Abstract
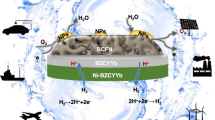
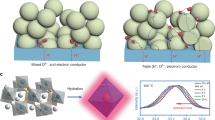
Protonic ceramic electrochemical cells hold promise for operation below 600 °C (refs. 1,2). Although the high proton conductivity of the bulk electrolyte has been demonstrated, it cannot be fully used in electrochemical full cells because of unknown causes3. Here we show that these problems arise from poor contacts between the low-temperature processed oxygen electrode–electrolyte interface. We demonstrate that a simple acid treatment can effectively rejuvenate the high-temperature annealed electrolyte surface, resulting in reactive bonding between the oxygen electrode and the electrolyte and improved electrochemical performance and stability. This enables exceptional protonic ceramic fuel-cell performance down to 350 °C, with peak power densities of 1.6 W cm−2 at 600 °C, 650 mW cm−2 at 450 °C and 300 mW cm−2 at 350 °C, as well as stable electrolysis operations with current densities above 3.9 A cm−2 at 1.4 V and 600 °C. Our work highlights the critical role of interfacial engineering in ceramic electrochemical devices and offers new understanding and practices for sustainable energy infrastructures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data supporting the findings in the present work are available in the manuscript or Supplementary Information. Additional data are available from the corresponding authors upon reasonable request.
References
Ding, H. et al. Self-sustainable protonic ceramic electrochemical cells using a triple conducting electrode for hydrogen and power production. Nat. Commun. 11, 1907 (2020).
Li, M. et al. Switching of metal–oxygen hybridization for selective CO2 electrohydrogenation under mild temperature and pressure. Nat. Catal. 4, 274–283 (2021).
Choi, S. et al. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 3, 202–210 (2018).
Duan, C. et al. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019).
Yang, L. et al. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3–δ. Science 326, 126–129 (2009).
Fabbri, E., Bi, L., Pergolesi, D. & Traversa, E. Towards the next generation of solid oxide fuel cells operating below 600 °C with chemically stable proton‐conducting electrolytes. Adv. Mater. 24, 195–208 (2012).
Rashid, N. L. R. M. et al. Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell. Ceram. Int. 45, 6605–6615 (2019).
Peng, C., Melnik, J., Luo, J.-L., Sanger, A. R. & Chuang, K. T. BaZr0.8Y0.2O3−δ electrolyte with and without ZnO sintering aid: preparation and characterization. Solid State Ion. 181, 1372–1377 (2010).
Shimada, H. et al. Effect of Ni diffusion into BaZr0.1Ce0.7Y0.1Yb0.1O3−δ electrolyte during high temperature co-sintering in anode-supported solid oxide fuel cells. Ceram. Int. 44, 3134–3140 (2018).
Okuyama, Y., Ebihara, N., Okuyama, K. & Mizutani, Y. Improvement of protonic ceramic fuel cells with thin film BCZY electrolyte. ECS Trans. 68, 2545 (2015).
Arabacı, A. & Öksüzömer, M. F. Preparation and characterization of 10 mol% Gd doped CeO2 (GDC) electrolyte for SOFC applications. Ceram. Int. 38, 6509–6515 (2012).
Zhang, S.-L., Chen, K., Zhang, A.-P., Li, C.-X. & Li, C.-J. Effect of Fe doping on the performance of suspension plasma-sprayed PrBa0.5Sr0.5Co2−xFexO5+δ cathodes for intermediate-temperature solid oxide fuel cells. Ceram. Int. 43, 11648–11655 (2017).
Zha, S., Moore, A., Abernathy, H. & Liu, M. GDC-based low-temperature SOFCs powered by hydrocarbon fuels. J. Electrochem. Soc. 151, A1128 (2004).
Melekh, B.-T. et al. Structure, phase transitions and optical properties of pure and rare earth doped BaCeO3, SrCeO3 prepared by inductive melting. Solid State Ion. 97, 465–470 (1997).
Yamanaka, S. et al. Thermophysical properties of BaZrO3 and BaCeO3. J. Alloys Compd. 359, 109–113 (2003).
An, H. et al. A 5×5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm–2 at 600 °C. Nat. Energy 3, 870–875 (2018).
Duan, C. et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349, 1321–1326 (2015).
Kim, J. et al. Triple‐conducting layered perovskites as cathode materials for proton‐conducting solid oxide fuel cells. Chem. Sus. Chem. 7, 2811–2815 (2014).
Nguyen, N. T. Q. & Yoon, H. H. Preparation and evaluation of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb) electrolyte and BZCYYb-based solid oxide fuel cells. J. Power Sources 231, 213–218 (2013).
Song, Y. et al. Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Joule 3, 2842–2853 (2019).
Bae, K. et al. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat. Commun. 8, 14553 (2017).
Park, B.-K., Zhang, Q., Voorhees, P. W. & Barnett, S. A. Conditions for stable operation of solid oxide electrolysis cells: oxygen electrode effects. Energy Environ. Sci. 12, 3053–3062 (2019).
Laguna-Bercero, M., Campana, R., Larrea, A., Kilner, J. & Orera, V. Electrolyte degradation in anode supported microtubular yttria stabilized zirconia-based solid oxide steam electrolysis cells at high voltages of operation. J. Power Sources 196, 8942–8947 (2011).
Graves, C., Ebbesen, S. D., Jensen, S. H., Simonsen, S. B. & Mogensen, M. B. Eliminating degradation in solid oxide electrochemical cells by reversible operation. Nat. Mater. 14, 239–244 (2015).
Dong, Y., Zhang, Z., Alvarez, A. & Chen, I.-W. Potential jumps at transport bottlenecks cause instability of nominally ionic solid electrolytes in electrochemical cells. Acta Mater. 199, 264–277 (2020).
Dong, Y. et al. Chemical and structural origin of hole states in yttria-stabilized zirconia. Acta Mater. 203, 116487 (2021).
Xue, W. et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 6, 495–505 (2021).
Xu, G.-L. et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes. Nat. Energy 4, 484–494 (2019).
Yoon, M. et al. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 6, 362–371 (2021).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017).
Acknowledgements
This work is supported by the US Department of Energy (DoE), Office of Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office under DoE Idaho Operations Office under contract no. DE-AC07-05ID14517. Y.D. and J.L. acknowledge support by the DoE, Basic Energy Sciences, under award number DE-SC0002633 (Chemomechanics of Far-From-Equilibrium Interfaces). C.J. and M.Z. thank the subcontracts from Idaho National Laboratory and M.Z. also acknowledges funding support from National Science Foundation (no. OIA-2119688).
Author information
Authors and Affiliations
Contributions
W.B., W.W., Y.D., J.L. and D.D. conceived the project. W.B. and W.W. fabricated the cells and conducted electrochemical measurements. Y.D. conducted the theoretical analysis. Y.D. and W.B. analysed the data. B.W. contributed to STEM characterizations. W.T. contributed to cell fabrications and SEM characterizations. M.Z. contributed to AFM characterizations. C.J. contributed to peeling tests. H.D. contributed to the development of the oxygen electrode. W.F. contributed to XPS measurements. W.B., W.W., Y.D., J.L. and D.D. wrote the paper. All authors discussed and contributed to writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Truls Norby and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28 and Supplementary Tables 1–9.
Rights and permissions
About this article
Cite this article
Bian, W., Wu, W., Wang, B. et al. Revitalizing interface in protonic ceramic cells by acid etch. Nature 604, 479–485 (2022). https://doi.org/10.1038/s41586-022-04457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04457-y
This article is cited by
-
Synergistic dual-phase air electrode enables high and durable performance of reversible proton ceramic electrochemical cells
Nature Communications (2024)
-
Robust tantalum tuned perovskite oxygen electrode for reversible protonic ceramic electrochemical cells
Rare Metals (2024)
-
Preparation of Plasma Sprayed GDC Electrolytes for Metal-Supported Solid Oxide Fuel Cells
Journal of Thermal Spray Technology (2024)
-
Direct methane protonic ceramic fuel cells with self-assembled Ni-Rh bimetallic catalyst
Nature Communications (2023)
-
Defective oxygen inert phase stabilized high-voltage nickel-rich cathode for high-energy lithium-ion batteries
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.