matsuo, T. & Suga, H. Phase transition in KOH-doped hexagonal ice. Nature 299, 810–812 (1982).
Kobayashi, K. & Yasuda, H. Phase transition of ice Ic to ice XI under electron beam irradiation. Chem. Phys. Lett. 547, 9–12 (2012).
Małolepsza, E. & Keyes, T. Water freezing and ice melting. J. Chem. Theory Comput. 11, 5613–5623 (2015).
Steytler, D. C., Dore, J. C. & Wright, C. J. Neutron diffraction study of cubic ice nucleation in a porous silica network. J. Phys. Chem. 87, 2458–2459 (1983).
Davies, M. B., Fitzner, M. & Michaelides, A. Routes to cubic ice through heterogeneous nucleation. Proc. Natl Acad. Sci. USA 118, e2025245118 (2021).
Zhu, L. et al. Visualizing anisotropic oxygen diffusion in ceria under activated conditions. Phys. Rev. Lett. 124, 056002 (2020).
Egerton, R. F., Li, P. & Malac, M. Radiation damage in the TEM and SEM. Micron 35, 399–409 (2004).
Heide, H.-G. Observations on ice layers. Ultramicroscopy 14, 271–278 (1984).
Wang, L. et al. Microscopic kinetics pathway of salt crystallization in graphene nanocapillaries. Phys. Rev. Lett. 126, 136001 (2021).
Wang, L. et al. Synthesis of honeycomb-structured beryllium oxide via graphene liquid cells. Angew. Chem. Int. Ed. 59, 15734–15740 (2020).
Koch, C. T. Determination of Core Structure Periodicity and Point Defect Density along Dislocations. PhD thesis, Arizona State Univ. (2002).
Molinero, V. & Moore, E. B. Water modeled as an intermediate element between carbon and silicon. J. Phys. Chem. B 113, 4008–4016 (2009).
Moore, E. B. & Molinero, V. Ice crystallization in water’s “no-man’s land”. J. Chem. Phys. 132, 244504 (2010).
Thompson, A. P. et al. LAMMPS - a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51991344, 11974388, 11888101, U1932153, 11974001, and 11974024), National Key R&D Program of China (2021YFA1400204, 2021YFA1400500, and 2019YFA0307800), the Program from Chinese Academy of Sciences (XDB33030200, XDB33010400 and ZDYZ2015-1), Beijing Natural Science Foundation (Z190011), and Guangdong Major Project of Basic and Applied Basic Research (2021B0301030002). L.W. is grateful for the support from the Youth Innovation Promotion Association of CAS (2020009). J.C. thanks the Shanghai Supercomputer Center and the Tianhe-1A Supercomputer Center for computational resources.
Author information
Authors and Affiliations
Contributions
L.W. and X.B. supervised the project. X.H., L.Liao, H.S. and J.W. performed the experiment. X.T., Z.X., W.W., L.W. and X.B. built the experimental set-up. K.L. performed the computational calculations. J.C., Y.J. and E.W. advised on the theoretical modelling. L.Liu advised the sample processing. L.W. and X.B. conceived the idea and wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Yoshiaki Sugimoto and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Alignment and summing up of an image stack.
a, A single frame in a TEM image stack (40 frames within an irradiation time of 2 s in total) obtained using single-electron counting mode via K2 camera with an electron beam dose rate of 196 e−Å2/s. b, Corresponding FFT of image in (a). c, Band-pass filter. d, As-filtered TEM image of (a). e, Cross-correlation between one frame with (a) as the reference frame. f, Image-drift profile determined from filtered image using cross-correlations. g, Image acquired with eight drift-corrected frames from one stack binned together. h, Image formed by directly summing up all 40 frames without cross-correlations in the same stack as in (g). i, Image acquired by summing up eight frames from the same image stack used for images in (g) and (h) with combined band-pass filter and cross-correlation.
Extended Data Fig. 2 Heterogeneous nucleation and growth of ice Ic.
a, b, Snapshots of bright-field TEM images and corresponding FFTs used for tracking nucleation and crystallization of one Ic crystallite on graphene at about 102 K. Each image was obtained by summing eight aligned frames in one corresponding low-dose image stack acquired at a frame rate of 40/s with an irradiation time of 2 s and a dose rate of 156 e−Å2/s. Bright spots in the high-resolution images indicate water dimer column positions in lattices. In-situ images showed that ice-like clusters nucleated on substrate first, exhibiting amorphous features, and then aggregated to ice Ic nucleus, which gradually crystallized to form one nanometre-sized crystallite in about 20 min.
Extended Data Fig. 3 Orientation statistics of ice Ic crystallites grown from vapour deposition on graphene at about 102 K.
Orientation statistics of fully-grown ice Ic crystallites on one graphene substrate based on real-space measurements, showing ice Ic crystallites with a preference for <110> orientation. A total of 92 ice Ic crystallites with different crystallographic axis orientations were observed and measured using the same conditions on one graphene substrate. The orientation and the corresponding number of the ice Ic crystallites is annotated in the chart.
Extended Data Fig. 4 Growth of ice Ic and ice XI crystallites.
a–i, Snapshots of in-situ imaging showing the long-term ice deposition process. b, FFT of image in (a) demonstrated amorphous clusters formed on graphene in the initial deposition stage. Each image was obtained by summing eight aligned frames in one corresponding low-dose image stack acquired at a frame rate of 40/s and a dose rate of 108 e−Å2/s with an irradiation time of 0.4 s. Individual crystallites were numbered based on the order of nuclei appearance. j, Corresponding FFTs for different crystallites, showing five randomly oriented ice Ic structures (1, 2, 4–6) and one <\(\bar{3}13\)> crystallography zone axis-oriented ice XI (3). k, Snapshots of ice XI cluster. l, High-magnification micrograph of the respective crystallites. The out-of-plane growth direction of the crystallite one deviates slightly from the <110> axis, resulting in the high-magnification TEM image and corresponding FFT with low resolution.
Extended Data Fig. 5 Real-time growth process of ice Ic from vapour deposition at 102 K.
a, b, Snapshots and corresponding FFT of in-situ imaging showing the long-term ice Ic growth process from vapour deposition. High density of defects on (1\(\bar{1}\)1) plane cause streaking in the diffraction spots of ice Ic crystallite. Liquid nitrogen was refilled to the Dewar of the cryo-holder every three hours to maintain a low temperature of 102 K. c, High-magnification image and corresponding FFT of defects in the ice Ic crystallite in (a) and another new ice Ic crystallite nearby showing the continuous nucleation and then crowded growth of ice Ic from prolonged vapour deposition. The crowded growth of multiple ice Ic crystallites result in multiple sets of diffraction spots to appear in the corresponding FFT of the TEM image. Each image was obtained by summing eight aligned frames in one corresponding low-dose image stack acquired at a frame rate of 40/s and a dose rate of 126 e−/Å2/s with an irradiation time of 0.4 s. Scale bars, 20 nm for (a), (b); 2 nm for (c).
Extended Data Fig. 6 Single-crystalline ice Ic crystallites grown by vapour deposition at 102 K.
a–c, Low-magnification TEM image, high-magnification micrograph overlaid with atomic structure model, corresponding FFT, and computed TEM image of individual single-crystalline ice Ic crystallites oriented along <001>, <110>, and <111> zone axes, respectively. The smallest lattice spacing in real space is marked in the <110> image. The measured resolution for reciprocal space is also annotated in the FFT image. Electron beam dose: 50.8 e−/Å2 for (a), 47.6 e−/Å2 for (b); 37 e−/Å2 for (c). Electron beam irradiation time: 0.4 s for (a–c).
Extended Data Fig. 7 TEM micrographs of ice Ih crystallite formation by vapour deposition at 102 K.
a, Sequential micrograph showing heterogeneous nucleation of one <001>-oriented ice Ih. Start time was defined as the time when the observation started. Each image was captured at an electron dose rate of 119 e−/Å2/s and a total dose of 47.6 e−/Å2. b–d, Low-magnification image, high-magnification image overlaid with atomic structure model, corresponding FFT, and computed image of separate ice Ih crystallite with a <001>, <011>, and <121> orientation, respectively. Illumination electron beam dose and time: ice Ih <001>, 50.4 e−/Å2 and 0.4 s; ice Ih <011>, 45.6 e−/Å2 and 0.4 s; ice Ih <121>, 43.2 e−/Å2 and 0.4 s.
Extended Data Fig. 8 Polymorphic ice I growth from vapour deposition at about 102 K.
a–c, Low-magnification image, corresponding electron diffraction pattern, and diffraction intensity profiles of deposits from different randomly-selected micrometre-range areas at different time, showing structural variation of vapour deposition on graphene. Skewed diffuse Bragg peaks are labelled by ice Ic planes for the initial crystalline phase. Bragg peak positions for ice Ih, which also increase gradually with deposition time, are marked on the final intensity profile. The broadening and splitting of the diffraction peaks indicate the mixing of the two polymorphs. d–f, Low-magnification image, corresponding electron diffraction pattern, and electron diffraction intensity profiles of ice deposition on ultrathin carbon from different randomly-selected micrometre-range areas at different time, indicating mainly amorphous solid water giving way to ice Ic during the embryonic stages. Skewed diffuse Bragg peaks indicate unmistakable contribution of ice Ih. Shoulders around Ic {111} peak position are annotated with the relevant planes of ice Ih in red colour. g, Micrograph of a region where two polymorphs coexist, the respective high-magnification image and the corresponding FFT of the two polymorphic crystallites, showing one <011>-oriented ice Ih and one <110>-oriented ice Ic formed together by vapour deposition on graphene after cooling for 8 h at about 102 K. h, Quantitatively calculated heterogeneous free energy profiles as a function of the cluster size r of two polymorphs, showing the experimentally observed results where the ice Ic dominates the polymorphic heterogeneous nucleation events. Electron beam dose rate: ~240 e−/Å2/s for (a)–(f). Irradiation time: 0.4 s for (a)–(f).
Extended Data Fig. 9 Evolution of planar defects during ice Ic growth.
a, Sequential TEM images of one defective ice Ic during growth. Illumination electron beam doses are annotated in each image. All images were obtained with an electron illumination time of 0.4 s. b–d, Zoom-in snapshot micrographs of different regions in (a) showing first aggregation and then annihilation of typical planar defects that are intermediate states to each other through slip planes in ice Ic during crystallite growth. Different defect types, including twin boundaries (TBs), stacking faults (SFs), and phase boundaries (PBs), are marked with dashed lines of different colours.
Extended Data Fig. 10 Defective ice Ic growth. Growth of one defective ice Ic crystallite.
a–d, Time-lapsed TEM images of one ice Ic crystallite (top row) and corresponding selected area FFTs (bottom row). The corresponding illumination electron beam dose of each image is 47 e−/Å2. Electron irradiation time for each image is 0.4 s. Facets were indexed as labelled on images. Area I is the single crystalline Ic matrix along <110> zone axis. Area II shows the high density of stacking faults on (\(1\bar{1}1\)) plane based on diffractometry information. Both single-crystalline Ic matrix and the defected area grew over time with no phase transition during the observation time.
Supplementary information
Supplementary Video 1 Visualization of the defect structure dynamics in ice Ic.
Real-time molecular-resolution snapshot TEM records of defects in an <110>-oriented ice Ic crystallite illuminated by 300-kV accelerated high-energy electron beam for 10 s, showing the slip and interlayer transfer behaviour of defective ice layers on close-packed planes. The bright spots in the video frame indicate the water-dimer column positions in the ice Ic lattice. With the increase in the electron-beam illumination time and dose, the defect layer and the water-dimer columns in the adjacent ice layer undergo cooperative distortion, which leads to the climbing of the defect layer along the close-packing direction.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Wang, L., Liu, K. et al. Tracking cubic ice at molecular resolution. Nature 617, 86–91 (2023). https://doi.org/10.1038/s41586-023-05864-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05864-5
This article is cited by
-
Observing growth and interfacial dynamics of nanocrystalline ice in thin amorphous ice films
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.