Abstract
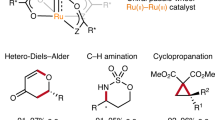
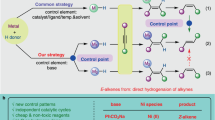
Asymmetric transition-metal catalysis represents a powerful strategy for accessing enantiomerically enriched molecules1,2,3. The classical strategy for inducing enantioselectivity with transition-metal catalysts relies on direct complexation of chiral ligands to produce a sterically constrained reactive metal site that allows formation of the major product enantiomer while effectively inhibiting the pathway to the minor enantiomer through steric repulsion4. The chiral-ligand strategy has proven applicable to a wide variety of highly enantioselective transition-metal-catalysed reactions, but important scenarios exist that impose limits to its successful adaptation. Here, we report a new approach for inducing enantioselectivity in transition-metal-catalysed reactions that relies on neutral hydrogen-bond donors (HBDs)5,6 that bind anions of cationic transition-metal complexes to achieve enantiocontrol and rate enhancement through ion pairing together with other non-covalent interactions7,8,9. A cooperative anion-binding effect of a chiral bis-thiourea HBD is demonstrated to lead to high enantioselectivity (up to 99% enantiomeric excess) in intramolecular ruthenium-catalysed propargylic substitution reactions10. Experimental and computational mechanistic studies show the attractive interactions between electron-deficient arene components of the HBD and the metal complex that underlie enantioinduction and the acceleration effect.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings in this work are available within the paper and Supplementary Information.
References
Noyori, R. Asymmetric catalysis: science and opportunities (Nobel Lecture). Angew. Chem. Int. Edn 41, 2008–2022 (2002).
Sharpless, K. B. Searching for new reactivity (Nobel Lecture). Angew. Chem. Int. Edn 41, 2024–2032 (2002).
Knowles, W. S. Asymmetric hydrogenations (Nobel Lecture 2001). Adv. Synth. Catal. 345, 3–13 (2003).
Pfaltz, A. & Drury, W. J. Design of chiral ligands for asymmetric catalysis: from C2-symmetric P,P- and N,N-ligands to sterically and electronically nonsymmetrical P,N-ligands. Proc. Natl Acad. Sci. USA 101, 5723–5726 (2004).
Nishikawa, Y. Recent topics in dual hydrogen bonding catalysis. Tetrahedron Lett. 59, 216–223 (2018).
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007).
Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Edn 52, 534–561 (2013).
García-Mancheño, O. Anion-Binding Catalysis (John Wiley & Sons, 2022).
Knowles, R. R. & Jacobsen, E. N. Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts. Proc. Natl Acad. Sci. USA 107, 20678–20685 (2010).
Nishibayashi, Y., Inada, Y., Hidai, M. & Uemura, S. Ruthenium-catalyzed carbon–carbon bond formation between propargylic alcohols and alkenes via the allenylidene-ene reaction. J. Am. Chem. Soc. 125, 6060–6061 (2003).
Ranieri, B., Escofet, I. & Echavarren, A. M. Anatomy of gold catalysts: facts and myths. Org. Biomol. Chem. 13, 7103–7118 (2015).
Michaelson, R. C., Palermo, R. E. & Sharpless, K. B. Chiral hydroxamic acids as ligands in the vanadium catalyzed asymmetric epoxidation of allylic alcohols by tert-butyl hydroperoxide. J. Am. Chem. Soc. 99, 1990–1992 (1977).
Yoshino, T., Satake, S. & Matsunaga, S. Diverse approaches for enantioselective C−H functionalization reactions using group 9 CpXMIII catalysts. Chem. Eur. J. 26, 7346–7357 (2020).
Phipps, R. J., Hamilton, G. L. & Toste, F. D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nat. Chem. 4, 603–614 (2012).
Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis: concept, definition, and applications. Angew. Chem. Int. Edn 52, 518–533 (2013).
Hamilton, G. L., Kang, E. J., Mba, M. & Toste, F. D. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science 317, 496–499 (2007).
Mukherjee, S. & List, B. Chiral counteranions in asymmetric transition-metal catalysis: highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 129, 11336–11337 (2007).
Satake, S. et al. Pentamethylcyclopentadienyl rhodium(III)–chiral disulfonate hybrid catalysis for enantioselective C–H bond functionalization. Nat. Catal. 1, 585–591 (2018).
Raducan, M., Moreno, M., Bour, C. & Echavarren, A. M. Phosphate ligands in the gold(I)-catalysed activation of enynes. Chem. Commun. 48, 52–54 (2012).
Kündig, E. P., Saudan, C. M. & Bernardinelli, G. A stable and recoverable chiral Ru Lewis acid: synthesis, asymmetric Diels–Alder catalysis and structure of the Lewis acid methacrolein complex. Angew. Chem. Int. Edn 38, 1219–1223 (1999).
Alvarez, S. Coordinating ability of anions, solvents, amino acids, and gases towards alkaline and alkaline-earth elements, transition metals, and lanthanides. Chem. Eur. J. 26, 4350–4377 (2020).
Nguyen, B. N. et al. Deconvolution of the mechanism of homogeneous gold-catalyzed reactions. Organometallics 31, 2395–2402 (2012).
Jindal, G. & Sunoj, R. B. Mechanistic insights on cooperative asymmetric multicatalysis using chiral counterions. J. Org. Chem. 79, 7600–7606 (2014).
Biasiolo, L. et al. Unexpected anion effect in the alkoxylation of alkynes catalyzed by N-heterocyclic carbene (NHC) cationic gold complexes. Chem. Eur. J. 20, 14594–14598 (2014).
Klausen, R. S. & Jacobsen, E. N. Weak Brønsted acid–thiourea co-catalysis: enantioselective, catalytic protio-Pictet–Spengler reactions. Org. Lett. 11, 887–890 (2009).
Xu, H., Zuend, S. J., Woll, M. G., Tao, Y. & Jacobsen, E. N. Asymmetric cooperative catalysis of strong Brønsted acid-promoted reactions using chiral ureas. Science 327, 986–990 (2010).
Banik, S. M., Levina, A., Hyde, A. M. & Jacobsen, E. N. Lewis acid enhancement by hydrogen-bond donors for asymmetric catalysis. Science 358, 761–764 (2017).
Trotta, A. H. & Jacobsen, E. N. in Anion-Binding Catalysis (ed. García-Mancheño, O.) 141–159 (John Wiley & Sons, 2022).
Mo, J. & Xiao, J. The Heck reaction of electron-rich olefins with regiocontrol by hydrogen-bond donors. Angew. Chem. Int. Edn 45, 4152–4157 (2006).
Ruan, J., Iggo, J. A., Berry, N. G. & Xiao, J. Hydrogen-bonding-promoted oxidative addition and regioselective arylation of olefins with aryl chlorides. J. Am. Chem. Soc. 132, 16689–16699 (2010).
Farney, E. P. et al. Discovery and elucidation of counteranion dependence in photoredox catalysis. J. Am. Chem. Soc. 141, 6385–6391 (2019).
Franchino, A., Martí, À., Nejrotti, S. & Echavarren, A. M. Silver-free Au(I) catalysis enabled by bifunctional urea- and squaramide-phosphine ligands via H-bonding. Chem. Eur. J. 27, 11989–11996 (2021).
Franchino, A., Martí, À. & Echavarren, A. M. H-bonded counterion-directed enantioselective Au(I) catalysis. J. Am. Chem. Soc. 144, 3497–3509 (2022).
Zhang, X. et al. Asymmetric azide–alkyne cycloaddition with Ir(I)/squaramide cooperative catalysis: atroposelective synthesis of axially chiral aryltriazoles. J. Am. Chem. Soc. 144, 6200–6207 (2022).
Hu, Q., He, Z., Peng, L. & Guo, C. Combining nickel and squaramide catalysis for the stereodivergent α-propargylation of oxindoles. Nat. Synth. 1, 322–331 (2022).
Li, M.-L., Yu, J.-H., Li, Y.-H., Zhu, S.-F. & Zhou, Q.-L. Highly enantioselective carbene insertion into N–H bonds of aliphatic amines. Science 366, 990–994 (2019).
Furniel, L. G., Echemendía, R. & Burtoloso, A. C. B. Cooperative copper-squaramide catalysis for the enantioselective N–H insertion reaction with sulfoxonium ylides. Chem. Sci. 12, 7453–7459 (2021).
Simlandy, A. K., Ghosh, B. & Mukherjee, S. Enantioselective [4 + 2]-annulation of azlactones with copper-allenylidenes under cooperative catalysis: synthesis of α-quaternary α-acylaminoamides. Org. Lett. 21, 3361–3366 (2019).
Guan, Y., Attard, J. W., Visco, M. D., Fisher, T. J. & Mattson, A. E. Enantioselective catalyst systems from copper(II) triflate and BINOL–silanediol. Chem. Eur. J. 24, 7123–7127 (2018).
Nishibayashi, Y., Wakiji, I. & Hidai, M. Novel propargylic substitution reactions catalyzed by thiolate-bridged diruthenium complexes via allenylidene intermediates. J. Am. Chem. Soc. 122, 11019–11020 (2000).
Miyake, Y., Uemura, S. & Nishibayashi, Y. Catalytic propargylic substitution reactions. Chem. Cat. Chem. 1, 342–356 (2009).
Nishibayashi, Y. et al. Ruthenium-catalyzed propargylic substitution reactions of propargylic alcohols with oxygen-, nitrogen-, and phosphorus-centered nucleophiles. Chem. Eur. J. 11, 1433–1451 (2005).
Nishibayashi, Y., Yoshikawa, M., Inada, Y., Hidai, M. & Uemura, S. Ruthenium-catalyzed propargylation of aromatic compounds with propargylic alcohols. J. Am. Chem. Soc. 124, 11846–11847 (2002).
Fukamizu, K., Miyake, Y. & Nishibayashi, Y. Ruthenium-catalyzed enantioselective carbon−carbon bond forming reaction via allenylidene-ene process: synthetic approach to chiral heterocycles such as chromane, thiochromane, and 1,2,3,4-tetrahydroquinoline derivatives. J. Am. Chem. Soc. 130, 10498–10499 (2008).
Reisman, S. E., Doyle, A. G. & Jacobsen, E. N. Enantioselective thiourea-catalyzed additions to oxocarbenium ions. J. Am. Chem. Soc. 130, 7198–7199 (2008).
Lehnherr, D., Ford, D. D., Bendelsmith, A. J., Kennedy, C. R. & Jacobsen, E. N. Conformational control of chiral amido-thiourea catalysts enables improved activity and enantioselectivity. Org. Lett. 18, 3214–3217 (2016).
Kennedy, C. R. et al. Mechanism-guided development of a highly active bis-thiourea catalyst for anion-abstraction catalysis. J. Am. Chem. Soc. 138, 13525–13528 (2016).
Park, Y. et al. Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions. Science 355, 162–166 (2017).
Levi, S. M., Li, Q., Rötheli, A. R. & Jacobsen, E. N. Catalytic activation of glycosyl phosphates for stereoselective coupling reactions. Proc. Natl Acad. Sci. USA 116, 35–39 (2019).
Mayfield, A. B., Metternich, J. B., Trotta, A. H. & Jacobsen, E. N. Stereospecific furanosylations catalyzed by bis-thiourea hydrogen-bond donors. J. Am. Chem. Soc. 142, 4061–4069 (2020).
Li, Q., Levi, S. M., Wagen, C. C., Wendlandt, A. E. & Jacobsen, E. N. Site-selective, stereocontrolled glycosylation of minimally protected sugars. Nature 608, 74–79 (2022).
Knowles, R. R., Lin, S. & Jacobsen, E. N. Enantioselective thiourea-catalyzed cationic polycyclizations. J. Am. Chem. Soc. 132, 5030–5032 (2010).
Ronchi, E., Paradine, S. M. & Jacobsen, E. N. Enantioselective, catalytic multicomponent synthesis of homoallylic amines enabled by hydrogen-bonding and dispersive interactions. J. Am. Chem. Soc. 143, 7272–7278 (2021).
Taylor, M. S., Tokunaga, N. & Jacobsen, E. N. Enantioselective thiourea-catalyzed acyl-Mannich reactions of isoquinolines. Angew. Chem. Int. Edn 44, 6700–6704 (2005).
Raheem, I. T., Thiara, P. S., Peterson, E. A. & Jacobsen, E. N. Enantioselective Pictet–Spengler-type cyclizations of hydroxylactams: H-bond donor catalysis by anion binding. J. Am. Chem. Soc. 129, 13404–13405 (2007).
Liao, S. & List, B. Asymmetric counteranion-directed transition-metal catalysis: enantioselective epoxidation of alkenes with manganese(III) salen phosphate complexes. Angew. Chem. Int. Edn 49, 628–631 (2010).
Myers, B. J. Common Solvents Used in Organic Chemistry: Table of Properties https://organicchemistrydata.org/solvents/ (2005).
Acknowledgements
This work was supported by the National Institutes of Health through grant no. GM43214, NSF predoctoral fellowship (DGE1745303) and Bristol-Myers Squibb Graduate Research fellowship to J.M.O., and Alfred Bader Fellowship in Chemistry to P.V. We thank S.-L. Zheng (Harvard University) for determination of the X-ray crystal structures, Q. Li for assistance with catalyst synthesis, and D. Diaz, J. Gair, C. Wagen and J. Wong for helpful discussions.
Author information
Authors and Affiliations
Contributions
E.N.J. conceived the work, P.V. and J.M.O. designed and conducted the experiments, E.N.J. supervised and directed the research, and all authors wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Anita Mattson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ovian, J.M., Vojáčková, P. & Jacobsen, E.N. Enantioselective transition-metal catalysis via an anion-binding approach. Nature 616, 84–89 (2023). https://doi.org/10.1038/s41586-023-05804-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05804-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.