Abstract
RNA splicing, the process of intron removal from pre-mRNA, is essential for the regulation of gene expression. It is controlled by the spliceosome, a megadalton RNA–protein complex that assembles de novo on each pre-mRNA intron through an ordered assembly of intermediate complexes1,2. Spliceosome activation is a major control step that requires substantial protein and RNA rearrangements leading to a catalytically active complex1,2,3,4,5. Splicing factor 3B subunit 1 (SF3B1) protein—a subunit of the U2 small nuclear ribonucleoprotein6—is phosphorylated during spliceosome activation7,8,9,10, but the kinase that is responsible has not been identified. Here we show that cyclin-dependent kinase 11 (CDK11) associates with SF3B1 and phosphorylates threonine residues at its N terminus during spliceosome activation. The phosphorylation is important for the association between SF3B1 and U5 and U6 snRNAs in the activated spliceosome, termed the Bact complex, and the phosphorylation can be blocked by OTS964, a potent and selective inhibitor of CDK11. Inhibition of CDK11 prevents spliceosomal transition from the precatalytic complex B to the activated complex Bact and leads to widespread intron retention and accumulation of non-functional spliceosomes on pre-mRNAs and chromatin. We demonstrate a central role of CDK11 in spliceosome assembly and splicing regulation and characterize OTS964 as a highly selective CDK11 inhibitor that suppresses spliceosome activation and splicing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All next-generation-sequencing source and processed data are available at the NCBI Gene Expression Omnibus (GSE185813). MS proteomics data are available through ProteomeXchange dataset PXD035189 (https://doi.org/10.25345/C59P2W974).
References
Wilkinson, M. E., Charenton, C. & Nagai, K. RNA splicing by the spliceosome. Annu. Rev. Biochem. 89, 359–388 (2020).
Wahl, M. C., Will, C. L. & Luhrmann, R. The spliceosome: design principles of a dynamic RNP machine. Cell 136, 701–718 (2009).
Bertram, K. et al. Cryo-EM structure of a pre-catalytic human spliceosome primed for activation. Cell 170, 701–713 (2017).
Townsend, C. et al. Mechanism of protein-guided folding of the active site U2/U6 RNA during spliceosome activation. Science 370, eabc3753 (2020).
Zhang, X. et al. Structure of the human activated spliceosome in three conformational states. Cell Res. 28, 307–322 (2018).
Sun, C. The SF3b complex: splicing and beyond. Cell. Mol. Life Sci. 77, 3583–3595 (2020).
Wang, C. et al. Phosphorylation of spliceosomal protein SAP 155 coupled with splicing catalysis. Genes Dev. 12, 1409–1414 (1998).
Agafonov, D. E. et al. Semiquantitative proteomic analysis of the human spliceosome via a novel two-dimensional gel electrophoresis method. Mol. Cell. Biol. 31, 2667–2682 (2011).
Bessonov, S. et al. Characterization of purified human Bact spliceosomal complexes reveals compositional and morphological changes during spliceosome activation and first step catalysis. RNA 16, 2384–2403 (2010).
Girard, C. et al. Post-transcriptional spliceosomes are retained in nuclear speckles until splicing completion. Nat. Commun. 3, 994 (2012).
Schneider-Poetsch, T., Chhipi-Shrestha, J. K. & Yoshida, M. Splicing modulators: on the way from nature to clinic. J. Antibiot. 74, 603–616 (2021).
Kotake, Y. et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 3, 570–575 (2007).
Thickman, K. R., Swenson, M. C., Kabogo, J. M., Gryczynski, Z. & Kielkopf, C. L. Multiple U2AF65 binding sites within SF3b155: thermodynamic and spectroscopic characterization of protein-protein interactions among pre-mRNA splicing factors. J. Mol. Biol. 356, 664–683 (2006).
Murthy, T. et al. Cyclin-dependent kinase 1 (CDK1) and CDK2 have opposing roles in regulating interactions of splicing factor 3B1 with chromatin. J. Biol. Chem. 293, 10220–10234 (2018).
Shi, Y., Reddy, B. & Manley, J. L. PP1/PP2A phosphatases are required for the second step of pre-mRNA splicing and target specific snRNP proteins. Mol. Cell 23, 819–829 (2006).
Loyer, P. et al. The RNA binding motif protein 15B (RBM15B/OTT3) is a functional competitor of serine-arginine (SR) proteins and antagonizes the positive effect of the CDK11p110–cyclin L2α complex on splicing. J. Biol. Chem. 286, 147–159 (2011).
Hu, D., Mayeda, A., Trembley, J. H., Lahti, J. M. & Kidd, V. J. CDK11 complexes promote pre-mRNA splicing. J. Biol. Chem. 278, 8623–8629 (2003).
Pak, V. et al. CDK11 in TREX/THOC regulates HIV mRNA 3′ end processing. Cell Host Microbe 18, 560–570 (2015).
Gajduskova, P. et al. CDK11 is required for transcription of replication-dependent histone genes. Nat. Struct. Mol. Biol. 27, 500–510 (2020).
Loyer, P. et al. Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: influence of cyclin L isoforms on splice site selection. J. Biol. Chem. 283, 7721–7732 (2008).
Trembley, J. H., Hu, D., Slaughter, C. A., Lahti, J. M. & Kidd, V. J. Casein kinase 2 interacts with cyclin-dependent kinase 11 (CDK11) in vivo and phosphorylates both the RNA polymerase II carboxyl-terminal domain and CDK11 in vitro. J. Biol. Chem. 278, 2265–2270 (2003).
Lin, A. et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 11, eaaw8412 (2019).
Matsuo, Y. et al. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci. Transl. Med. 6, 259ra145 (2014).
Hu, Q. F. et al. Design, synthesis and biological evaluation of novel 1-phenyl phenanthridin-6(5H)-one derivatives as anti-tumor agents targeting TOPK. Eur. J. Med. Chem. 162, 407–422 (2019).
Czudnochowski, N., Bosken, C. A. & Geyer, M. Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition. Nat. Commun. 3, 842 (2012).
Vasta, J. D. et al. Quantitative, wide-spectrum kinase profiling in live cells for assessing the effect of cellular ATP on target engagement. Cell Chem. Biol. 25, 206–214 (2018).
Wells, C. I. et al. Quantifying CDK inhibitor selectivity in live cells. Nat. Commun. 11, 2743 (2020).
Chao, S. H. & Price, D. H. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J. Biol. Chem. 276, 31793–31799 (2001).
Greifenberg, A. K. et al. Structural and functional analysis of the Cdk13/cyclin K complex. Cell Rep. 14, 320–331 (2016).
Caizzi, L. et al. Efficient RNA polymerase II pause release requires U2 snRNP function. Mol. Cell 81, 1920–1934 (2021).
Effenberger, K. A. et al. Coherence between cellular responses and in vitro splicing inhibition for the anti-tumor drug pladienolide B and its analogs. J. Biol. Chem. 289, 1938–1947 (2014).
Koga, M., Hayashi, M. & Kaida, D. Splicing inhibition decreases phosphorylation level of Ser2 in Pol II CTD. Nucleic Acids Res. 43, 8258–8267 (2015).
Nojima, T. et al. Mammalian NET-Seq reveals genome-wide nascent transcription coupled to RNA processing. Cell 161, 526–540 (2015).
Luo, M. J. & Reed, R. Splicing is required for rapid and efficient mRNA export in metazoans. Proc. Natl Acad. Sci. USA 96, 14937–14942 (1999).
AJ, C. Q., Bugai, A. & Barboric, M. Cracking the control of RNA polymerase II elongation by 7SK snRNP and P-TEFb. Nucleic Acids Res. 44, 7527–7539 (2016).
Pilarova, K., Herudek, J. & Blazek, D. CDK12: cellular functions and therapeutic potential of versatile player in cancer. NAR Cancer 2, zcaa003 (2020).
Seghezzi, W. et al. Cyclin E associates with components of the pre-mRNA splicing machinery in mammalian cells. Mol. Cell. Biol. 18, 4526–4536 (1998).
Lopez, M. S., Kliegman, J. I. & Shokat, K. M. The logic and design of analog-sensitive kinases and their small molecule inhibitors. Methods Enzymol. 548, 189–213 (2014).
Yokoi, A. et al. Biological validation that SF3b is a target of the antitumor macrolide pladienolide. FEBS J. 278, 4870–4880 (2011).
Paruch, K. et al. Discovery of dinaciclib (SCH 727965): a potent and selective inhibitor of cyclin-dependent kinases. ACS Med. Chem. Lett. 1, 204–208 (2010).
Sanso, M. et al. P-TEFb regulation of transcription termination factor Xrn2 revealed by a chemical genetic screen for Cdk9 substrates. Genes Dev. 30, 117–131 (2016).
Loyer, P., Trembley, J. H., Lahti, J. M. & Kidd, V. J. The RNP protein, RNPS1, associates with specific isoforms of the p34cdc2-related PITSLRE protein kinase in vivo. J. Cell Sci. 111, 1495–1506 (1998).
Zarnack, K. et al. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell 152, 453–466 (2013).
Briese, M. et al. A systems view of spliceosomal assembly and branchpoints with iCLIP. Nat. Struct. Mol. Biol. 26, 930–940 (2019).
Van Nostrand, E. L. et al. Principles of RNA processing from analysis of enhanced CLIP maps for 150 RNA binding proteins. Genome Biol. 21, 90 (2020).
Kfir, N. et al. SF3B1 association with chromatin determines splicing outcomes. Cell Rep. 11, 618–629 (2015).
Carrocci, T. J. & Neugebauer, K. M. Pre-mRNA splicing in the nuclear landscape. Cold Spring Harb. Symp. Quant. Biol. 84, 11–20 (2019).
Tresini, M. et al. The core spliceosome as target and effector of non-canonical ATM signalling. Nature 523, 53–58 (2015).
Deckert, J. et al. Protein composition and electron microscopy structure of affinity-purified human spliceosomal B complexes isolated under physiological conditions. Mol. Cell. Biol. 26, 5528–5543 (2006).
Makarov, E. M. et al. Small nuclear ribonucleoprotein remodeling during catalytic activation of the spliceosome. Science 298, 2205–2208 (2002).
Allen, J. J. et al. A semisynthetic epitope for kinase substrates. Nat. Methods 4, 511–516 (2007).
Stejskal, K., Potesil, D. & Zdrahal, Z. Suppression of peptide sample losses in autosampler vials. J. Proteome Res. 12, 3057–3062 (2013).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Kramer, A. et al. Optimization of pyrazolo[1,5-a]pyrimidines lead to the identification of a highly selective casein kinase 2 inhibitor. Eur. J. Med. Chem. 208, 112770 (2020).
Huppertz, I. et al. iCLIP: protein-RNA interactions at nucleotide resolution. Methods 65, 274–287 (2014).
Mukherjee, P., Raghava Kurup, R. & Hundley, H. A. RNA immunoprecipitation to identify in vivo targets of RNA editing and modifying enzymes. Methods Enzymol. 658, 137–160 (2021).
Teng, T. et al. Splicing modulators act at the branch point adenosine binding pocket defined by the PHF5A–SF3b complex. Nat. Commun. 8, 15522 (2017).
Vichai, V. & Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 1, 1112–1116 (2006).
Ritz, C., Baty, F., Streibig, J. C. & Gerhard, D. Dose-response analysis using R. PLoS ONE 10, e0146021 (2015).
Chirackal Manavalan, A. P. et al. CDK12 controls G1/S progression by regulating RNAPII processivity at core DNA replication genes. EMBO Rep. 20, e47592 (2019).
Radle, B. et al. Metabolic labeling of newly transcribed RNA for high resolution gene expression profiling of RNA synthesis, processing and decay in cell culture. J. Vis. Exp. 8, 50195 (2013).
Duffy, E. E. & Simon, M. D. Enriching s4U-RNA using methane thiosulfonate (MTS) chemistry. Curr. Protoc. Chem. Biol. 8, 234–250 (2016).
Fedorov, O., Niesen, F. H. & Knapp, S. Kinase inhibitor selectivity profiling using differential scanning fluorimetry. Methods Mol. Biol. 795, 109–118 (2012).
Asquith, C. R. M. et al. SGC-GAK-1: a chemical probe for cyclin G associated kinase (GAK). J. Med. Chem. 62, 2830–2836 (2019).
Bonfert, T., Kirner, E., Csaba, G., Zimmer, R. & Friedel, C. C. ContextMap 2: fast and accurate context-based RNA-seq mapping. BMC Bioinform. 16, 122 (2015).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Kluge, M. & Friedel, C. C. Watchdog—a workflow management system for the distributed analysis of large-scale experimental data. BMC Bioinform. 19, 97 (2018).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
R Core Team. R: A Language and Environment for Statistical Computing http://www.R-project.org/ (R Foundation for Statistical Computing, 2016).
Caizzi, L. et al. Efficient RNA polymerase II pause release requires U2 snRNP function. Mol. Cell 81, 1920–1934 (2021).
Di Tommaso, P. et al. Nextflow enables reproducible computational workflows. Nat. Biotechnol. 35, 316–319 (2017).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9, e1003118 (2013).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Ramirez, F., Dundar, F., Diehl, S., Gruning, B. A. & Manke, T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–W191 (2014).
Acknowledgements
We thank all of the members of the Blazek laboratory for discussions throughout the project and comments on the manuscript; M. Hallegger for protocols and advice regarding in vitro splicing assays; M. Šebesta for providing GST–RNAPIICTD, T. Bárta for providing anti-CDK2 antibodies; K. Hanáková for assistance with proteomic experiments; and the staff at Life Science Editors for editing services. This research was supported by grants from the Czech Science Foundation (21-19266S); the CEITEC (Project CEITEC-Central-European Institute of Technology (CZ.1.05/1.1.00/02.0068)); GAMU MUNI/G/1129/2021 to D.B.; the Deutsche Forschungsgemeinschaft (DFG; grants FR2938/9-1 and FR2938/10-1) to C.C.F.; the European Union’s Horizon 2020 research and innovation programme (835300-RNPdynamics) to J.U; European Structural and Investment Funds, Operational Programme Research, Development and Education—“Preclinical Progression of New Organic Compounds with Targeted Biological Activity” (Preclinprogress)—CZ.02.1.01/0.0/0.0/16_025/0007381, project no. LQ1605 from the National Program of Sustainability II (MEYS CR); the project CZ-OPENSCREEN: National Infrastructure for Chemical Biology (LM2018130); Bader Philanthropies; and GAMU MUNI/G/1129/2021 to K.P. CIISB, Instruct-CZ Centre of Instruct-ERIC EU consortium, funded by MEYS CR infrastructure project LM2018127, is acknowledged for the financial support of the measurements at the CEITEC Proteomics Core Facility. Computational resources were supplied by the project “e-Infrastruktura CZ” (e-INFRA CZ ID: 90140) supported by the Ministry of Education, Youth and Sports of the Czech Republic. S.K. and B.-T.B. are grateful for support from the SGC, a registered charity (no. 1097737) that receives funds from AbbVie, Bayer, Boehringer Ingelheim, the Canada Foundation for Innovation, Eshelman Institute for Innovation, Genentech, Genome Canada through Ontario Genomics Institute (OGI-196), EU/EFPIA/OICR/McGill/KTH/Diamond, Innovative Medicines Initiative 2 Joint Undertaking (EUbOPEN grant 875510), Janssen, Merck, Merck & Co, Pfizer, Takeda and Wellcome; S.K. from the German translational cancer network (DKTK) and the Frankfurt Cancer Institute (FCI); and S.K. and B.-T.B. from the collaborative research center 1399 “Mechanisms of drug sensitivity and resistance in small cell lung cancer”.
Author information
Authors and Affiliations
Contributions
M.H., P.G., M.R., B.-T.B., Z.S., D.P. and D.B. performed experiments. M.H. performed in vitro splicing, immunoprecipitation assays and IVKA with analogue-sensitive CDK11, validated and used P-SF3B1 antibodies for western blotting analyses of SF3B1 phosphorylation in cells and purified SF3B1 for MS/MS analyses of phosphosites. P.G. performed IVKA with the RNAPIICTD substrate, western blotting analyses of RNAPIICTD phosphorylation after OTS964 treatment, iCLIP, RNA-seq, RNA immunoprecipitation and RT–qPCR/RT–PCR experiments and isolated and analysed chromatin-associated spliceosome components. M.R. performed 4SU–seq, ChIP–seq and western blotting analyses of the RNAPIICTD phosphorylation after pladi B treatment. B.-T.B. performed DSF, ITC and NanoBRET assays. Z.S. performed GST pull-down, viability and cell cycle assays, IVKA with the SF3B1 substrate and generated drug-resistant cell lines. D.B. performed purification of CDK11-associated proteins. D.P. performed and analysed MS/MS experiments. I.R.d.L.M., M.K., E.W. and C.C.F. performed bioinformatics analyses. I.R.d.L.M. analysed iCLIP and splicing ratio from RNA-seq. M.K. analysed ChIP–seq and RNA-seq data. E.W. analysed promoter-proximal pausing and C.C.F analysed 4SU–seq data. K.P. analysed kinome-wide profiling and radiometric assays. All of the authors provided feedback, discussed the design of experiments and analysed data. D.B. wrote the initial draft of the manuscript and all of the authors edited and approved the manuscript. J.U., S.K., Z.Z., K.P., C.C.F. and D.B. supervised research and acquired funding.
Corresponding author
Ethics declarations
Competing interests
B.-T.B. is a co-founder and the CEO of CELLinib. D.B. has received a consulting fee from Proxygen. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Robert Fisher, Clara Kielkopf and Juan Valcarcel for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Characterization of potential off-targets of OTS964.
a, b, Graphs show percentage of CDK9/CyclinT1 (a) and TOPK (b) kinase activities in the presence of increasing concentrations of OTS964 in IVKAs. Resulting IC50 from n = 2 replicates are shown in the graphs. c, Isothermal Titration Calorimetry (ITC) of TOPK and OTS964. d, Differential Scanning Fluorimetry (DSF) curve of TOPK and OTS964, staurosporine, DMSO and blank. e, f, Graphs show percentage of normalized NanoBRET ratio for CDK9/Cyclin T2 upon OTS964 or control dinaciclib treatment (n = 2 biologically independent replicates, a representative replicate is shown) (e) and for TYK2 upon OTS964 or control AT9283 treatment (n = 1 biological replicate) (f).
Extended Data Fig. 2 OTS964 decreases bulk phosphorylation of all the CTD residues in a CDK11-dependent manner in cells.
a, Immunoblot of proteins after treatment of HCT116 cells with indicated concentrations of OTS964 for indicated times. b, Immunoblot of proteins after treatment of WT or CDK11 G579S HCT116 cells with 50 nM OTS964 or 200 nM flavopiridol for 4 h. c, d, Graphs show relative levels of mRNA of 14 histone genes in WT or CDK11 G579S HCT116 cells either treated with DMSO or with 50 nM OTS964 (c) or 200 nM flavopiridol (d) for 4 h. mRNA levels were normalized to Ppia mRNA expression and expression in DMSO was set as 1. n = 4 biologically independent experiments, error bars=SEM. e, Sulforhodamine B growth assays in WT and CDK11 G579S HCT116 cells treated with increasing concentrations of OTS964. n = 3 replicates of biologically independent experiments. f, Percentage of HCT116 WT and CDK11 G579S cells in individual cell cycle phases based on flowcytometry profiles of a representative replicate treated with the indicated concentrations of OTS964 for 16 h. n = 3 replicates of biologically independent experiments.
Extended Data Fig. 3 OTS964 and pladi B globally disrupt splicing in a similar way.
a, IGV genome browser view of Brd2 and Ddx47 genes from RNA-seq treated with 30 nM OTS964 or DMSO (control) for 4 h. b, Box plots showing ratio of spliced reads over total unspliced and spliced reads in RNA-seq after treatment with DMSO (control) or 30 nM OTS964 for 4 h. First, middle and last introns in selected 6222 isoforms were considered. Boxes represent the range between the first and third quartiles for each condition. Black horizontal lines in boxes show the median. The ends of the whiskers extend the box by 1.5 times the inter-quartile range. n=2 biologically independent experiments. c, Graph shows change in expression of transcripts of five genes in WT or CDK11 G579S HCT116 cells either treated with DMSO or 200 nM flavopiridol for 4 h. mRNA levels were normalized to Ppia mRNA expression and expression in DMSO was set as 1. n = 4 biologically independent experiments, error bars=SEM. E-E=primers spanning exon-exon junctions; E-In=primers spanning exon-intron junctions. d, Box plots showing the ratio of intronic reads over total intronic and exonic reads in 4SU–seq after treatment with DMSO, 50 nM OTS964 and 186 nM pladi B in pulse (upper) and chase (lower) experiment. Two biological replicates (REP1, REP2) are shown. Boxes represent the range between the first and third quartiles for each condition. Black horizontal lines in boxes show the median. The ends of the whiskers extend the box by 1.5 times the inter-quartile range. e, IGV genome browser view of Dusp4 and Fosl1 genes from pulse and chase 4SU–seq experiments. f, Scatterplots comparing intron ratios in individual genes between DMSO (control) and either OTS964 (left) or pladi B (right) treated HCT116 cells in 4SU–seq pulse experiment. g, Scatterplots comparing intron ratios in individual genes between OTS964 and pladi B treated HCT116 cells in 4SU–seq pulse (left) and chase (right) experiments. h, Boxplots showing distribution of log2 fold changes of intron ratios for individual genes in OTS964 over DMSO and pladi B over DMSO in 4SU–seq chase experiment. n = 2 biologically independent experiments. Boxes represent the range between the first and third quartiles for each condition. Black horizontal lines in boxes show the median. The ends of the whiskers (vertical lines) extend the box by 1.5 times the inter-quartile range. Data points outside this range (outliers) are shown as small circles. The y-axis was limited to the range between −2 and 4.
Extended Data Fig. 4 CDK11 inhibition alters total and modified RNAPII occupancies on genes.
a, Immunoblot of proteins after treatment of HCT116 cells with indicated concentrations of pladi B for indicated times. b, Metagene analyses of RNAPII ChIP-seq occupancies of the 7500 protein-coding genes with highest RNAPII occupancy in DMSO treated control. Only genes separated by ≥5,000 nt from other genes are included. Dotted lines indicate transcription start site (TSS), 1,500 nucleotides downstream of TSS, 1,500 nucleotides upstream of transcription termination site (TTS) and TTS. The colour track at the bottom indicates the significance of paired two-sided Wilcoxon tests comparing DMSO treated control cells and cells treated with 30 nM OTS964 for 4 h. Colour code: red = adjusted P-value ≤ 10−15, orange = adjusted P-value ≤ 10−10, yellow = adjusted P-value ≤ 10−3. c, d, Metagene analyses of RNAPII ChIP-seq occupancies over down-, up- and non-regulated genes in RNA-seq (c) and over transcripts with indicated lengths (d) upon 30 nM OTS964 or control DMSO treatment in HCT116 cells for 4 h. e, Scatter plot comparing PI between OTS964 and control DMSO treatment. Genes in red have ≥ 2-fold increase in PI, in green have ≥ 2-fold decrease and in blue are within the 2-fold range. This analysis includes only well-expressed genes with an RPKM ≥ 1 either on the promoter or gene body in control DMSO treatment. f, Pie chart showing proportion of genes with indicated increases or decreases in PI or with no change. g, Boxplots showing the distribution of gene body RPKM (reads per kilo base per million mapped reads) for control DMSO and OTS964 treatment for genes with either a decrease or increase in PI. n = 2 biologically independent experiments. Boxes represent the range between the first and third quartiles for each condition. Black horizontal lines in boxes show the median. The ends of the whiskers (vertical lines) extend the box by 1.5 times the inter-quartile range. Data points outside this range (outliers) are shown as small circles. The y-axis was limited to the range between 0 and 2. h, i, Metagene analyses of P-Ser5 (h) and P-Ser7 (i) occupancies over 7,500 genes upon 30 nM OTS964 or control DMSO treatment for 4 h in HCT116 cells.
Extended Data Fig. 5 CDK11 inhibition alters transcription dynamics.
a, Metagene analyses of P-Ser2 ChIP-seq occupancies over 7500 genes. See Extended Data Fig. 4b for legend. b, c, Metagene analyses of P-Ser2 ChIP-seq occupancies over down-, up- and non-regulated genes in RNA-seq (b) and over transcripts with indicated lengths (c) upon 30 nM OTS964 or control DMSO treatment in HCT116 cells for 4 h. d, IGV gene tracks for gene Cdc25a and Riok3 showing RNAPII, P-Ser2, P-Ser5 and P-Ser7 ChIP-seq occupancies in control DMSO and OTS964 treated HCT116 cells for 4 h.
Extended Data Fig. 6 OTS964 blocks spliceosome before the first catalytic step of splicing.
a, b, Native gel analyses of spliceosome complex formation on radiolabelled AdML pre-mRNA in HeLa nuclear extracts treated with DMSO or indicated concentrations of OTS964 for indicated times. Identities of spliceosome complexes E, A, B and C are depicted on the side. -ATP corresponds to ATP-depleted nuclear extracts. c, Native gel analyses of spliceosome complexes upon treatment with OTS964 or indicated concentrations of control compounds or under condition of low concentration of MgCl2. See Extended Data Fig. 6a, b for legend.
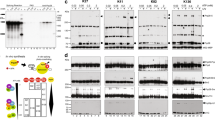
Extended Data Fig. 7 CDK11 binds and phosphorylates the threonine-proline-rich N terminus of SF3B1.
a, Immunoblot analyses of immunoprecipitations of endogenous CDK11 in HEK293 cells. Detected proteins are indicated on the right. CK2a is known CDK11 interacting partner (ref. 21), IgG=antibody control. b, Immunoblot analyses of in vitro binding assays of GST-tagged SF3B1 (1-463) purified from E. coli and Flag-tagged CDK11 purified from HCT116 cells. Antibodies used are shown on the right. c, Schematic view of SF3B1 protein and its deletion mutants used in (d). d, Immunoblot analyses of IVKA using Flag-tagged AS CDK11 and Flag-tagged SF3B1, SF3B1 (1-463), and SF3B1 (464-1304) substrates. AS CDK11 remains catalytically active, but has mutated a gatekeeper methionine to glycine (M503G) in its catalytic site to allow the usage of thiophosphate ATP analogues (ref. 38). This can be detected in substrates by western blotting using an anti-thiophosphate ester (TPE) antibody (ref. 52). The blots were probed with Flag, CDK11, SF3B1 and TPE antibodies as indicated. Inputs represent aliquots of individual proteins added to the IVKA. e, Immunoblot of proteins after treatment of WT or SF3B1 R1074H HCT116 cells with 50 nM OTS964 for 3 min. f, Depiction of SF3B1 peptides found by IP-MS/MS in HCT116 cells with stably integrated Flag-tagged-SF3B1 treated with control DMSO or 50 nM OTS964 for 1 h; shown peptides were found in at least 3 replicates of control- but not OTS964-treated cells. Phosphorylated threonines and serines are shown in red; bold indicates the phosphorylated residues found in Bact complex (ref. 10). n = 4 replicates. g, Immunoblot of proteins after treatment of WT HCT116 cells with 20 nM dinaciclib for indicated times. h, Denaturing gel analyses of the radiolabelled AdML pre-mRNA and spliced products from in vitro splicing reactions in HeLa nuclear extracts treated with DMSO or 1 µM dinaciclib for indicated times (left panel). Native gel analyses of spliceosome assembly on radiolabelled AdML pre-mRNA in HeLa nuclear extracts treated with DMSO or indicated concentrations of dinaciclib for indicated times (right panel). See Fig. 3a, b for further legend.
Extended Data Fig. 8 OTS964 and pladi B affect splicing via different mechanisms.
a, Immunoblot of proteins after treatment of WT or SF3B1 R1074H HCT116 cells with 186 nM pladi B for indicated times. b, Immunoblot of proteins after treatment of SF3B1 R1074H HCT116 cells with 50 nM OTS964 for indicated times. c, Immunoblot of proteins after treatment of CDK11 G579S HCT116 cells with 186 nM pladi B for indicated times. d, e, DNA gel-visualized RT-PCR analyses of splicing of Riok3 (d) and Ccnl1 (e) genes in WT, CDK11 G579S and SF3B1 R1074H HCT116 cells treated with control DMSO, 50 nM OTS964 or 186 nM pladi B for indicated times. Schema of unspliced and spliced products are depicted on the right and their ratio (spliced/unspliced) on bottom of gels. Markers on the left indicate size in nucleotides (nt) and DNA corresponds to genomic DNA control. f, Immunoblot analyses of HCT116 cell lysates treated with either 50 nM OTS964 for 4 h or untreated control. Pan P-RS = pan-phospho-RS specific antibody. Long and short correspond to long and short exposures of the film, respectively.
Extended Data Fig. 9 CDK11 inhibition stalls spliceosome assembly prior to formation of Bact complex.
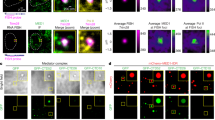
a, b, c, Autoradiography visualization of RNA-U2AF65 (a), -SF3B1 (b) and -AQR (c) complexes resolved on SDS-PAGE gel upon indicated treatments. Clamps on the side of the panels and asterisks indicate RNA-protein complexes upon low (L) and collapsed RNA-protein band upon high (H) RNase I treatments, respectively. REP1=replicate 1; REP2=replicate 2. d, Correlation analyses between indicated biological replicates of U2AF65, SF3B1 and AQR iCLIP libraries (10 kb bins). Numbers correspond to R2 (Pearson correlation coefficient) between indicated replicates and correlation strength is indicated by the colour code. e, f, g, Graphs show percentage of significantly bound genomic regions (FDR>0.05) and normalized to region length for U2AF65 (e), SF3B1 (f) and AQR (g) iCLIP upon indicated treatments. REP1=replicate 1; REP2=replicate 2. h, IGV genome browser view of SF3B1, U2AF65 and AQR iCLIP binding upon indicated treatments at Rpl27a transcript. i, Immunoblot analyses of association of indicated splicing factors in nucleoplasmic (soluble) and chromatin (chrom) fractions upon treatment with OTS964 in either HCT116 (WT) or HCT116 CDK11 G579S cells. Presence of the factors in specific spliceosome complexes is indicated on the right. Arrows mark phosphorylated (upper) and non-phosphorylated (lower) forms of RNAPII and SF3B1.
Extended Data Fig. 10 Phosphorylated SF3B1 mediates interaction with snRNAs in the CDK11-dependent manner/Model.
a, b, Graphs represent RIP analyses of total (a) and P-Thr235 (b) SF3B1 binding to indicated snRNAs in HCT116 cells treated with either control DMSO or 50 nM OTS964 for 2 h. n = 4 biologically independent experiments, error bars=SEM, no Ab corresponds to no antibody control immunoprecipitation. Lower panels represent zoom-in of upper panels. c, Graph presents ratios of P-Thr235-SF3B1 and total SF3B1 RIP-qPCR signals on indicated snRNAs. n = 4 biologically independent experiments, error bars=SEM. Lower panel represents zoom-in of the upper panel. d, Working model: CDK11 and its inhibition by OTS964 in pre-mRNA splicing. In untreated cells (no inhibitor), U1 and U2 snRNPs (U1 and U2) are bound on 5′ and 3′ splice sites, respectively and unphosphorylated SF3B1 is bound on BP adenosine (A) via HEAT-repeat domain in its “closed” formation, rendering the A complex. Unwinding of U1 and recruitment of U4/U6.U5 tri-snRNPs (U4/U6, U5) together with B-specific proteins (B-spec.) marks formation of the B complex. During its conversion into Bact complex, CDK11 phosphorylates (P) the N terminus of SF3B1, U4 and B-specific proteins are evicted, and RES and IBC complexes and other Bact-specific proteins (Bact spec.) are incorporated into the spliceosome. These events are prerequisite for formation of catalytically active spliceosome and normal pre-mRNA splicing (upper panel). OTS964 inhibits the ability of CDK11 to phosphorylate SF3B1 (dotted arrow) at the stage of B complex causing block of its transformation into the Bact complex and splicing defect (middle panel). In contrast, pladi B forces SF3B1 to remain in “open” conformation on BS adenosine, stalling spliceosome in A-like complex with outcome on splicing essentially the same as after the OTS964 treatment (lower panel). Black box=exon, black line=intron, 5′ and 3′ marks 5′ and 3′ ends of the transcript, respectively. e, Working model: Outcome of splicing inhibition on transcription and RNAPIICTD phosphorylation. Inhibition of splicing by inhibitors targeting SF3B1 and spliceosome assembly (OTS964, pladi B) results in suboptimal transcription, likely affecting promoter-proximal pausing, elongation, and termination and in relatively unspecific bulk dephosphorylation of Tyr1 (Y1), Ser2 (S2), Thr4 (T4), Ser5 (S5) and Ser7(S7) in the RNAPIICTD.
Supplementary information
Supplementary Fig. 1
Source data for raw gels and blots.
Supplementary Fig. 2
Supplementary Data File 1 with IC50 graphs for OTS964 (MU1602).
Supplementary Fig. 3
The gating strategy for flow cytometry data in Extended Data Fig. 2f.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hluchý, M., Gajdušková, P., Ruiz de los Mozos, I. et al. CDK11 regulates pre-mRNA splicing by phosphorylation of SF3B1. Nature 609, 829–834 (2022). https://doi.org/10.1038/s41586-022-05204-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05204-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.