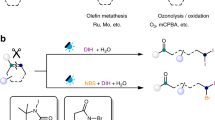
Abstract
The oxidative cleavage of alkenes is an integral process that converts feedstock materials into high-value synthetic intermediates1,2,3. The most viable method to achieve this in one chemical step is with ozone4,5,6,7; however, this poses technical and safety challenges owing to the explosive nature of ozonolysis products8,9. Here we report an alternative approach to achieve oxidative cleavage of alkenes using nitroarenes and purple-light irradiation. We demonstrate that photoexcited nitroarenes are effective ozone surrogates that undergo facile radical [3+2] cycloaddition with alkenes. The resulting ‘N-doped’ ozonides are safe to handle and lead to the corresponding carbonyl products under mild hydrolytic conditions. These features enable the controlled cleavage of all types of alkenes in the presence of a broad array of commonly used organic functionalities. Furthermore, by harnessing electronic, steric and mediated polar effects, the structural and functional diversity of nitroarenes has provided a modular platform to obtain site selectivity in substrates containing more than one alkene.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (D.L.) upon reasonable request.
References
Baumann, H. et al. Natural fats and oils—renewable raw materials for the chemical industry. Angew. Chem. Int. Ed. 27, 41–62 (1988).
Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411–2502 (2007).
Köckritz, A. & Martin, A. Oxidation of unsaturated fatty acid derivatives and vegetable oils. Eur. J. Lipid Sci. Technol. 110, 812–824 (2008).
Bailey P. S. Ozonization in Organic Chemistry Vol. 1. (Academic Press, 1978).
Fisher, T. J. & Dussault, P. H. Alkene ozonolysis. Tetrahedron 73, 4233–4258 (2017).
Surburg, H. & Panten, J. Common Fragrance and Flavor Materials (Wiley-VHC, 2006).
Caron, S., Dugger, R. W., Ruggeri, S. G., Ragan, J. A. & Ripin, D. H. B. Large-scale oxidations in the pharmaceutical industry. Chem. Rev. 106, 2943–2989 (2006).
Allian, A. in Managing Hazardous Reactions and Compounds in Process Chemistry (eds Pesti, J. A. & Abdel-Magid, A. F.) 353–382 (ACS, 2014).
Kula, J. Safer ozonolysis reactions: a compilation of laboratory experience. Chem. Health Saf. 6, 21–22 (1999).
Van Ornum, S. G., Champeau, R. M. & Pariza, R. Ozonolysis applications in drug synthesis. Chem. Rev. 106, 2990–3001 (2006).
Gabric, A., Hodnik, Z. & Pajk, S. Oxidation of drugs during drug product development: problems and solutions. Pharamaceutics 14, 325 (2000).
Hoelderich, W. F. & Kollmer, F. Oxidation reactions in the synthesis of fine and intermediate chemicals using environmentally benign oxidants and the right reactor system. Pure Appl.Chem. 72, 1273–1287 (2000).
High-Throughput Screening in Drug Discovery (ed. Hüser, J.) Vol. 35 (Wiley-VCH, 2006).
Spannring, P., Bruijnincx, P. C. A., Weckhuysen, B. M. & Klein Gebbink, R. J. M. Transition metal-catalyzed oxidative double bond cleavage of simple and bio-derived alkenes and unsaturated fatty acids. Catal. Sci. Technol. 4, 2182–2209 (2014).
Yang, D. & Zhang, C. Ruthenium-catalyzed oxidative cleavage of alkenes to aldehydes. J. Org. Chem. 14, 4814–4818 (2001).
Yu, W., Mei, Y., Kang, Y., Hua, Z. & Jin, Z. Improved procedure for the oxidative cleavage of alkenes by OsO4–NaIO4. Org. Lett. 6, 3217–3219 (2004).
Maithani, M., Raturi, R., Sharma, P., Gupta, V. & Bansal, P. Elemental impurities in pharmaceutical products adding fuel to the fire. Regul. Toxicol. Pharmacol. 108, 104435 (2019).
Urgoitia, G., SanMartin, R., Herrero, M. T. & Domínguez, E. Aerobic cleavage of alkenes and alkynes into carbonyl and carboxyl compounds. ACS Catal. 7, 3050–3060 (2017).
Huang, Z. et al. Oxidative cleavage of alkenes by O2 with a non-heme manganese catalyst. J. Am. Chem. Soc. 143, 10005–10013 (2021).
Ranganathan, S., Ranganathan, D., Ramachandran, P. V., Mahanty, M. K. & Bamezai, S. A chemical and thermochemical study of non-observed symmetry allowed reactions. Tetrahedron 37, 4171–4184 (1981).
Leitich, J. 1,3,2-Dioxazolidines by thermal 1,3-cycloaddition of nitro groups to strained alkenes. Angew. Chem. Int. Ed. 15, 372–373 (1976).
Buchi, G. & Ayer, D. E. Light catalyzed organic reactions. IV. The oxidation of alkenes with nitrobenzene. J. Am. Chem. Soc. 78, 689–690 (1956).
De Mayo, P., Charlton, J. L. & Liao, C. C. Photochemical synthesis. XXXV. Addition of aromatic nitro compounds to alkenes. J. Am. Chem. Soc. 93, 2463–2471 (1971).
Okada, K., Saito, Y. & Oda, M. Photochemical reaction of polynitrobenzenes with adamantylideneadamantane: the X-ray structure analysis and chemical properties of the dispiro N-(2,4,6-trinitrophenyl)-1,3,2-dioxazolidine product. J. Chem. Soc. Chem. Commun. 1731–1732 (1992).
D’Auria, M., Esposito, V. & Mauriello, G. Photochemical reactivity of aromatic and heteroaromatic nitroderivatives in the presence of arylalkenes. Tetrahedron 52, 14253–14272 (1996).
Lu, C. et al. Intramolecular reductive cyclization of o-nitroarenes via biradical recombination. Org. Lett. 21, 1438–1443 (2019).
Gang, L. et al. Light-promoted C–N coupling of aryl halides with nitroarenes. Angew. Chem. Int. Ed. 133, 5290–5294 (2021).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Albini, A., Bettinetti, G. & Minola, G. Photodecomposition of some para-substituted 2-pyrazolylphenyl azides. substituents affect the phenylnitrene S-T gap more than the barrier to ring expansion. J. Am. Chem. Soc. 121, 3104–3113 (1999).
Carra, C., Bally, T. & Albini, A. Role of conformation and electronic structure in the chemistry of ground and excited state o-pyrazolylphenylnitrenes. J. Am. Chem. Soc. 127, 5552–5562 (2005).
Partridge, K. M., Guzei, I. A. & Yoon, T. P. Carbonyl imines from oxaziridines: generation and cycloaddition of N–O=C dipoles. Angew. Chem. Int. Ed. 49, 930–934 (2010).
Zhao, E., Zhou, F. & Zhao, Y. Lewis acids promoted 3 + 2 cycloaddition of oxaziridines and cyclic allylic alcohols through carbonyl imine intermediates. J. Org. Chem. 84, 4282–4293 (2019).
Murahashi, S.-I. & Imada, Y. Synthesis and transformations of nitrones for organic synthesis. Chem. Rev. 119, 4684–4716 (2019).
Hurley, R. & Testa, A. C. Photochemical n → π* excitation of nitrobenzene. J. Am. Chem. Soc. 88, 4330–4332 (1966).
Hurley, R. & Testa, A. C. Nitrobenzene photochemistry. II. Protonation in the excited state. J. Am. Chem. Soc. 89, 6917–6919 (1967).
Bietti, M. Activation and deactivation strategies promoted by medium effects for selective aliphatic C–H bond functionalization. Angew. Chem. Int. Ed. 57, 16618–16637 (2018).
Strieth-Kalthoff, F. & Glorius, F. Triplet energy transfer photocatalysis: unlocking the next level. Chem 6, 1888–1903 (2020).
Huisgen, R. 1,3-Dipolar cycloadditions. 76. Concerted nature of 1,3-dipolar cycloadditions and the question of diradical intermediates. J. Org. Chem. 41, 403–419 (1976).
Acknowledgements
D.L. thanks EPSRC for a Fellowship (EP/P004997/1) and a grant (EP/V046799/1), the European Research Council for a research grant (758427), the Leverhulme Trust for additional support (Philip Leverhulme Prize to D.L.). We acknowledge I. J. Vitorica-Yrezabal (University of Manchester) for solving the X-ray crystal structure of 4, and N. S. Sheikh for discussions.
Author information
Authors and Affiliations
Contributions
M.S. and D.L. designed the project; M.S. directed the work; M.S., A.R. and C.H. performed all synthetic and mechanistic experiments. M.S. and D.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Martins Oderinde and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–26—see Contents page for details.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruffoni, A., Hampton, C., Simonetti, M. et al. Photoexcited nitroarenes for the oxidative cleavage of alkenes. Nature 610, 81–86 (2022). https://doi.org/10.1038/s41586-022-05211-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05211-0
This article is cited by
-
Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes
Nature Chemistry (2024)
-
Characterization of A π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis
Nature Communications (2024)
-
Carbonyl cross-metathesis via deoxygenative gem-di-metal catalysis
Nature Chemistry (2024)
-
C(alkyl)–C(vinyl) bond cleavage enabled by Retro-Pallada-Diels-Alder reaction
Nature Communications (2023)
-
Carbon-to-nitrogen single-atom transmutation of azaarenes
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.