Abstract
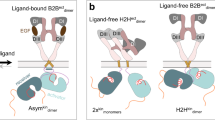
Human epidermal growth factor receptor 2 (HER2) and HER3 form a potent pro-oncogenic heterocomplex1,2,3 upon binding of growth factor neuregulin-1β (NRG1β). The mechanism by which HER2 and HER3 interact remains unknown in the absence of any structures of the complex. Here we isolated the NRG1β-bound near full-length HER2–HER3 dimer and, using cryo-electron microscopy, reconstructed the extracellular domain module, revealing unexpected dynamics at the HER2–HER3 dimerization interface. We show that the dimerization arm of NRG1β-bound HER3 is unresolved because the apo HER2 monomer does not undergo a ligand-induced conformational change needed to establish a HER3 dimerization arm-binding pocket. In a structure of the oncogenic extracellular domain mutant HER2(S310F), we observe a compensatory interaction with the HER3 dimerization arm that stabilizes the dimerization interface. Both HER2–HER3 and HER2(S310F)–HER3 retain the capacity to bind to the HER2-directed therapeutic antibody trastuzumab, but the mutant complex does not bind to pertuzumab. Our structure of the HER2(S310F)–HER3–NRG1β–trastuzumab Fab complex reveals that the receptor dimer undergoes a conformational change to accommodate trastuzumab. Thus, similar to oncogenic mutations, therapeutic agents exploit the intrinsic dynamics of the HER2–HER3 heterodimer. The unique features of a singly liganded HER2–HER3 heterodimer underscore the allosteric sensing of ligand occupancy by the dimerization interface and explain why extracellular domains of HER2 do not homo-associate via a canonical active dimer interface.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Three-dimensional cryo-EM density maps have been deposited in the Electron Microscopy Data Bank under the accession numbers EMD-23916 (HER2–HER3–NRG1b), EMD-23917 (HER2(S310F)–HER3–NRG1b) and EMD-23918 (HER2(S310F)–HER3–NRG1b–herceptin Fab). Atomic coordinates for the atomic models have been deposited in the RCSB Protein Data Bank under the accession numbers 7MN5 (HER2–HER3–NRG1b), 7MN6 (HER2(S310F)–HER3–NRG1b) and 7MN8 (HER2(S310F)–HER3–NRG1b–herceptin Fab). Source data are provided with this paper.
Change history
07 February 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41586-021-04299-0
References
Sliwkowski, M. X. et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J. Biol. Chem. 269, 14661–14665 (1994).
Wallasch, C. et al. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 14, 4267–4275 (1995).
Moasser, M. M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26, 6469–6487 (2007).
Sierke, S. L., Cheng, K., Kim, H. & Koland, J. G. Biochemical characterization of the protein tyrosine kinase domain of the ErbB3 (HER3) receptor protein. Biochem. J. 322, 757–763 (1997).
Jura, N., Shan, Y., Cao, X., Shaw, D. E. & Kuriyan, J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc. Natl Acad. Sci. USA 106, 21608–21613 (2009).
Zhang, X., Gureasko, J., Shen, K., Cole, P. A. & Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125, 1137–1149 (2006).
Littlefield, P. et al. Structural analysis of the EGFR/HER3 heterodimer reveals the molecular basis for activating HER3 mutations. Sci. Signal. 7, ra114 (2015).
Ferguson, K. et al. EGF activates its receptor by removing interactions with autoinhibit ectodomain dimerization. Mol. Cell 11, 507–517 (2003).
Bouyain, S., Longo, P. A., Li, S., Ferguson, K. M. & Leahy, D. J. The extracellular region of ErbB4 adopts a tethered conformation in the absence of ligand. Proc. Natl Acad. Sci. USA 102, 15024–15029 (2005).
Cho, H.-S. & Leahy, D. J. Structure of the extracellular region of HER3 reveals an interdomain tether. Science 297, 1330–1333 (2002).
Garrett, J. T. et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor α. Cell 110, 763–773 (2002).
Ogiso, H. et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 110, 775–787 (2002).
Liu, P. et al. A single ligand is sufficient to activate EGFR dimers. Proc. Natl Acad. Sci. USA 109, 10861–10866 (2012).
Cho, H.-S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760, (2003).
Hao, Y., Yu, X., Bai, Y., McBride, H. J. & Huang, X. Cryo-EM Structure of HER2–trastuzumab–pertuzumab complex. PLoS ONE 14, e0216095 (2019).
Alvarado, D., Klein, D. E. & Lemmon, M. A. ErbB2 resembles an autoinhibited invertebrate epidermal growth factor receptor. Nature 461, 287–291 (2009).
Ferguson, K. M., Darling, P. J., Mohan, M. J., Macatee, T. L. & Lemmon, M. A. Extracellular domains drive homo- but not hetero- dimerization of erbB receptors. EMBO J. 19, 4632–4643 (2000).
Hanker, A. B. et al. Co-occurring gain-of-function mutations in HER2 and HER3 modulate HER2/HER3 activation, oncogenesis, and HER2 inhibitor sensitivity. Cancer Cell, 39, 1099–1114.e8 (2021).
Freed, D. M. et al. EGFR ligands differentially stabilize receptor dimers to specify signaling kinetics. Cell 171, 683–695 e618 (2017).
Lu, C. et al. Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor. Mol. Cell. Biol. 30, 5432–5443, (2010).
Huang, Y. et al. A molecular mechanism for the generation of ligand-dependent differential outputs by the epidermal growth factor receptor. Preprint at https://doi.org/10.1101/2020.12.08.417006 (2021).
Greulich, H. et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc. Natl Acad. Sci. USA 109, 14476–14481 (2012).
Wang, T. et al. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 108, 671–677 (2017).
The Cancer Genome Atlas Network. Comprehensive molecular portraits for human breast tumours. Nature 490, 61–70 (2012).
Franklin, M. C. et al. Insights into ErbB signaling from the structure of the ErbB2–pertuzumab complex. Cancer Cell 5, 317–328 (2004).
Alvarado, D., Klein, D. E. & Lemmon, M. A. Structural basis for negative cooperativity in growth factor binding to an EGF receptor. Cell 142, 568–579 (2010).
Arkhipov, A., Shan, Y., Kim, E. T., Dror, R. O. & Shaw, D. E. Her2 activation mechanism reflects evolutionary preservation of asymmetric ectodomain dimers in the human EGFR family. eLife 2, e00708 (2013).
Garrett, J. T. et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol. Cell 11, 495–505 (2003).
Xu, W. et al. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat. Struct. Mol. Biol. 12, 120–126 (2005).
Jaiswal, B. S. et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell 23, 603–617 (2013).
Palovcak, E. et al. A simple and robust procedure for preparing graphene-oxide cryo-EM grids. J. Struct. Biol. 204, 80–84 (2018).
Wang, F. et al. General and robust covalently linked graphene oxide affinity grids for high-resolution cryo-EM. Proc. Natl Acad. Sci. USA 117, 24269–24273 (2020).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
de la Rosa-Trevin, J. M. et al. Scipion: A software framework toward integration, reproducibility and validation in 3D electron microscopy. J. Struct. Biol. 195, 93–99 (2016).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Kucukelbir, A., Sigworth, F. & Tagare, H. Quantifying the local resolution of cryo-EM density maps. Nature Methods 11, 63–65 (2014).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793-796 (2017).
Asarnow, D., Palovcak, E. & Cheng, Y. UCSF pyem v0.5. Zenodo (2019).
DiMaio, F. et al. Atomic-accuracy models from 4.5-A cryo-electron microscopy data with density-guided iterative local refinement. Nat. Methods 12, 361–365 (2015).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D 74, 519–530 (2018).
Frenz, B. et al. Automatically fixing errors in glycoprotein structures with Rosetta. Structure 27, 134–139.e133 (2019).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Agirre, J. et al. Privateer: software for the conformational validation of carbohydrate structures. Nat. Struct. Mol. Biol. 22, 833–834 (2015).
Acknowledgements
We thank A. Manglik for advice on receptor expression; D. Bulkley, G. Gilbert, E. Tse and Z. Yu from the UCSF EM facility; members of the Verba and Jura laboratories for their helpful discussions; E. Linossi and H. Torosyan for critical comments on the manuscript; and D. Suveges for his experimental contributions to the generation of our first HER expression constructs. This work was funded through UCSF Program for Breakthrough Biomedical Research to K.A.V. and N.J., NIH/NIGMS R35-GM139636 to N.J., Genentech research grant to N.J., NIH/NCI U54-CA209891 to N.J. and D.A.A., NIH/NIGMS R35-GM118099 to D.A.A., DFG German Research Foundation GZ: TR 1668/1-1 to R.T. and NIH/NCI 1F30CA247147 to D.D.
Author information
Authors and Affiliations
Contributions
N.J. conceived the project and D.D., R.T., K.A.V. and N.J. designed the research approach. D.D. and R.T. performed all expression and purification, electron microscopy imaging and processing, structural modelling and in vitro experiments. T.M.T. provided initial receptor expression constructs. F.W. and D.A.A. provided holey carbon graphene-oxide grids for cryo-EM. D.D., R.T., K.A.V. and N.J. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
N.J. is a member of the Scientific Advisory Board and a shareholder of Turning Point Therapeutics, SUDO Biosciences and Type6 Therapeutics. The Jura laboratory has received sponsored research support from Genentech. The other authors declare no competing interests.
Additional information
Peer review information Nature thanks Michael Eck, Stevan Hubbard, Zhi-Jie Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Purification, characterization, and reconstruction of the near full-length HER2/HER3/NRG1β heterocomplex.
a, Schematic summary of the HER2/HER3/NRG1β complex purification strategy. b, A representative Coomassie-stained SDS-PAGE gel analysis of the HER2/HER3/NRG1β complex after purification showing bands corresponding to the HER2 and HER3 receptors. The gel is representative of three independent experiments. c, Representative size exclusion chromatography profile of the HER2/HER3/NRG1β complex resolved on a Superose6 10/300 Increase column (GE Healthcare). The major peak at ~14 ml elution volume is marked grey and corresponds to the fractions used for structural studies. We routinely obtained a 1-2 mAu peaks for the complex preparations from 120 ml of mammalian culture, which was sufficient for the structural studies. d, Mass photometry of the peak sample indicates that the majority of particles have an average mass of ~326 kDa with a standard deviation of 17 kDa. The mass is consistent with that of the HER2/HER3/NRG1β complex (predicted ~280 kDa without accounting for micelle mass, ~340 kDa assuming a ~60 kDa DDM micelle mass). e, Representative negative stain electron microscopy 2D class averages of sample from HER2/HER3/NRG1β complex fractions (ECDs (extracellular domains), ICDs (intracellular domains)). f, Near full-length reconstruction of HER2-S310F/HER3/NRG1β after particle recentering with center of mass at the micelle in RELION from a ~45,000 particle stack. Rod-shaped density consistent with the asymmetric kinase domain dimer is visible below the micelle. g, The reconstruction accommodates models of the HER2-S310F/HER3/NRG1β extracellular domains from this study, two helical transmembrane domains and juxtamembrane-A (JM-A) segments from HER2 (PDB ID 2N2A), and kinases arranged in the asymmetric dimer (homology model generated based on structures of the EGFR kinase from PDB ID 3KEX and PDB ID 3PP0). HER2 is colored in blue, HER3 in golden yellow, and NRG1β in teal.
Extended Data Fig. 2 Resolution estimation, map quality and the workflow for processing of the HER2/HER3/NRG1β dataset.
a, Representative micrograph of the HER2/HER3/NRG1β sample on graphene oxide grids, from a dataset with 2035 GO-containing micrographs. The scale bar corresponds to 0.5 μm. b, Example 2D cryo-EM class averages. c, Local resolution estimation from ResMap. d, Gold Standard Fourier Shell Correlation (GSFSC) of the final map used for model building from CryoSPARC2 with a reported resolution of 2.93 Å. e, Directional FSCs calculated by 3DFSC server. Map sphericity was calculated to be 0.927. f, Workflow for processing the HER2/HER3/NRG1β dataset. Blue box indicates model and associated particle stack used for downstream processing. The final model is indicated with a red box. The map in the yellow box represents a slightly different low-occupancy species. The insets show that the HER3 dimerization arm is still poorly resolved in that class.
Extended Data Fig. 3 Q-score analysis of the cryo-EM maps and a structural comparison of the HER2/HER3/NRG1β heterocomplex with crystal structures of previously reported HER receptor structures.
a, Zoomed-in view of the cryo-EM density and model of WT HER2/HER3 (residues 63-150 of HER3) showing features appropriate for the reported resolution. b, WT HER2/HER3 model colored by estimated per residue Q-score ranging from 0 (red) to 0.75 (blue). The color bar shows corresponding estimated resolution in Å for each Q-score. Expected Q-score for 2.9Å map is 0.604. c, Zoomed-in view of the cryo-EM density and model of HER2 S310F/HER3 (residues 63-150 of HER3) showing features appropriate for the reported resolution. d, HER2-S310F/HER3 model colored by estimated Q-score with the same scale as in b. Expected Q-score for 3.1Å structure is 0.569. e, Overlay of the HER2/HER3/NRG1β heterocomplexes with symmetric structures of EGFR/EGF (PDB ID 3NJP), EGFR/TGFα (PDB ID 1MOX) and HER4/NRG1β (PDB ID 3U7U). f, Overlay of the HER2/HER3/NRG1β heterocomplexes with asymmetric structures of EGFR/EREG (PDB ID 5WB7), doubly liganded dEGFR/SPITZ (PDB ID 3LTF) and singly liganded dEGFR/SPITZ (PDB ID 3LTG). All structures were aligned on HER3. Differences between the heterodimer and the homodimers are primarily appreciated in overlays on the HER2 monomer. The heterodimer more closely resembles asymmetric homodimers than symmetric homodimers but reflects a unique conformation that is not seen in previous structures.
Extended Data Fig. 4 Comparison of the domain II dimerization interface between HER2/HER3/NRG1β complex domain with crystal structures of previously reported HER receptor homodimers.
a–e, The domain II interfaces of select HER receptor dimers are shown with the number of hydrogen bonds and the total buried surface area between domains I-III indicated below. Domain IV was excluded from this analysis because it is not resolved in all structures. Hydrogen bonds are shown as red dotted lines, highlighting more substantial interfaces for symmetric homodimers (EGFR/EGF (PDB ID 3NJP), HER4/NRG1β (PDB ID 3U7U) than asymmetric dimers (HER2/HER3/NRG1β, EGFR/EREG (PDB ID 5WB7)), with the exception of the mutant HER2-S310F/HER3/NRG1β heterocomplex in which the mutation stabilizes the domain II interface. All interface hydrogen bonds are formed within domain II, except for an additional hydrogen bond in EGFR with domain III which is not shown here and marked (*) in table. Numbering for all HER receptors includes the signal sequence peptide.
Extended Data Fig. 5 In-depth structural and functional analysis of the NRG1β binding-site and the HER2/HER3 dimerization interface.
a, Left, HER4 bound to NRG1β (PDB ID 3U7U) with salt-bridge interactions highlighted. Middle, HER3 bound to NRG1β in our structure with salt bridge interactions highlighted. NRG1β engages with HER3 primarily through an extensive interaction network at its C-terminus (total buried surface area: 2,803 Å2) stabilized by salt bridges between R207 (NRG1β) and D112 in HER3 domain I, and R220 (NRG1β) and D371 in HER3 domain III, bringing domains I and III into close proximity. Right, overlay between the two structures shows that the overall orientation of the ligand and some salt-bridge interactions are shared between HER3 and HER4, but overall HER3 forms fewer salt bridges with NRG1β than HER4. b, Structure of HER2 in our HER2/HER3/NRG1β dimer structure is overlayed with the crystal structure of the HER2 extracellular domain bound to trastuzumab Fab (PDB ID 1N8Z), the cryo-EM structure of the HER2 extracellular domain bound to pertuzumab and trastuzumab Fabs (PDB ID 6OGE) and the crystal structure of the rat HER2 extracellular domain (PDB ID 1N8Y). The structures are almost identical with root mean squared deviations (RMSDs) as following: 1.01 Å (1N8Z), 0.74 Å (1N8Y), 0.97 Å (6OGE). Minor conformational changes are observed in the dimerization arm and domain IV. c, Detailed view of the dimerization interface between domains II of HER2 and HER3 in the HER2/HER3/NRG1β structure with all polar contacts between receptors highlighted in the boxes to the left and to the right. d, Calculated buried surface area at the dimerization interface for HER2/HER3/NRG1β complex and the known structures of the dimeric HER extracellular domain complexes. The following structures were used for the analysis: EGFR/EGF (PDB ID 3NJP), EGFR/EREG (PDB ID 5WB7), HER4/NRG1β (PDB ID 3U7U). e, Representative Western Blot analysis of the HER2 and HER3 (wild-type HER3 and HER3 GS-arm) constructs co-transfected in the EXPI293 cells and pulled-down via NRG1β immobilized on FLAG beads. TS – Twin Strep tag (present on both HER2 and HER3). Receptors were detected with the Strep-Tactin® HRP conjugate (anti-TS). For gel source data, see Supplementary Figure 1. f, Quantification of data shown in panel e. Values are presented as mean values +/− SD of mean intensity ratios of HER2 over HER3 for each blot in n = 3 independent biological replicates. The HER2/HER3 pulldown ratios are 0.18 +/− 0.06 for HER3-WT and 0.22 +/− 0.07 for HER3-GS-Arm complexes. Significance was determined via unpaired, two-tailed t-test via GraphPad Prism. p = 0.5463, t = 0.6582, df = 4. g, Anti-phospho-HER3 (pHER3, Y1289), anti-HER3 and anti-HER2 Western Blot analysis of lysates from COS7 cells transfected with indicated full-length receptors (wild type or carrying the indicated mutations), serum-starved and stimulated with 10 nM NRG1β for 10 min. HER3-V945R is a negative control due to the presence of mutation in the active kinase dimer interface. For gel source data, see Supplementary Figure 1. h, Quantification of data shown in panel g. Values are presented as mean values +/− SD of pHER3 intensities of the mutant relative to wild-type HER3 for each blot in n = 5 independent biological replicates. The mean intensity values are 0.90 +/− 0.19 for the HER3-GS-Arm and 0.13 +/− 0.04 for HER3-V945R relative to the WT with defined values of 1. Significance was determined via unpaired, two-tailed t-test via GraphPad Prism. HER3 GS-arm vs HER3: p = 0.2887, t = 1.136, df = 8, HER3 vs HER3 V945R: p<0.0001, t = 43.89, df = 8.
Extended Data Fig. 6 The oncogenic HER2-S310F/Y mutations increase the yields of the HER2/HER3/NRG1β complex purification.
a, Representative Western Blot analysis of the HER2 (wild type, S310F or S310F mutant) and HER3 constructs co-transfected in the EXPI293 cells, and pulled-down via NRG1β immobilized on FLAG beads. TS – Twin Strep tag (present on both HER2 and HER3). Receptors were detected with the Strep-Tactin® HRP conjugate (anti-TS). For gel source data, see Supplementary Figure 1. b, Densitometry analysis of blots for data shown in panel a. Values are presented as mean values +/− SD of mean intensity ratios of HER2 over HER3 for each blot in n=3 independent biological replicates. The HER2/HER3 pulldown ratios are 0.09 +/− 0.04 for HER2-WT, 0.20 +/− 0.06 for HER2-S310F and 0.35 +/− 0.09 for HER2-S310Y complexes. Significance was determined via unpaired, two-tailed t-test via GraphPad Prism. WT vs S310F: p = 0.0605, t = 2.592, df = 4. WT vs. S310Y: p = 0.0107, t = 4.516, df = 4. c, Overlay of representative size exclusion chromatogram profiles from a Superose6 10/300 Increase column (GE Healthcare) of WT and oncogenic HER2-S310F heterocomplexes. Heterodimer peaks are denoted by asterisks. d, Cartoon representation of the cryo-EM structure of HER2-S310F/HER3/NRG1β complex overlayed on HER2/HER3/NRG1β. The HER2-S310F mutation is shown in red.
Extended Data Fig. 7 Resolution estimation, map quality and the workflow for processing of the HER2-S310F/HER3/NRG1β and HER2-S310F/HER3/NRG1β trastuzumab datasets.
a, Cryo-EM map colored by local resolution as estimated from ResMap. b, Gold Standard Fourier Shell Correlation (GSFSC) of the final map used for model building from CryoSPARC2 with a reported resolution of 3.10 Å. c, Directional FSCs calculated by 3DFSC server. Map sphericity was calculated to be 0.949.d, Workflow for processing the HER2/HER3/NRG1β/trastuzumab dataset. The final model is indicated with a red box. e, Cryo-EM map colored by local resolution as estimated from ResMap. f, Gold Standard Fourier Shell Correlation (GSFSC) of the final map used for model building from CryoSPARC2 with a reported resolution of 3.45Å. g, Directional FSCs calculated by 3DFSC server. Map sphericity was calculated to be 0.941.
Extended Data Fig. 8 Functional and structural analysis of interactions between the therapeutic Fabs and HER receptors in the HER2/HER3/NRG1β complex.
a, Overlay of the HER2/trastuzumab structure (PDB ID 6OGE) with the HER2-S310F/HER3/NRG1β structure reveals a steric clash between the Fab light chain constant domain and HER3 domain III. b, Left, overlay of the trastuzumab Fab variable domains in the HER2/trastuzumab structure (PDB ID 1N8Z) with the trastuzumab Fab in the HER2/HER3/NRG1β complex (HER2 domain IV in blue, trastuzumab Fab variable domains in magenta). The structures were aligned on HER2 domains I-III. Right, the HER2 domain IV bound to Fab variable domain from each structure was overlayed (RMSD: 1.569 Å) demonstrating that a Fab-binding epitope on HER2 is the same. Trastuzumab therefore avoids a steric clash with HER3 by inducing a rigid body rotation of HER2 domain IV relative to HER3. c, Overlay of the HER2/pertuzumab structure (PDB: 6OGE) with the HER2-S310F/HER3/NRG1β structure reveals a steric clash between the Fab variable domains, the HER3 dimerization arm, and HER3 domain II. d, Anti-phospho-HER3 (pHER3, Y1289), anti-HER3 and anti-HER2 Western Blot analysis of lysates from COS7 cells transfected with indicated full-length wild-type or mutant receptors. Cells were serum-starved, pre-incubated with pertuzumab or trastuzumab for 1h, as indicated, and then stimulated with 10 nM NRG1β. HER3-EGQR is HER3-E928G/Q809R. Representative Western Blot is shown, n=3. For gel source data, see Supplementary Figure 1. e, D31 of the pertuzumab variable light chain forms a polar contact with HER2 S310. f, HER2-S310F removes the polar contact with D31 and introduces a steric clash with T30 of the pertuzumab variable light chain. The predicted net effect of the oncogenic HER2-S310F mutation would be a decrease in pertuzumab affinity for HER2 (PDB ID 6OGE).
Supplementary information
Supplementary Figure 1
Unprocessed and uncropped gel images for Fig. 4a, Extended Data Figs. 5e, g, 6a, 8d. Dotted boxes indicate where the gels were cropped. Fig. 4a blot was processed on film, and the position of the molecular weight marker was manually marked on the film by overlay with the blot. For Extended Data Figs. 5e, g, 6a, 8d, blots were imaged using BioRad Imager. A colour image for each blot was obtained using a separate channel, and overlaid on the chemiluminescent image to obtain exact position of the molecular weight marker. The overlays are presented in their uncropped versions.
Rights and permissions
About this article
Cite this article
Diwanji, D., Trenker, R., Thaker, T.M. et al. Structures of the HER2–HER3–NRG1β complex reveal a dynamic dimer interface. Nature 600, 339–343 (2021). https://doi.org/10.1038/s41586-021-04084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04084-z
This article is cited by
-
Neuregulin-1 and ALS19 (ERBB4): at the crossroads of amyotrophic lateral sclerosis and cancer
BMC Medicine (2024)
-
SIBP-03, a novel anti-HER3 antibody, exerts antitumor effects and synergizes with EGFR- and HER2-targeted drugs
Acta Pharmacologica Sinica (2024)
-
Structure and dynamics of the EGFR/HER2 heterodimer
Cell Discovery (2023)
-
Tuning phenylalanine fluorination to assess aromatic contributions to protein function and stability in cells
Nature Communications (2023)
-
Glioblastoma mutations alter EGFR dimer structure to prevent ligand bias
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.