Abstract
The global terrestrial carbon sink is increasing1,2,3, offsetting roughly a third of anthropogenic CO2 released into the atmosphere each decade1, and thus serving to slow4 the growth of atmospheric CO2. It has been suggested that a CO2-induced long-term increase in global photosynthesis, a process known as CO2 fertilization, is responsible for a large proportion of the current terrestrial carbon sink4,5,6,7. The estimated magnitude of the historic increase in photosynthesis as result of increasing atmospheric CO2 concentrations, however, differs by an order of magnitude between long-term proxies and terrestrial biosphere models7,8,9,10,11,12,13. Here we quantify the historic effect of CO2 on global photosynthesis by identifying an emergent constraint14,15,16 that combines terrestrial biosphere models with global carbon budget estimates. Our analysis suggests that CO2 fertilization increased global annual photosynthesis by 11.85 ± 1.4%, or 13.98 ± 1.63 petagrams carbon (mean ± 95% confidence interval) between 1981 and 2020. Our results help resolve conflicting estimates of the historic sensitivity of global photosynthesis to CO2, and highlight the large impact anthropogenic emissions have had on ecosystems worldwide.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data used to support the findings of this study are publicly available. TRENDY model simulations are available on request from TRENDY coordinator S. Sitch (s.a.sitch@exeter.ac.uk; https://blogs.exeter.ac.uk/trendy/). The Multivariate ENSO Index is available from https://psl.noaa.gov/enso/mei/. The GIMMS fAPAR data are available on request from R. Myneni (rmyneni@bu.edu). Climate forcings used are available from the Climate Research Unit at East Anglia University (https://crudata.uea.ac.uk/cru/data/hrg/). Upscaled GPP data are available from the Max Planck Institute for Biogeochemistry (https://www.bgc-jena.mpg.de/geodb/projects/Home.php). Locations for FLUXNET tower sites are available at www.fluxnet.org.
Code availability
Code used to support the findings of this study is publicly available at www.github.com/trevorkeenan/gpp-co2.
Change history
16 March 2022
Editor's Note: Readers are alerted that the uncertainties reported in this manuscript are currently in question. Appropriate editorial action will be taken once this matter is resolved.
30 May 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41586-022-04869-w
References
Friedlingstein, P. et al. Global carbon budget 2019. Earth Syst. Sci. Data 11, 1783–1838 (2019).
Ballantyne, A. P., Alden, C. B., Miller, J. B., Tans, P. P. & White, J. W. C. Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years. Nature 488, 70–72 (2012).
Sitch, S. et al. Recent trends and drivers of regional sources and sinks of carbon dioxide. Biogeosciences 12, 653–679 (2015).
Keenan, T. F. et al. Recent pause in the growth rate of atmospheric CO2 due to enhanced terrestrial carbon uptake. Nat. Commun. 7, 13428 (2016).
Schimel, D., Stephens, B. B. & Fisher, J. B. Effect of increasing CO2 on the terrestrial carbon cycle. Proc. Natl Acad. Sci. USA 112, 436–441 (2015).
Huntzinger, D. N. et al. Uncertainty in the response of terrestrial carbon sink to environmental drivers undermines carbon-climate feedback predictions. Sci. Rep. 7, 4765 (2017).
Walker, A. P. et al. Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2. New Phytol. 229, 2383–2385 (2020).
Sun, Z. et al. Evaluating and comparing remote sensing terrestrial GPP models for their response to climate variability and CO2 trends. Sci. Total Environ. 668, 696–713 (2019).
Smith, W. K. et al. Large divergence of satellite and Earth system model estimates of global terrestrial CO2 fertilization. Nat. Clim. Change 6, 306–310 (2016).
Li, W. et al. Recent changes in global photosynthesis and terrestrial ecosystem respiration constrained from multiple observations. Geophys. Res. Lett. 45, 1058–1068 (2018).
Wenzel, S., Cox, P. M., Eyring, V. & Friedlingstein, P. Projected land photosynthesis constrained by changes in the seasonal cycle of atmospheric CO2. Nature 538, 499–501 (2016).
Ehlers, I. et al Detecting long-term metabolic shifts using isotopomers: CO2-driven suppression of photorespiration in C3 plants over the 20th century. Proc. Natl Acad. Sci. USA 112, 15585–15590 (2015).
Campbell, J. E. et al. Large historical growth in global terrestrial gross primary production. Nature 544, 84–87 (2017).
Eyring, V. et al. Taking climate model evaluation to the next level. Nat. Clim. Change 9, 102–110 (2019).
Winkler, A. J., Myneni, R. B. & Brovkin, V. Investigating the applicability of emergent constraints. Earth Syst. Dyn. 10, 501–523 (2019).
Hall, A., Cox, P., Huntingford, C. & Klein, S. Progressing emergent constraints on future climate change. Nat. Clim. Change 9, 269–278 (2019).
Keenan, T. F. & Williams, C. A. The terrestrial carbon sink. Annu. Rev. Environ. Resour. 43, 219–243 (2018).
Ryu, Y., Berry, J. A. & Baldocchi, D. D. What is global photosynthesis? History, uncertainties and opportunities. Remote Sens. Environ. 223, 95–114 (2019).
Winkler, A. J., Myneni, R. B., Alexandrov, G. A. & Brovkin, V. Earth system models underestimate carbon fixation by plants in the high latitudes. Nat. Commun. 10, 95 (2019).
Ainsworth, E. A. & Long, S. P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372 (2005).
De Kauwe, M. G., Keenan, T. F., Medlyn, B. E., Prentice, I. C. & Terrer, C. Satellite based estimates underestimate the effect of CO2 fertilization on net primary productivity. Nat Clim. Change 6, 892–893 (2016).
Cernusak, L. A. et al Robust response of terrestrial plants to rising CO2. Trends Plant Sci. 24, 578–586 (2019).
Piao, S. et al. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob. Change Biol. 19, 2117–2132 (2013).
Haverd, V. et al. Higher than expected CO2 fertilization inferred from leaf to global observations. Glob. Change Biol. 26, 2390–2402 (2020).
Friedlingstein, P. et al. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J. Clim. 27, 511–526 (2014).
Zhao, F. et al. Role of CO2, climate and land use in regulating the seasonal amplitude increase of carbon fluxes in terrestrial ecosystems: a multimodel analysis. Biogeosciences 13, 5121–5137 (2016).
Le Quéré, C. et al. Global carbon budget 2017. Earth Syst. Sci. Data 10, 405–448 (2018).
Running, S. W. & Zhao, M. Daily GPP and Annual NPP (MOD17A2/A3) Products NASA Earth Observing System MODIS Land Algorithm User’s Guide v. 3 (MODIS Land Team, 2015).
Jung, M. et al. Global patterns of land-atmosphere fluxes of carbon dioxide, latent heat, and sensible heat derived from eddy covariance, satellite, and meteorological observations. J. Geophys. Res. 116, https://doi.org/10.1029/2010JG001566 (2011).
Zeng, N. et al. Agricultural Green Revolution as a driver of increasing atmospheric CO2 seasonal amplitude. Nature 515, 394–397 (2014).
Long, S. P. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant Cell Environ. 14, 729–739 (1991).
Stevens, N., Lehmann, C. E. R., Murphy, B. P. & Durigan, G. Savanna woody encroachment is widespread across three continents. Glob. Change Biol. 23, 235–244 (2017).
Fleischer, K. et al. Amazon forest response to CO2 fertilization dependent on plant phosphorus acquisition. Nat. Geosci. 12, 736–741 (2019).
Myneni, R. B. et al. Global products of vegetation leaf area and fraction absorbed PAR from year one of MODIS data. Remote Sens. Environ. 83, 214–231 (2002).
Cernusak, L. A. et al. Tropical forest responses to increasing atmospheric CO2: current knowledge and opportunities for future research. Funct. Plant Biol. 40, 531–551 (2013).
Ainsworth, E. A. & Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 30, 258–270 (2007).
Baig, S., Medlyn, B. E., Mercado, L. M. & Zaehle, S. Does the growth response of woody plants to elevated CO2 increase with temperature? A model-oriented meta-analysis. Glob. Change Biol. 21, 4303–4319 (2015).
Yang, J. et al. Low sensitivity of gross primary production to elevated CO2 in a mature eucalypt woodland. Biogeosciences 17, 265–279 (2020).
McMurtrie, R. E., Comins, H. N., Kirschbaum, M. U. F. & Wang, Y. P. Modifying existing forest growth models to take account of effects of elevated CO2. Aust. J. Bot. 40, 657–677 (1992).
Luo, Y., Sims, D. A., Thomas, R. B., Tissue, D. T. & Ball, J. T. Sensitivity of leaf photosynthesis to CO2 concentration is an invariant function for C3 plants: a test with experimental data and global applications. Global Biogeochem. Cycles 10, 209–222 (1996).
Li, Q. et al. Leaf area index identified as a major source of variability in modeled CO2 fertilization. Biogeosciences 15, 6909–6925 (2018).
Graven, H. D. et al. Enhanced seasonal exchange of CO2 by northern ecosystems since 1960. Science 341, 1085–1089 (2013).
Zaehle, S. et al. Evaluation of 11 terrestrial carbon-nitrogen cycle models against observations from two temperate free-air CO2 enrichment studies. New Phytol. 202, 803–822 (2014).
De Kauwe, M. G. et al. Where does the carbon go? A model-data intercomparison of vegetation carbon allocation and turnover processes at two temperate forest free-air CO2 enrichment sites. New Phytol. 203, 883–899 (2014).
Stocker, B. D. et al Drought impacts on terrestrial primary production underestimated by satellite monitoring. Nat. Geosci. 12, 264–270 (2019).
Williamson, M. S. et al Emergent constraints on climate sensitivities. Rev. Mod. Phys. 93, 025004 (2021).
Sanderson, B. et al. On structural errors in emergent constraints. Earth Syst. Dyn. Discuss. https://doi.org/10.5194/esd-2020-85 (2021).
Fisher, J. B., Huntzinger, D. N., Schwalm, C. R. & Sitch, S. Modeling the terrestrial biosphere. Annu. Rev. Environ. Resour. 39, 91–123 (2014).
Arora, V. K. et al. Carbon-concentration and carbon-climate feedbacks in CMIP5 earth system models. J. Clim. 26, 5289–5314 (2013).
Ballantyne, A. et al. Accelerating net terrestrial carbon uptake during the warming hiatus due to reduced respiration. Nat. Clim. Change 7, 148–152 (2017).
Forkel, M. et al. Enhanced seasonal CO2 exchange caused by amplified plant productivity in northern ecosystems. Science 351, 696–699 (2016).
Friedlingstein, P. et al. On the contribution of CO2 fertilization to the missing biospheric sink. Global Biogeochem. Cycles 9, 541–556 (1995).
Farquhar, G. D., von Caemmerer, S. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 (1980).
Myneni, R. B., Keeling, C. D., Tucker, C. J., Asrar, G. & Nemani, R. R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386, 698–702 (1997).
Zhu, Z. et al. Greening of the Earth and its drivers. Nat. Clim. Change 6, 791–795 (2016).
Keenan, T. F. et al. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499, 324–327 (2013).
Ukkola, A. M., Keenan, T. F., Kelley, D. I. & Prentice, I. C. Vegetation plays an important role in mediating future water resources. Environ. Res. Lett. 11, 094022 (2016).
Donohue, R. J., Roderick, M. L., McVicar, T. R. & Farquhar, G. D. Impact of CO2 fertilization on maximum foliage cover across the globe’s warm, arid environments. Geophys. Res. Lett. 40, 3031–3035 (2013).
Smith, N. G. & Dukes, J. S. Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Glob. Change Biol. 19, 45–63 (2013).
De Kauwe, M. G. et al. A test of the ‘one-point method’ for estimating maximum carboxylation capacity from field-measured, light-saturated photosynthesis. New Phytol. 210, 1130–1144 (2016).
Maire, V. et al. The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS ONE 7, e0038345 (2012).
Smith, N. G. & Keenan, T. F. Mechanisms underlying leaf photosynthetic acclimation to warming and elevated CO2 as inferred from least-cost optimality theory. Glob. Change Biol. 26, 806–834 (2020).
Lloyd, J. & Farquhar, G. The CO2 dependence of photosynthesis, plant growth responses to elevated atmospheric CO2 concentrations and their interaction with soil nutrient status. I. General principles and forest ecosystems. Funct. Ecol. 10, 4–32 (1996).
Ehleringer, J. & Björkman, O. Quantum yields for CO2 uptake in C3 and C4 plants: dependence on temperature, CO2, and O2 concentration. Plant Physiol. 59, 86–90 (1997).
Bernacchi, C. J., Singsaas, E. L., Pimentel, C., Portis, A. R. Jr & Long, SP. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell Environ. 24, 253–259 (2001).
Prentice, I. C., Dong, N., Gleason, S. M., Maire, V. & Wright, I. J. Balancing the costs of carbon gain and water transport: testing a new theoretical framework for plant functional ecology. Ecol. Lett. 17, 82–91 (2014).
Wang, H. et al. Towards a universal model for carbon dioxide uptake by plants. Nat. Plants 3, 734–741 (2017).
Huber, M. L. et al. New international formulation for the viscosity of H2O. J. Phys. Chem. Ref. Data 38, 101–125 (2009).
Still, C. J., Berry, J. A., Collatz, G. J. & DeFries, R. S. Global distribution of C3 and C4 vegetation: carbon cycle implications. Global Biogeochem. Cycles 17, 6-1–6-14 (2003).
Zhu, Z. et al. Global data sets of vegetation leaf area index (LAI)3g and fraction of photosynthetically active radiation (FPAR)3g derived from global inventory modeling and mapping studies (GIMMS) normalized difference vegetation index (NDVI3g) for the period 1981 to 2. Remote Sens. 5, 927–948 (2013).
Zhao, M. & Running, S. W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329, 940–943 (2010).
Gallego-Sala, A. et al. Bioclimatic envelope model of climate change impacts on blanket peatland distribution in Great Britain. Clim. Res. 45, 151–162 (2010).
Veroustraete, F. On the use of a simple deciduous forest model for the interpretation of climate change effects at the level of carbon dynamics. Ecol. Modell. 75–76, 221–237 (1994).
Jiang, C. & Ryu, Y. Multi-scale evaluation of global gross primary productivity and evapotranspiration products derived from Breathing Earth System Simulator (BESS). Remote Sens. Environ. 186, 528–547 (2016).
Zhang, S. et al. Evaluation and improvement of the daily boreal ecosystem productivity simulator in simulating gross primary productivity at 41 flux sites across Europe. Ecol. Modell. 368, 205–232 (2018).
Liu, Y., Hejazi, M., Li, H., Zhang, X. & Leng, G. A hydrological emulator for global applications-HE v1.0.0. Geosci. Model Dev. 11, 1077–1092 (2018).
Yuan, W. et al. Increased atmospheric vapor pressure deficit reduces global vegetation growth. Sci. Adv. 5, aax1396 (2019).
Haverd, V. et al. A new version of the CABLE land surface model (Subversion revision r4601) incorporating land use and land cover change, woody vegetation demography, and a novel optimisation-based approach to plant coordination of photosynthesis. Geosci. Model Dev. 11, 2995–3026 (2018).
Melton, J. R. & Arora, V. K. Competition between plant functional types in the Canadian Terrestrial Ecosystem Model (CTEM) v. 2.0. Geosci. Model Dev. 9, 323–361 (2016).
Oleson, K. W. et al. Technical Description of Version 4.0 of the Community Land Model (CLM) (National Center for Atmospheric Research, 2013).
Tian, H. et al. North American terrestrial CO2 uptake largely offset by CH4 and N2O emissions: toward a full accounting of the greenhouse gas budget. Clim. Change 129, 413–426 (2015).
Jain, A. K., Meiyappan, P., Song, Y. & House, J. I. CO2 emissions from land-use change affected more by nitrogen cycle, than by the choice of land-cover data. Glob. Change Biol. 19, 2893–2906 (2013).
Reick, C. H., Raddatz, T., Brovkin, V. & Gayler, V. Representation of natural and anthropogenic land cover change in MPI-ESM. J. Adv. Model Earth Syst. 5, 459–482 (2013).
Clark, D. B. et al. The Joint UK Land Environment Simulator (JULES), model description—Part 2: Carbon fluxes and vegetation dynamics. Geosci. Model Dev. 4, 701–722 (2011).
Smith, B. et al. Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model. Biogeosciences 11, 2027–2054 (2014).
Sitch, S. et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob. Chang. Biol. 9, 161–185 (2003).
Keller, K. M. et al. 20th century changes in carbon isotopes and water-use efficiency: tree-ring-based evaluation of the CLM4.5 and LPX-Bern models. Biogeosciences 14, 2641–2673 (2017).
Krinner, G. et al. A dynamic global vegetation model for studies of the coupled atmosphere-biosphere system. Global Biogeochem. Cycles 19, GB1015 (2005).
Guimberteau, M. et al. ORCHIDEE-MICT (v8.4.1), a land surface model for the high latitudes: model description and validation. Geosci. Model Dev. 11, 121–163 (2018).
Zeng, N., Mariotti, A. & Wetzel, P. Terrestrial mechanisms of interannual CO2 variability. Global Biogeochem. Cycles 19, https://doi.org/10.1029/2004GB002273 (2005).
Kato, E., Kinoshita, T., Ito, A., Kawamiya, M. & Yamagata, Y. Evaluation of spatially explicit emission scenario of land-use change and biomass burning using a process-based biogeochemical model. J. Land Use Sci. 8, 104–122 (2013).
Fernández-Martínez, M. et al. Atmospheric deposition, CO2, and change in the land carbon sink. Sci. Rep. 7, 9632 (2017).
Ciais, P. et al. Large inert carbon pool in the terrestrial biosphere during the Last Glacial Maximum. Nat. Geosci. 5, 74–79 (2012).
Cheng, L. et al. Recent increases in terrestrial carbon uptake at little cost to the water cycle. Nat. Commun. 8, 110 (2017).
Ueyama, M. et al. Inferring CO2 fertilization effect based on global monitoring land-atmosphere exchange with a theoretical model. Environ. Res. Lett. 15, 084009 (2020).
Pastorello, G. et al. The FLUXNET2015 dataset and the ONEFlux processing pipeline for eddy covariance data. Sci. Data 7, 225 (2020).
Acknowledgements
T.F.K., X.L. and Y.Z. acknowledge primary support from the NASA IDS Award NNH17AE86I. T.F.K. acknowledges additional support from NASA award 80NSSC21K1705 and by the Director, Office of Science, Office of Biological and Environmental Research of the US Department of Energy (DOE) under Contract DE-AC02-05CH11231 as part of the RUBISCO SFA and a DOE ECRP Award DE-SC0021023. M.G.D.K. acknowledges support from the Australian Research Council (ARC) Centre of Excellence for Climate Extremes (CE170100023), the ARC Discovery Grant (DP190101823) and the NSW Research Attraction and Acceleration Program. I.C.P. acknowledges the Imperial College initiative on Grand Challenges in Ecosystems and the Environment and the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (grant agreement no. 787203 REALM). N.G.S. acknowledges support from NSF DEB-2045968 and Texas Tech University. B.D.S. was funded by the Swiss National Science Foundation grant no. PCEFP2_181115. C.T. was supported by a Lawrence Fellow award through Lawrence Livermore National Laboratory (LLNL), the DOE LLNL contract DE-AC52-07NA27344, and the LLNL-LDRD Program project 20-ERD-055. We thank R. Myneni and Z. Zhu for the provision of the fAPAR dataset, the Max Planck Institute for Biogeochemistry Department of Biogeochemical Integration for the provision of the upscaled GPP data. We thank the TRENDY team for the provision of the DGVM simulations, and the researchers of the Global Carbon Project for making their data publicly available. We thank A. Walker for useful discussions on interpreting the deuterium isotopomer results, and acknowledge the stimulating discussions during the Integrating CO2 Fertilization Evidence Streams and Theory (ICOFEST) meeting September 2018, part of the FACE model Data-Synthesis project funded by the US Department of Energy, Office of Science, Office of Biological and Environmental Research.
Author information
Authors and Affiliations
Contributions
T.F.K. designed the study, performed the analysis and wrote the manuscript. X.L. aided in the regridding of the TRENDY model data. M.G.D.K., B.D.S., I.C.P., H.W., N.G.S., B.E.M., X.L. and S.Z. provided feedback on the remote sensing implementation. S.Z. and Y.Z. provided feedback on the emergent constraint implementation. B.S. provided feedback on the TRENDY model data interpretation. All authors discussed and commented on the results and the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Peter Cox, Alexander Winkler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41586-022-04869-w
Extended data figures and tables
Extended Data Fig. 1 The relationship between the sensitivity of global primary photosynthesis (GPP) to CO2 \(({{\boldsymbol{\beta }}}_{{\bf{R}}}^{{\bf{GPP}}})\) and the terrestrial carbon sink (SLAND, PgC y−1).
The emergent constraint on \({\beta }_{{\rm{R}}}^{{\rm{GPP}}}\) is comparable to that derived using the normalized SLAND, though the associated uncertainty is considerably higher due to the unexplained variance in the \({\beta }_{{\rm{R}}}^{{\rm{GPP}}}\)~SLAND relationship. The red line and shaded area show the best linear fit across models, and the associated 95% prediction intervals.
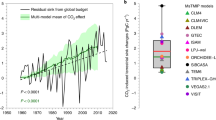
Extended Data Fig. 2 A multiple linear model of the terrestrial biosphere model predictions of the global carbon sink.
a, The terrestrial biosphere model (TBM) predictions of the global carbon sink are predicted as a function of the modeled sensitivity of photosynthesis to CO2\(({\beta }_{{\rm{R}}}^{{\rm{GPP}}})\), the modeled sensitivity of respiration to CO2\(({\beta }_{{\rm{R}}}^{{\rm{Reco}}})\) and the magnitude of the modeled non-respired carbon flux (\({\rm{\gamma }}\)) (Extended Data Table 2). The red line and shaded area show the best linear fit across models, and the associated 95% prediction intervals. b, the effect size of each of the terms included in the model (mean, 95% CI), which estimates main effect on the response from changing each predictor value, averaging out the effects of the other predictors. TBM names and details are provided in Extended Data Table 1. Details of the linear model used are provided in Extended Data Table 2.
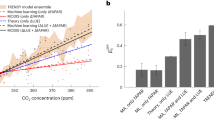
Extended Data Fig. 3 An emergent constraint on the sensitivity of global photosynthesis to CO2.
a, The relationship between the sensitivity of global primary photosynthesis (GPP) to CO2 and the modeled terrestrial carbon sink (PgC y-1), in relative terms (∆GPP (%)). The vertical gray shading shows the range of the observed terrestrial residual carbon sink over the period of 1982 to 2012, as estimated by the Global Carbon Project. The red line and shaded area show the best linear fit across models, and the associated 95% prediction intervals, and the horizontal dashed line shows the implied emergent constraint on the sensitivity of GPP to CO2. This figure reproduces Fig. 1a, but includes model names, which correspond to labels given in Extended Data Table 1. See Extended Data Fig. 1 for the underlying relationship between the sensitivity of GPP to CO2 and the terrestrial carbon sink. b, Uncertainty contributions to the constrained sensitivity of global photosynthesis to CO2. The unconstrained probability density function (PDF) distribution of \({\beta }_{{\rm{R}}}^{{\rm{GPP}}}\) across models (black line, gray bars), which assumes that all of the TRENDY models are equally likely conditional to be correct and that they come from a Gaussian distribution. The orange area represents the probability distribution derived by applying the constraint from (a) to the across model relationship, with dashed and dotted lines in the orange area indicating the relative contribution of different sources of uncertainty (see methods).
Extended Data Fig. 4 Assessment of the effect of choice of period on the sensitivity of global primary photosynthesis (GPP) to CO2 \(({{\boldsymbol{\beta }}}_{{\bf{R}}}^{{\bf{GPP}}})\).
Estimates of the residual terrestrial sink (SLAND) from the Global Carbon Project (GCP) used in this study were split into two 15-year periods (1982-1997 (a, b) and 1998-2012 (c, d)) and the emergent constraint approach (see methods) was applied to each independently, using GCP estimates of the land sink for those periods to estimate a constrained value of \({\beta }_{{\rm{R}}}^{{\rm{GPP}}}\) from the TRENDY dynamic global vegetation models (Extended Data Table 1). Estimated SLAND in panel a and c is SLAND ~ 1 + \({\beta }_{{\rm{R}}}^{{\rm{GPP}}}\) + \({\beta }_{{\rm{R}}}^{{\rm{Reco}}}\) + \({\beta }_{{\rm{R}}}^{{\rm{Reco}}}\):\({\gamma }\). The vertical dashed lines in a and c indicate the GCP estimate of the mean residual sink for that period. The red lines and shaded areas in a and c show the best linear fit across models, and the associated 95% prediction intervals.
Extended Data Fig. 5 Long-term changes in annual gross primary production (GPP) of global tropical forests.
GPP estimated by terrestrial biosphere models (TBMs) in the TRENDY model ensemble considers either temporally dynamic CO2 and fixed climate and land use (orange, experiment S1), temporally dynamic CO2 and climate, and fixed land use (red, experiment S2), or temporally dynamic CO2, climate, and land use (purple, experiment S3). Shaded areas represent the mean and standard error of the annual estimate across the TRENDY ensemble. Remote sensing (RS) GPP considers temporally dynamic climate and land use, and either fixed (blue) or varying (red) CO2. Tropical forests represent the Evergreen Broadleaf Forest classification within tropical latitudes (23.5°N: 23.5°S).
Extended Data Fig. 6 Assessment of the effect of CO2 on global primary photosynthesis (GPP) at sites included in the FLUXNET 2015 dataset.
(a) The distribution of the length of the observational record at each of the 206 sites in the FLUXNET 2015 open access database. The vertical red line indicates the median site record length (5 years). (b) The expected effect of CO2 on GPP at all sites, demonstrated by comparing the GPP predicted by the original (x-axis) and updated (y-axis) remote sensing-based methods for all site months of observations in the FLUXNET 2015 database96. The mean expected difference across sites is 2.39%.
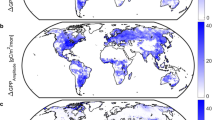
Extended Data Fig. 7 Global and high latitude changes in the terrestrial carbon cycle.
Both the global (a, b, c) and northern land (high latitude, > 45°N) (d, e, f) contribution of CO2 (orange shaded area, derived from TRENDYv6 CO2-only simulations (S1)) and climate (red shaded area, derived from the difference between TRENDYv6 CO2-only simulations and CO2 + Climate simulations (S2-S1)), to long term (1900-2016) changes in annual net ecosystem productivity (NEP), gross primary production (GPP) and ecosystem respiration (RECO). The shaded areas represent the annual mean and standard error across the TRENDY model ensemble. The impact of climate change is large in high latitude ecosystems, increasing both GPP (e) and RECO (f). This does not however translate to a large impact on the global carbon cycle (a–c).
Supplementary information
About this article
Cite this article
Keenan, T.F., Luo, X., De Kauwe, M.G. et al. RETRACTED ARTICLE: A constraint on historic growth in global photosynthesis due to increasing CO2. Nature 600, 253–258 (2021). https://doi.org/10.1038/s41586-021-04096-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04096-9
This article is cited by
-
Boosting a practical Li-CO2 battery through dimerization reaction based on solid redox mediator
Nature Communications (2024)
-
Warming inhibits increases in vegetation net primary productivity despite greening in India
Scientific Reports (2023)
-
Future socio-ecosystem productivity threatened by compound drought–heatwave events
Nature Sustainability (2023)
-
Recent decrease of the impact of tropical temperature on the carbon cycle linked to increased precipitation
Nature Communications (2023)
-
Increasing sensitivity of dryland vegetation greenness to precipitation due to rising atmospheric CO2
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.