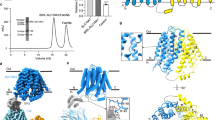
Abstract
Cyclic dinucleotides (CDNs) are ubiquitous signalling molecules in all domains of life1,2. Mammalian cells produce one CDN, 2′3′-cGAMP, through cyclic GMP–AMP synthase after detecting cytosolic DNA signals3,4,5,6,7. 2′3′-cGAMP, as well as bacterial and synthetic CDN analogues, can act as second messengers to activate stimulator of interferon genes (STING) and elicit broad downstream responses8,9,10,11,12,13,14,15,16,17,18,19,20,21. Extracellular CDNs must traverse the cell membrane to activate STING, a process that is dependent on the solute carrier SLC19A122,23. Moreover, SLC19A1 represents the major transporter for folate nutrients and antifolate therapeutics24,25, thereby placing SLC19A1 as a key factor in multiple physiological and pathological processes. How SLC19A1 recognizes and transports CDNs, folate and antifolate is unclear. Here we report cryo-electron microscopy structures of human SLC19A1 (hSLC19A1) in a substrate-free state and in complexes with multiple CDNs from different sources, a predominant natural folate and a new-generation antifolate drug. The structural and mutagenesis results demonstrate that hSLC19A1 uses unique yet divergent mechanisms to recognize CDN- and folate-type substrates. Two CDN molecules bind within the hSLC19A1 cavity as a compact dual-molecule unit, whereas folate and antifolate bind as a monomer and occupy a distinct pocket of the cavity. Moreover, the structures enable accurate mapping and potential mechanistic interpretation of hSLC19A1 with loss-of-activity and disease-related mutations. Our research provides a framework for understanding the mechanism of SLC19-family transporters and is a foundation for the development of potential therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The cryo-EM maps have been deposited into the Electron Microscopy Data Bank under accession numbers EMD-33386 (apo hSLC19A1), EMD-33389 (hSLC19A1 + 2′3′-cGAMP), EMD-33387 (hSLC19A1 + 3′3′-CDA), EMD-33388 (hSLC19A1 + 2′3′-CDAS), EMD-34176 (hSLC19A1 + 5-MTHF) and EMD-34177 (hSLC19A1 + PMX). The coordinates have been deposited at the Protein Data Bank under accession numbers 7XPZ (apo hSLC19A1), 7XQ2 (hSLC19A1 + 2′3′-cGAMP), 7XQ0 (hSLC19A1 + 3′3′-CDA), 7XQ1 (hSLC19A1 + 2′3′-CDAS), 8GOE (hSLC19A1 + 5-MTHF) and 8GOF (hSLC19A1 + PMX).
References
Danilchanka, O. & Mekalanos, J. J. Cyclic dinucleotides and the innate immune response. Cell 154, 962–970 (2013).
Zaver, S. A. & Woodward, J. J. Cyclic dinucleotides at the forefront of innate immunity. Curr. Opin. Cell Biol. 63, 49–56 (2020).
Wu, J. X. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013).
Sun, L. J., Wu, J. X., Du, F. H., Chen, X. & Chen, Z. J. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Gao, P. et al. Cyclic [G(2′,5′) pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153, 1094–1107 (2013).
Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013).
Diner, E. J. et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 3, 1355–1361 (2013).
Zhang, X. et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51, 226–235 (2013).
Gao, P. et al. Structure-function analysis of STING activation by c[G(2′,5′) pA(3′,5′)p] and targeting by antiviral DMXAA. Cell 154, 748–762 (2013).
Shang, G. J., Zhang, C. G., Chen, Z. J. J., Bai, X. C. & Zhang, X. W. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature 567, 389–393 (2019).
Hopfner, K. P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Zhang, C. G. et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature 567, 394–398 (2019).
Zhao, B. Y. et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature 569, 718–722 (2019).
Liu, S. Q. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, 1217–U1217 (2015).
Zhao, B. Y. et al. Structural basis for concerted recruitment and activation of IRF-3 by innate immune adaptor proteins. Proc. Natl Acad. Sci. USA 113, E3403–E3412 (2016).
Mann, C. C. D. et al. Modular architecture of the STING C-terminal tail allows interferon and NF-κB signaling adaptation. Cell Rep. 27, 1165–1175 (2019).
Gui, X. et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 567, 262–266 (2019).
Burdette, D. L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011).
McWhirter, S. M. et al. A host type I interferon response is induced by cytosolic sensing of the bacterial second messenger cyclic-di-GMP. J. Exp. Med. 206, 1899–1911 (2009).
Woodward, J. J., Iavarone, A. T. & Portnoy, D. A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328, 1703–1705 (2010).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Luteijn, R. D. et al. SLC19A1 transports immunoreactive cyclic dinucleotides. Nature 573, 434–438 (2019).
Ritchie, C., Cordova, A. F., Hess, G. T., Bassik, M. C. & Li, L. Y. SLC19A1 is an importer of the immunotransmitter cGAMP. Mol. Cell 75, 372–381 (2019).
Matherly, L. H. & Hou, Z. J. Structure and function of the reduced folate carrier: a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitam. Horm. 79, 145–184 (2008).
Zhao, R., Matherly, L. H. & Goldman, I. D. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 11, e4 (2009).
Deng, L. F. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Wang, H. et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl Acad. Sci. USA 114, 1637–1642 (2017).
Demaria, O. et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl Acad. Sci. USA 112, 15408–15413 (2015).
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 7, 283ra52 (2015).
Marcus, A. et al. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity 49, 754–763 (2018).
Carozza, J. A. et al. Extracellular cGAMP is a cancer cell-produced immunotransmitter involved in radiation-induced anti-cancer immunity. Nat. Cancer 1, 184–196 (2020).
Ahn, J., Son, S., Oliveira, S. C. & Barber, G. N. STING-dependent signaling underlies IL-10 controlled inflammatory colitis. Cell Rep. 21, 3873–3884 (2017).
Maltbaek, J. H. et al. ABCC1 transporter exports the immunostimulatory cyclic dinucleotide cGAMP. Immunity 55, 1799–1812 (2022).
Ganapathy, V., Smith, S. B. & Prasad, P. D. SLC19: the folate/thiamine transporter family. Pflugers Arch. 447, 641–646 (2004).
Marchant, J. S., Subramanian, V. S., Parker, I. & Said, H. M. Intracellular trafficking and membrane targeting mechanisms of the human reduced folate carrier in mammalian epithelial cells. J. Biol. Chem. 277, 33325–33333 (2002).
Liu, X. Y., Witt, T. L. & Matherly, L. H. Restoration of high-level transport activity by human reduced folate carrier/ThTr1 thiamine transporter chimaeras: role of the transmembrane domain 6/7 linker region in reduced folate carrier function. Biochem. J. 369, 31–37 (2003).
Lahey, L. J. et al. LRRC8A:C/E heteromeric channels are ubiquitous transporters of cGAMP. Mol. Cell 80, 578–591 (2020).
Zhou, C. et al. Transfer of cGAMP into bystander cells via LRRC8 volume-regulated anion channels augments STING-mediated interferon responses and anti-viral immunity. Immunity 52, 767–781 (2020).
Cordova, A. F., Ritchie, C., Bohnert, V. & Li, L. Y. Human SLC46A2 is the dominant cGAMP importer in extracellular cGAMP-sensing macrophages and monocytes. ACS Cent. Sci. 7, 1073–1088 (2021).
Parker, J. L. et al. Structural basis of antifolate recognition and transport by PCFT. Nature 595, 130–134 (2021).
Wright, N. J. et al. Methotrexate recognition by the human reduced folate carrier SLC19A1. Nature 609, 1056–1062 (2022).
Ritchie, T. et al. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 (2009).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Wu, C., Huang, X., Cheng, J., Zhu, D. & Zhang, X. High-quality, high-throughput cryo-electron microscopy data collection via beam tilt and astigmatism-free beam-image shift. J. Struct. Biol. 208, 107396 (2019).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Wang, N. et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370–383 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
The PyMOL molecular graphics system. Version 1.0 (Schrodinger, 2010).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Visentin, M., Zhao, R. & Goldman, I. D. Augmentation of reduced folate carrier-mediated folate/antifolate transport through an antiport mechanism with 5-aminoimidazole-4-carboxamide riboside monophosphate. Mol. Pharmacol. 82, 209–216 (2012).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35 1997–2004 (2014).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Acknowledgements
Cryo-EM data collection was carried out at the Center for Biological Imaging, Core Facilities for Protein Science at the Institute of Biophysics, Chinese Academy of Sciences. Computation work was performed using the high-performance computing resources at the Center for Biological Imaging, Institute of Biophysics, Chinese Academy of Science. All radioactivity experiments were performed at the Radioactive Isotope Laboratory (Institute of Biophysics, CAS), with guidance from H. J. Zhang in handling radioactive materials. We thank F. Wang and his group members for their help in antibody screening. The plasmid expressing membrane scaffold protein 1D1 (MSP1D1) was a gift from Z. Liu’s group. This work was supported by grants from National Key R&D Program of China (2018YFA0507203, 2018YFA0508000), National Natural Science Foundation of China (32130057, 31922037, 81921005, 32171219 and 82001681), Beijing Natural Science Foundation (Z220018), CAS Project for Young Scientists in Basic Research (YSBR-074), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB37030203 and XDB29050100) and China Postdoctoral Science Foundation (BX2021039).
Author information
Authors and Affiliations
Contributions
Q.Z., Y. Zhu and P.S. purified proteins, prepared cryo-EM samples, collected and processed cryo-EM data, and reconstructed density maps. Y. Zhu, Q.Z. and P.G. built and refined models. X.Z. and Q.Z. performed antibody screening and validation. X.Z. performed cellular assays. Y. Zhang, Z.L. and J.L. performed docking and molecular dynamics simulation. Liwei Zhang, J.M., X.N. and L. Zeng assisted with antibody screening. Y.G. assisted with cryo-EM data process and reconstruction. S.L. and A.G. assisted with cell culture and protein expression. P.G., Liguo Zhang and A.G. initiated the project and directed the research. P.G. wrote the manuscript with the help of all of the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Larry Matherly and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Structure of hSLC19A1 and distribution of critical mutations.
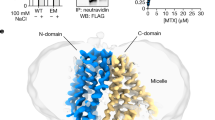
a, Cut-open side view (left), side view (middle), and cytosolic bottom view (right) of hSLC19A1‘s conservation surface mapping using ConSurf. The cytosolic entrance is indicated by dashed white oval. b, Extracellular top view of the hSLC19A1 inward-open structure, with extracellular loops and TM1/2/7/8 helices highlighted in red. c, Hydrophobic interactions of IL6-7 (aa 204–214) (salmon) with surrounding elements (grey). Key residues are shown as sticks and dots. d, Hydrogen bonding interactions of IL6-7 (aa 204–214) with surrounding elements. Key residues are shown as sticks and hydrogen bonds are represented as dashed lines. e,f, Effect of IL6-7 (aa 204-214) mutant on extracellular CDN signalling. 293T-STING cells were transfected with empty vector, WT hSLC19A1, or IL6-7 (aa 204–214) mutant, and followed by 2′3′-cGAMP stimulation or left untreated. Immunoblot analyses were carried out using the antibodies of IRF3, phospho-IRF3, STING, α-Tubulin and SLC19A1. Representative results from four independent experiments are shown. Data are mean ± s.e.m. of n = 4 independent experiments. **, p ≤ 0.01 by unpaired, two-tailed Student‘s t-test. For gel sorce data, see Supplementary Fig. 3. g, Distribution of previously proposed important residues of hSLC19A1, as well as hSLC19A1 mutations found in antifolate drug resistant cell lines and cancer patients (from BioMuta database with functional prediction probability >0.99). For the corresponding human residue number, references and study strategies, see Supplementary Table 2.
Extended Data Fig. 2 Cryo-EM data processing of hSLC19A1 bound to 3′3′-CDA.
a, Representative raw micrograph of hSLC19A1-Fab-3′3′-CDA complex in nanodisc. b, Selected 2D class averages of hSLC19A1-Fab-3′3′-CDA complex particles. c, Data processing flow chart. d, Gold-standard Fourier shell correlation curves of the final reconstruction. e, Local resolution distribution of hSLC19A1-Fab-3′3′-CDA complex. f, Cryo-EM densities of transmembrane helices and intracellular loop IL6-7 at 5σ.
Extended Data Fig. 3 Cryo-EM data processing of hSLC19A1 bound to 2′3′-CDAS.
a, Representative raw micrograph of hSLC19A1-Fab-2′3′-CDAS complex in nanodisc. b, Selected 2D class averages of hSLC19A1-Fab-2′3′-CDAS complex particles. c, Data processing flow chart. d, Gold-standard Fourier shell correlation curves of the final reconstruction. e, Local resolution distribution of hSLC19A1-Fab-2′3′-CDAS complex. f, Cryo-EM densities of transmembrane helices and intracellular loop IL6-7 at 5σ.
Extended Data Fig. 4 Cryo-EM data processing of hSLC19A1 bound to 2′3′-cGAMP.
a, Representative raw micrograph of hSLC19A1-Fab-2′3′-cGAMP complex in nanodisc. b, Selected 2D class averages of hSLC19A1-Fab-2′3′-cGAMP complex particles. c, Data processing flow chart. d, Gold-standard Fourier shell correlation curves of the final reconstruction. e, Local resolution distribution of hSLC19A1-Fab-2′3′-cGAMP complex. f, Cryo-EM densities of transmembrane helices and intracellular loop IL6-7 at 5σ.
Extended Data Fig. 5 Chemical structures, densities, structural superimposition, and MD simulations of CDNs.
a–c, Chemical structures of 3′3′-CDA (a), 2′3′-CDAS (b), and 2′3′-cGAMP (c). d–f, Cryo-EM densities of 3′3′-CDA (d), 2′3′-CDAS (e), and 2′3′-cGAMP (f) at 8σ, 7σ, and 6σ, respectively. Two bound CDN molecules in all three structures are coloured as in Fig. 2. g, Structural superimposition for hSLC19A1 structures in apo and CDN-bound states. h, Cryo-EM densities of 3'3'-CDA at 2.5:1 (left; 7σ) and 0.5:1 (right; 5σ) ligand:protein molar ratios. i, The backbone RMSD of hSLC19A1 except F211-V250 from the cryo-EM structures for the simulation (top). The heavy atom RMSD of 3′3′-CDA from the initial position for the simulation (bottom). j–l, Alignments of initial state (violet) and final state (green) of the simulation for 3′3′-CDA-dimer (j), 3′3′-CDA-monomer-upper (k), and 3′3′-CDA-monomer-lower (l). m, Different conformations of the bound 3'3'-CDA, 2′3′-CDAS, and 2′3′-cGAMP based on structural superimposition results.
Extended Data Fig. 6 Interactions between hSLC19A1 and 3′3′-CDA/2′3′-CDAS.
a,c, Cut-open side view of electrostatic potential surface of hSLC19A1-3′3′-CDA (a) or hSLC19A1-2′3′-CDAS (c) complex. b,d, Slab through the surface of hSLC19A1 in a cytosolic bottom view, which highlights the binding position of 3′3′-CDA (b) or 2′3′-CDAS (d) relative to the cavity. e,f, Two views of stacking interactions between hSLC19A1 and 3′3′-CDA (e) or 2′3′-CDAS (f). g–j, Hydrogen bonding interactions between hSLC19A1 and 3′3′-CDA (g,h) or 2′3′-CDAS (i,j) from bottom/intracellular (g,i) and top/extracellular (h,j) views. All panels share similar representations and colour codes as in Fig. 3.
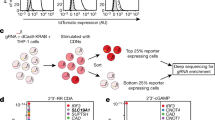
Extended Data Fig. 7 Effects of CDN-binding pocket mutants on extracellular CDN signalling.
a, Small conformational changes of the residues participated in CDN binding in 3′3′-CDA-, 2′3′-CDAS-, and 2′3′-cGAMP-bound structures. b,c, Effects of CDN-binding pocket mutants on extracellular CDN signalling. Experiments and quantification were performed as in Extended Data Fig. 1e, f. Data are mean ± s.e.m. of n = 3 independent experiments. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; NS, not significant. For gel sorce data, see Supplementary Fig. 3. d, Identification of SLC19A1 mutations in THP-1 and HeLa cell lines. The table (left) summaries the deletions and insertions for SLC19A1 alleles in THP-1 and HeLa cell lines. The diagrams (right) depict the predicted coding DNA sequence of SLC19A1 alleles in SLC19A1−/− cells. Boxes represent exons of SLC19A1 and horizontal lines represent introns. e, Expression of hSLC19A1 and its mutants in SLC19A1−/− THP-1 cells. Wild type and mutants were cloned into the lentiviral vector fusion with P2A-GFP which enables SLC19A1 and its mutant expression to be quantified via the expression of green fluorescence protein (GFP). SLC19A1 and its mutants (P2A-GFP+) were sorted and analysed by FACS. f,g Conserved function of SLC19A1-mediated CDN-transport. Comparison of CDN transport activities of human (h), mouse (m), and xenopus (x) SLC19A1. 293T-STING cells were transfected with SLC19A1 from different species, then the transfected cells were stimulated with 2′3′-cGAMP or left unstimulated. CDN transport activity was quantified by phospho-IRF3 and normalized to GFP (a surrogate for SLC19A1 expression). Data are mean ± s.e.m. of n=3 independent experiments. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001 by two-tailed unpaired Student‘s t-test. For gel sorce data, see Supplementary Fig. 3.
Extended Data Fig. 8 Cryo-EM data processing of hSLC19A1 bound to 5-MTHF.
a, Representative raw micrograph of hSLC19A1-Fab-5-MTHF complex in nanodisc. b, Selected 2D class averages of hSLC19A1-Fab-5-MTHF complex particles. c, Data processing flow chart. d, Gold-standard Fourier shell correlation curves of the final reconstruction. e, Local resolution distribution of hSLC19A1-Fab-5-MTHF complex. f, Chemical structure of 5-MTHF. g, Cryo-EM densities of 5-MTHF at 8σ.
Extended Data Fig. 9 Cryo-EM data processing of hSLC19A1 bound to PMX.
a, Representative raw micrograph of hSLC19A1-Fab-PMX complex in nanodisc. b, Selected 2D class averages of hSLC19A1-Fab-PMX complex particles. c, Data processing flow chart. d, Gold-standard Fourier shell correlation curves of the final reconstruction. e, Local resolution distribution of hSLC19A1-Fab-PMX complex. f, Chemical structure of PMX. g, Cryo-EM densities of PMX at 3σ.
Extended Data Fig. 10 hSLC19A1-PMX interaction, protein expression, and different binding strategies for multiple substrates.
a, Hydrogen bonding interactions between PMX (sticks; wheat) and hSLC19A1 (grey). Key residues are shown as sticks and hydrogen bonds are represented as dashed lines. b, Hydrophobic interactions between PMX (sticks; wheat) and hSLC19A1 (grey). Key residues are shown as sticks and dots. c, Expression of hSLC19A1 and its mutants in SLC19A1−/− HeLa cells. Wild type and folate (left) or CDN (right) pocket mutants (P2A-GFP+) were sorted and quantified in HeLa cells by FACS. d–i, Structural superimposition between the pyrimidine-pyrrole moiety of PMX (sticks; wheat) and the adenine base of 3′3′-CDA (sticks; different conformations shown in different colours) was shown in the upper box. Slab through the surface of hSLC19A1 in a cytosolic bottom view (lower layer) highlights the steric hindrance effects formed between the modelled 3′3′-CDA (space-filling) and the cavity. The models of 3′3′-CDA in different conformations were obtained from: (d) PDB-4qsh, (e) the current work, (f) PDB-4qk9, (g) PDB-4qsh, and (h,i) predicted structures. j, Models of the CDN monomer docked into the cavity of hSLC19A1 using AutoDock Vina. k, Chemical structures of 5-MTHF (left), Folic acid (middle) and PT523 (right). l, Hydrophobic interactions between the methyl group on N5 of 5-MTHF (sphere; cyan) and Glu45, Ile48 and Tyr126 of hSLC19A1 (dots, grey). m, Hydrogen bond between N8 of 5-MTHF (sticks; cyan) and the carbonyl oxygen of Glu123 of hSLC19A1 (sticks; grey). n, Alignments of 50 ns structure snapshots from two independent MD simulations on hSLC19A1-PT523 models. o, Overall positively charged interior cavity (left) and cytosolic entrance (right) of hSCL19A1. NTD and CTD are coloured in yellow and blue, respectively; key residues are shown as sticks.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Supplementary Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Zhang, X., Zhu, Y. et al. Recognition of cyclic dinucleotides and folates by human SLC19A1. Nature 612, 170–176 (2022). https://doi.org/10.1038/s41586-022-05452-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05452-z
This article is cited by
-
Substrate and drug recognition mechanisms of SLC19A3
Cell Research (2024)
-
Transport and inhibition mechanism for VMAT2-mediated synaptic vesicle loading of monoamines
Cell Research (2024)
-
Membrane transporters in drug development and as determinants of precision medicine
Nature Reviews Drug Discovery (2024)
-
Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME
Journal of Biomedical Science (2023)
-
cGAMP-activated cGAS–STING signaling: its bacterial origins and evolutionary adaptation by metazoans
Nature Structural & Molecular Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.