Abstract
Resolving the early evolution of euarthropods is one of the most challenging problems in metazoan evolution1,2. Exceptionally preserved fossils from the Cambrian period have contributed important palaeontological data to deciphering this evolutionary process3,4. Phylogenetic studies have resolved Radiodonta (also known as anomalocaridids) as the closest group to all euarthropods that have frontalmost appendages on the second head segment (Deuteropoda)5,6,7,8,9. However, the interrelationships among major Cambrian euarthropod groups remain disputed1,2,4,7, which impedes our understanding of the evolutionary gap between Radiodonta and Deuteropoda. Here we describe Kylinxia zhangi gen. et. sp. nov., a euarthropod from the early Cambrian Chengjiang biota of China. Kylinxia possesses not only deuteropod characteristics such as a fused head shield, a fully arthrodized trunk and jointed endopodites, but also five eyes (as in Opabinia) as well as radiodont-like raptorial frontalmost appendages. Our phylogenetic reconstruction recovers Kylinxia as a transitional taxon that bridges Radiodonta and Deuteropoda. The most basal deuteropods are retrieved as a paraphyletic lineage that features plesiomorphic raptorial frontalmost appendages and includes Kylinxia, megacheirans, panchelicerates, ‘great-appendage’ bivalved euarthropods and isoxyids. This phylogenetic topology supports the idea that the radiodont and megacheiran frontalmost appendages are homologous, that the chelicerae of Chelicerata originated from megacheiran great appendages and that the sensorial antennae in Mandibulata derived from ancestral raptorial forms. Kylinxia thus provides important insights into the phylogenetic relationships among early euarthropods, the evolutionary transformations and disparity of frontalmost appendages, and the origin of crucial evolutionary innovations in this clade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data analysed in this paper, including the phylogenetic datasets, are available as part of the Article, Extended Data Figs. 1–10 or Supplementary Information. The nomenclatural acts in this publication have been registered at ZooBank (LSID: urn:lsid:zoobank.org:pub:3B79BB94-239D-4C0A-93E5-62E90DAE6469).
References
Giribet, G. & Edgecombe, G. D. The phylogeny and evolutionary history of arthropods. Curr. Biol. 29, R592–R602 (2019).
Budd, G. E. & Telford, M. J. The origin and evolution of arthropods. Nature 457, 812–817 (2009).
Edgecombe, G. D. & Legg, D. A. Origins and early evolution of arthropods. Palaeontology 57, 457–468 (2014).
Daley, A. C., Antcliffe, J. B., Drage, H. B. & Pates, S. Early fossil record of Euarthropoda and the Cambrian Explosion. Proc. Natl Acad. Sci. USA 115, 5323–5331 (2018).
Daley, A. C., Budd, G. E., Caron, J.-B., Edgecombe, G. D. & Collins, D. The Burgess Shale anomalocaridid Hurdia and its significance for early euarthropod evolution. Science 323, 1597–1600 (2009).
Legg, D. A., Sutton, M. D. & Edgecombe, G. D. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nat. Commun. 4, 2485 (2013).
Ortega-Hernández, J. Making sense of ‘lower’ and ‘upper’ stem-group Euarthropoda, with comments on the strict use of the name Arthropoda von Siebold, 1848. Biol. Rev. 91, 255–273 (2016).
Aria, C. & Caron, J.-B. Burgess Shale fossils illustrate the origin of the mandibulate body plan. Nature 545, 89–92 (2017).
Aria, C. & Caron, J.-B. A middle Cambrian arthropod with chelicerae and proto-book gills. Nature 573, 586–589 (2019).
Whittington, H. B. The enigmatic animal Opabinia regalis, Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. R. Soc. Lond. B 271, 1–43 (1975).
Ortega-Hernández, J. Homology of head sclerites in Burgess Shale euarthropods. Curr. Biol. 25, 1625–1631 (2015).
Daley, A. C. & Edgecombe, G. D. Morphology of Anomalocaris canadensis from the Burgess Shale. J. Paleontol. 88, 68–91 (2014).
Cong, P. et al. New radiodonts with gnathobase-like structures from the Cambrian Chengjiang biota and implications for the systematics of Radiodonta. Pap. Palaeontol. 4, 605–621 (2018).
Chen, J., Waloszek, D. & Maas, A. A new ‘great-appendage’ arthropod from the Lower Cambrian of China and homology of chelicerate chelicerae and raptorial antero-ventral appendages. Lethaia 37, 3–20 (2004).
Hou, X. New rare bivalved arthropods from the Lower Cambrian Chengjiang Fauna, Yunnan, China. J. Paleontol. 73, 102–116 (1999).
Fu, D., Zhang, X. & Shu, D. Soft anatomy of the early Cambrian arthropod Isoxys curvirostratus from the Chengjiang biota of south China with a discussion on the origination of great appendages. Acta Palaeontol. Pol. 56, 843–852 (2011).
Legg, D. A. & Vannier, J. The affinities of the cosmopolitan arthropod Isoxys and its implications for the origin of arthropods. Lethaia 46, 540–550 (2013).
Hou, X. et al. The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal Life 2nd edn (John Wiley & Sons, 2017).
Aria, C., Zhao, F., Zeng, H., Guo, J. & Zhu, M. Fossils from South China redefine the ancestral euarthropod body plan. BMC Evol. Biol. 20, 4 (2020).
Ortega-Hernández, J., Lerosey-Aubril, R. & Pates, S. Proclivity of nervous system preservation in Cambrian Burgess Shale-type deposits. Proc. R. Soc. Lond. B 286, 20192370 (2019).
Yang, J., Ortega-Hernández, J., Butterfield, N. J. & Zhang, X. Specialized appendages in fuxianhuiids and the head organization of early euarthropods. Nature 494, 468–471 (2013).
Tanaka, G., Hou, X., Ma, X., Edgecombe, G. D. & Strausfeld, N. J. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502, 364–367 (2013).
Haug, J. T., Waloszek, D., Maas, A., Liu, Y. & Haug, C. Functional morphology, ontogeny and evolution of mantis shrimp-like predators in the Cambrian. Palaeontology 55, 369–399 (2012).
Ortega-Hernández, J., Janssen, R. & Budd, G. E. Origin and evolution of the panarthropod head — a palaeobiological and developmental perspective. Arthropod Struct. Dev. 46, 354–379 (2017).
Scholtz, G. & Edgecombe, G. D. The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 216, 395–415 (2006).
Cong, P., Ma, X., Hou, X., Edgecombe, G. D. & Strausfeld, N. J. Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513, 538–542 (2014).
Stein, M. A new arthropod from the Early Cambrian of North Greenland, with a ‘great appendage’-like antennula. Zool. J. Linn. Soc. 158, 477–500 (2010).
Yang, J., Ortega-Hernández, J., Lan, T., Hou, J. & Zhang, X. A predatory bivalved euarthropod from the Cambrian (Stage 3) Xiaoshiba Lagerstätte, South China. Sci. Rep. 6, 27709 (2016).
Fu, D., Zhang, X., Budd, G. E., Liu, W. & Pan, X. Ontogeny and dimorphism of Isoxys auritus (Arthropoda) from the Early Cambrian Chengjiang biota, South China. Gondwana Res. 25, 975–982 (2014).
Aria, C. & Caron, J.-B. Cephalic and limb anatomy of a new isoxyid from the Burgess Shale and the role of “stem bivalved arthropods” in the disparity of the frontalmost appendage. PLoS ONE 10, e0124979 (2015).
Zhao, F., Caron, J.-B., Hu, S. & Zhu, M. Quantitative analysis of taphofacies and paleocommunities in the Early Cambrian Chengjiang Lagerstätte. Palaios 24, 826–839 (2009).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Lewis, P. O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925 (2001).
Rambaut, A., Drummond, A. J., Xie, D., Baele, G. & Suchard, M. A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904 (2018).
Goloboff, P. A. Estimating character weights during tree search. Cladistics 9, 83–91 (1993).
Goloboff, P. A. & Catalano, S. A. TNT, version 1.5, with a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238 (2016).
Acknowledgements
This study was supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB26000000 and XDB18000000), the National Natural Science Foundation of China (41921002, 41902014 and 41925008), Jiangsu Basic Research Project (BK20191102), State Key Laboratory of Paleobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, CAS (20192111 and 20181105) and IGCP Project 668. H.Z. was supported by a Smithsonian post-doctoral fellowship. We thank J. Chen and S. Hu for access to their collections of Chengjiang fossils, J. Li and T. Alima (Electron Microscopy Laboratory, IGGCAS) for efforts to maintain operation during scanning electron microscopy and energy-dispersive X-ray spectroscopy experiments, H. Xu and Y. Chen (Geobiodiversity Database, NIGPAS) for maintaining the computers used in the phylogenetic analysis, J. Sun for artistic reconstructions, D. Briggs for discussion, J.-B. Caron for access to the radiodont specimens at the Royal Ontario Museum, and D. Erwin for comments on the manuscript and access to the Burgess Shale collection at the Smithsonian Institution.
Author information
Authors and Affiliations
Contributions
D.H. designed the research. M.Z. secured the funding and prompted completion of the project. F.Z. collected all of the material of Kylinxia zhangi except for the paratype, which was collected by K.N. H.Z. conducted the geochemical and phylogenetic analyses. H.Z. wrote the paper with input from all other authors. All authors participated in the interpretation of the material and the discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Jean Vannier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
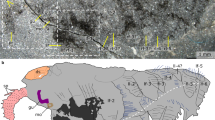
Extended Data Fig. 2 Additional specimens of Kylinxia zhangi in different post-embryonic developmental stages.
a, b, Nearly complete specimen, NIGP 171305a, part. c, d, Incomplete specimen showing anterior body part, NIGP 171306. e–g, Complete larger juvenile specimen, NIGP 171307a, part. h, i, Complete smaller juvenile specimen, NIGP 171308. a, c, e, g, Whole specimens. b, d, f, i, Head regions in a, c, e, h, respectively. g, Magnification of the alimentary canal shown in e, showing paired digestive glands. ts, spine on head shield. Other abbreviations as in Figs. 1, 2.
Extended Data Fig. 3 Head and eyes of Cambrian euarthropods discussed in this study.
a–d, g, h, Opabinia regalis. a, USNM 155600b. b, USNM 205259. c, USNM 205258. d, USNM 131217. e, Anomalocaris sp., ELRC 20001. f, Haikoucaris ercaiensis, ELRC EC11051a. g–i, Ocular regions in c–e, respectively. j, l, Odaraia alata, USNM 189232. k, m, Helmetia expansa, USNM 83952. l, m, Ocular regions in j, k, respectively. n, o, Head regions of Kylinxia zhangi in Extended Data Fig. 1a, b, respectively, NIGP 171304. n, Part, NIGP 171304a. o, Counterpart, NIGP 171304b. as, anterior sclerite; me, median eye. Other abbreviations as in Figs. 1, 2.
Extended Data Fig. 4 Drawings of the head region in Kylinxia and comparison of frontalmost appendages in Kylinxia and Anomalocaris.
a–g, K. zhangi. a–e, Camera lucida drawings of head regions, see colours of different anatomical structures at the bottom. a, YLSNHM 01124, as in Fig. 2g. b, NIGP 171304a, as in Extended Data Fig. 2d. c, NIGP 171307a, as in Extended Data Fig. 2f. d, NIGP 171305a, as in Extended Data Fig. 2b. e, NIGP 171308, as in Extended Data Fig. 2i. f, g, Frontalmost appendages of Kylinxia. f, YLSNHM 01124. g, NIGP 171304a. h–k, Frontal appendages of Anomalocaris canadensis. h, i, Full appendages. h, GSC 75535. i, ROMIP 51212. j, k, Shaft region. j, ROMIP 51215. k, ROMIP 59947. Abbreviations as in Figs. 1, 2 and Extended Data Fig. 2.
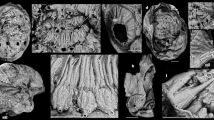
Extended Data Fig. 5 Anatomy and geochemical analysis of cephalic soft tissues in Kylinxia zhangi, holotype NIGP 171304a.
a, Magnification of the head and anteriormost trunk under visible light. b, Interpretive drawing of a by integrating observations under visible light and from geochemical analysis, see Extended Data Fig. 4 for a colour key. c, Backscatter scanning electron image of a under a scanning electronic microscope. d–h, Elemental maps of a from energy-dispersive X-ray spectroscopy. d, C map, showing alimentary canal with positive signal. e, Fe map, showing nervous tissue with positive signal. f, Overlay image of C and Fe maps with the blending mode of filtering colour in Adobe Photoshop CS6, showing the compositional differences between alimentary canal and nervous tissues. g, P map, showing putative digestive glands with positive signal. h, Si map, showing soft tissues in negative relief. pr, protocerebral tissue. Other abbreviations as in Figs. 1, 2.
Extended Data Fig. 6 Morphological details of trunk in Kylinxia zhangi.
a–d, Soft tissue in trunk. Arrowheads indicate putative bifurcating nerves into paired trunk appendages. a, YLSNHM 01124. b, NIGP 171304a. c, NIGP 171305a. d, NIGP 171306. e–h, Tail region. e, NIGP 171307a. f, NIGP 171308. g, NIGP 171304a. h, YLSNHM 01124. i, Magnification of the tail flaps in g, showing setae. a, trunk appendage; st, setae. Other abbreviations as in Figs. 1, 2.
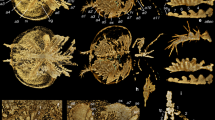
Extended Data Fig. 7 Frontalmost appendages of Cambrian megacheirans, isoxyids and artiopodans.
a–i, Great appendages of megacheirans. a, d, Haikoucaris ercaiensis, NIGP 171309. a, Whole specimen. d, Great appendage in a. b, e, Fortiforceps foliosa, NIGP 169954. b, Whole specimen. e, Great appendage in b. c, f, g, Yohoia tenuis, USNM 179053. c, Whole specimen. f, Great appendage in c. g, Endites in f, arrowheads indicate auxiliary spines. h, i, Parapeytoia yunnanensis, NIGP 171310. h, Great appendage. i, Endite showing auxiliary spines in h. j–q, Frontalmost appendages showing raptorial features in isoxyids and artiopodans. j, k, Isoxys curvirostratus, NIGP 171311. j, Whole specimen. k, Frontalmost appendage in j, note the frontalmost appendages are disarticulated and oriented downward. l–o, I. auritus. l, m, YDKS 43. n, o, NIGP 171312. l, n, Whole specimen. m, o, Frontalmost appendage in l, n, respectively. p, q, Kuamaia lata, NIGP 172294. p, Head region. q, Antenna in p. Yellow arrowheads indicate endites. l, m, Specimen courtesy of S. Hu. ba, basis of great appendage; pd, peduncle podomere. Other abbreviations as in Figs. 1, 2.
Extended Data Fig. 8 Ecological reconstruction of Kylinxia zhangi in the early Cambrian Chengjiang biota.
Artistic reconstruction by J. Sun.
Extended Data Fig. 9 Consensus trees from Bayesian and parsimony analyses of panarthropod relationships based on a matrix of 81 taxa and 283 characters.
a, The 50% majority-rule consensus tree from a Bayesian analysis. Nodal supports are posterior probabilities. Major taxonomic groups are indicated by bars on the right of tips. b, Strict consensus of a single most parsimonious tree of 51.91166 steps (consistency index = 0.507; retention index = 0.871) from a parsimony analysis using implied character weighting (concavity constant k = 3). Nodal supports are group present and contradicted frequency differences. In a, b, nodal supports of 100% are not shown, and the ordering of tips is the same. The consensus results from Bayesian analysis and parsimony analyses with equal and various implied character weighting settings (concavity constant k = 2, 3, 5, 10) are consistent on the position of Kylinxia zhangi (in red) and the main topologies between major taxonomic groups (indicated by bars in a), except for the alternatively monophyletic or paraphyletic grouping of great-appendage bivalved forms and Isoxyida and the questionable relationships of mandibulate-related groups including Hymenocarina (indicated by asterisks in a, b).
Extended Data Fig. 10 Consensus trees from Bayesian and parsimony analyses of panarthropod relationships with Kylinxia zhangi omitted.
a, The 50% majority-rule consensus tree from a Bayesian analysis. Nodal supports are posterior probabilities. Major taxonomic groups are indicated by bars on the right of tips. b, Strict consensus of a single most parsimonious tree of 49.60546 steps (consistency index = 0.518, retention index = 0.874) from a parsimony analysis using implied character weighting (concavity constant k = 3). Nodal supports are group present and contradicted frequency differences. In a, b, nodal supports of 100% are not shown. Note that Isoxyida and great-appendage bivalved forms (both in red) are placed at a more basal position than Megacheira and Panchelicerata when K. zhangi is omitted, which is a major difference from the results with K. zhangi included (Extended Data Fig. 9).
Supplementary information
Supplementary Discussion
This file contains detailed morphological description, additional remarks and the character list used for phylogenetic analyses.
Supplementary Data 1
Morphological matrix, MrBayes code and consensus trees found in the phylogenetic analyses with Kylinxia zhangi included.
Supplementary Data 2
Morphological matrix, MrBayes code and consensus trees found in the phylogenetic analyses with Kylinxia zhangi omitted.
Rights and permissions
About this article
Cite this article
Zeng, H., Zhao, F., Niu, K. et al. An early Cambrian euarthropod with radiodont-like raptorial appendages. Nature 588, 101–105 (2020). https://doi.org/10.1038/s41586-020-2883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2883-7
This article is cited by
-
Phanerozoic oceanic and climatic perturbations in the context of Tethyan evolution
Science China Earth Sciences (2023)
-
The velvet worm brain unveils homologies and evolutionary novelties across panarthropods
BMC Biology (2022)
-
Ordovician opabiniid-like animals and the role of the proboscis in euarthropod head evolution
Nature Communications (2022)
-
Exites in Cambrian arthropods and homology of arthropod limb branches
Nature Communications (2021)
-
Current understanding on the Cambrian Explosion: questions and answers
PalZ (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.