Abstract
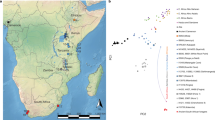
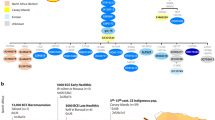
Our knowledge of ancient human population structure in sub-Saharan Africa, particularly prior to the advent of food production, remains limited. Here we report genome-wide DNA data from four children—two of whom were buried approximately 8,000 years ago and two 3,000 years ago—from Shum Laka (Cameroon), one of the earliest known archaeological sites within the probable homeland of the Bantu language group1,2,3,4,5,6,7,8,9,10,11. One individual carried the deeply divergent Y chromosome haplogroup A00, which today is found almost exclusively in the same region12,13. However, the genome-wide ancestry profiles of all four individuals are most similar to those of present-day hunter-gatherers from western Central Africa, which implies that populations in western Cameroon today—as well as speakers of Bantu languages from across the continent—are not descended substantially from the population represented by these four people. We infer an Africa-wide phylogeny that features widespread admixture and three prominent radiations, including one that gave rise to at least four major lineages deep in the history of modern humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The aligned sequences are available through the European Nucleotide Archive under accession number PRJEB32086. Genotype data used in analysis are available at https://reich.hms.harvard.edu/datasets. Any other relevant data are available from the corresponding author upon reasonable request.
References
de Maret, P. in Aspects of African Archaeology: Papers from the 10th Congress of the Pan-African Association of Prehistory and Related Studies (eds Pwiti, G. & Soper, R.) 274–279 (Univ. of Zimbabwe Publications, Harare, 1996).
Ribot, I., Orban, R. & de Maret, P. The Prehistoric Burials of Shum Laka Rockshelter (North-West Cameroon) (Annales du Musée Royal de l’Afrique Centrale vol. 164) (Musée Royal de l’Afrique Centrale, Tervuren, 2001).
Lavachery, P. The Holocene archaeological sequence of Shum Laka rock shelter (Grassfields, western Cameroon). Afr. Archaeol. Rev. 18, 213–247 (2001).
de Maret, P. in The Oxford Handbook of African Archaeology (eds Mitchell, P. & Lane, P.) 627–643 (Oxford Univ. Press, 2013).
Cornelissen, E. in The Oxford Handbook of African Archaeology (eds Mitchell, P. & Lane, P.) 403–417 (Oxford Univ. Press, 2013).
Vansina, J. New linguistic evidence and ‘the Bantu expansion’. J. Afr. Hist. 36, 173–195 (1995).
Tishkoff, S. A. et al. The genetic structure and history of Africans and African Americans. Science 324, 1035–1044 (2009).
Berniell-Lee, G. et al. Genetic and demographic implications of the Bantu expansion: insights from human paternal lineages. Mol. Biol. Evol. 26, 1581–1589 (2009).
Bostoen, K. et al. Middle to late Holocene Paleoclimatic change and the early Bantu expansion in the rain forests of Western Central Africa. Curr. Anthropol. 56, 354–384 (2015).
Patin, E. et al. Dispersals and genetic adaptation of Bantu-speaking populations in Africa and North America. Science 356, 543–546 (2017).
Bostoen, K. in Oxford Research Encyclopedia of African History (ed. Spear, T.) https://oxfordre.com/africanhistory/view/10.1093/acrefore/9780190277734.001.0001/acrefore-9780190277734-e-191 (Oxford Univ. Press, 2018).
Mendez, F. L. et al. An African American paternal lineage adds an extremely ancient root to the human Y chromosome phylogenetic tree. Am. J. Hum. Genet. 92, 454–459 (2013).
Krahn, T., Schrack, B., Fomine, F. L. M. & Krahn, A.-M. Searching for our most distant (paternal) cousins in Cameroon. Institute for Genetic Genealogy 2016 Conference, San Diego (2016).
Rohland, N., Harney, E., Mallick, S., Nordenfelt, S. & Reich, D. Partial uracil-DNA-glycosylase treatment for screening of ancient DNA. Phil. Trans. R. Soc. Lond. B 370, 20130624 (2015).
Gonder, M. K., Mortensen, H. M., Reed, F. A., de Sousa, A. & Tishkoff, S. A. Whole-mtDNA genome sequence analysis of ancient African lineages. Mol. Biol. Evol. 24, 757–768 (2007).
Batini, C. et al. Phylogeography of the human mitochondrial L1c haplogroup: genetic signatures of the prehistory of Central Africa. Mol. Phylogenet. Evol. 43, 635–644 (2007).
Wood, E. T. et al. Contrasting patterns of Y chromosome and mtDNA variation in Africa: evidence for sex-biased demographic processes. Eur. J. Hum. Genet. 13, 867–876 (2005).
Karmin, M. et al. A recent bottleneck of Y chromosome diversity coincides with a global change in culture. Genome Res. 25, 459–466 (2015).
Mendez, F. L., Poznik, G. D., Castellano, S. & Bustamante, C. D. The divergence of Neandertal and modern human Y chromosomes. Am. J. Hum. Genet. 98, 728–734 (2016).
Fan, S. et al. African evolutionary history inferred from whole genome sequence data of 44 indigenous African populations. Genome Biol. 20, 82 (2019).
Schlebusch, C. M. et al. Southern African ancient genomes estimate modern human divergence to 350,000 to 260,000 years ago. Science 358, 652–655 (2017).
Skoglund, P. et al. Reconstructing prehistoric African population structure. Cell 171, 59–71 (2017).
Gallego Llorente, M. et al. Ancient Ethiopian genome reveals extensive Eurasian admixture in Eastern Africa. Science 350, 820–822 (2015).
Gronau, I., Hubisz, M. J., Gulko, B., Danko, C. G. & Siepel, A. Bayesian inference of ancient human demography from individual genome sequences. Nat. Genet. 43, 1031–1034 (2011).
Mallick, S. et al. The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature 538, 201–206 (2016).
van de Loosdrecht, M. et al. Pleistocene North African genomes link Near Eastern and sub-Saharan African human populations. Science 360, 548–552 (2018).
Plagnol, V. & Wall, J. D. Possible ancestral structure in human populations. PLoS Genet. 2, e105 (2006).
Hammer, M. F., Woerner, A. E., Mendez, F. L., Watkins, J. C. & Wall, J. D. Genetic evidence for archaic admixture in Africa. Proc. Natl Acad. Sci. USA 108, 15123–15128 (2011).
Durvasula, A. & Sankararaman, S. Recovering signals of ghost archaic admixture in the genomes of present-day Africans. Preprint at bioRxiv https://doi.org/10.1101/285734 (2018).
Hey, J. et al. Phylogeny estimation by integration over isolation with migration models. Mol. Biol. Evol. 35, 2805–2818 (2018).
Ragsdale, A. P. & Gravel, S. Models of archaic admixture and recent history from two-locus statistics. PLoS Genet. 15, e1008204 (2019).
Huysecom, E. et al. The emergence of pottery in Africa during the 10th millennium calBC: new evidence from Ounjougou (Mali). Antiquity 83, 905–917 (2009).
Gasse, F. Hydrological changes in the African tropics since the Last Glacial Maximum. Quat. Sci. Rev. 19, 189–211 (2000).
Triska, P. et al. Extensive admixture and selective pressure across the Sahel belt. Genome Biol. Evol. 7, 3484–3495 (2015).
Laval, G., Patin, E., Barreiro, L. B. & Quintana-Murci, L. Formulating a historical and demographic model of recent human evolution based on resequencing data from noncoding regions. PLoS ONE 5, e10284 (2010).
Soares, P. et al. The expansion of mtDNA haplogroup L3 within and out of Africa. Mol. Biol. Evol. 29, 915–927 (2012).
Behar, D. M. et al. A “Copernican” reassessment of the human mitochondrial DNA tree from its root. Am. J. Hum. Genet. 90, 675–684 (2012).
Poznik, G. D. et al. Punctuated bursts in human male demography inferred from 1,244 worldwide Y-chromosome sequences. Nat. Genet. 48, 593–599 (2016).
Pickrell, J. et al. The genetic prehistory of southern Africa. Nat. Commun. 3, 1143 (2012).
Scerri, E. in Oxford Research Encyclopedia of African History (ed. Spear, T.) https://oxfordre.com/africanhistory/view/10.1093/acrefore/9780190277734.001.0001/acrefore-9780190277734-e-137 (Oxford University Press, 2017).
Hublin, J.-J. et al. New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature 546, 289–292 (2017).
Harvati, K. et al. The later Stone Age calvaria from Iwo Eleru, Nigeria: morphology and chronology. PLoS ONE 6, e24024 (2011).
Scerri, E. M., Blinkhorn, J., Niang, K., Bateman, M. D. & Groucutt, H. S. Persistence of Middle Stone Age technology to the Pleistocene/Holocene transition supports a complex hominin evolutionary scenario in West Africa. J Archaeol. Sci. Rep. 11, 639–646 (2017).
Scerri, E. M. L. et al. Did our species evolve in subdivided populations across Africa, and why does it matter? Trends Ecol. Evol. 33, 582–594 (2018).
Henn, B. M., Steele, T. E. & Weaver, T. D. Clarifying distinct models of modern human origins in Africa. Curr. Opin. Genet. Dev. 53, 148–156 (2018).
Dabney, J. et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl Acad. Sci. USA 110, 15758–15763 (2013).
Korlević, P. et al. Reducing microbial and human contamination in DNA extractions from ancient bones and teeth. Biotechniques 59, 87–93 (2015).
Briggs, A. W. et al. Removal of deaminated cytosines and detection of in vivo methylation in ancient DNA. Nucleic Acids Res. 38, e87 (2010).
Lipson, M. et al. Ancient genomes document multiple waves of migration in Southeast Asian prehistory. Science 361, 92–95 (2018).
Fu, Q. et al. DNA analysis of an early modern human from Tianyuan Cave, China. Proc. Natl Acad. Sci. USA 110, 2223–2227 (2013).
Haak, W. et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522, 207–211 (2015).
Fu, Q. et al. An early modern human from Romania with a recent Neanderthal ancestor. Nature 524, 216–219 (2015).
Mathieson, I. et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528, 499–503 (2015).
Lazaridis, I. et al. Genomic insights into the origin of farming in the ancient Near East. Nature 536, 419–424 (2016).
Kircher, M., Sawyer, S. & Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3 (2012).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26, 589–595 (2010).
Weissensteiner, H. et al. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 44, W58–W63 (2016).
Skoglund, P., Storå, J., Götherström, A. & Jakobsson, M. Accurate sex identification of ancient human remains using DNA shotgun sequencing. J. Archaeol. Sci. 40, 4477–4482 (2013).
Korneliussen, T. S., Albrechtsen, A. & Nielsen, R. ANGSD: analysis of next generation sequencing data. BMC Bioinformatics 15, 356 (2014).
Giresse, P., Maley, J. & Brenac, P. Late Quaternary palaeoenvironments in the Lake Barombi Mbo (West Cameroon) deduced from pollen and carbon isotopes of organic matter. Palaeogeogr. Palaeoclimatol. Palaeoecol. 107, 65–78 (1994).
Lohse, J. C., Culleton, B. J., Black, S. L. & Kennett, D. J. A precise chronology of middle to late Holocene bison exploitation in the far southern Great Plains. J. Texas Archeol. Hist. 1, 94–126 (2014).
van Klinken, G. J. Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 26, 687–695 (1999).
Lavachery, P. De la Pierre au Métal: Archéologie des Dépôts Holocènes de l’Abri de Shum Laka (Cameroun). PhD thesis, Université Libre de Bruxelles (1997).
Bronk Ramsey, C., Higham, T. F., Owen, D., Pike, A. & Hedges, R. E. Radiocarbon dates from the Oxford AMS system: archaeometry datelist 31. Archaeometry 44, 1–150 (2002).
Ward, G. K. & Wilson, S. R. Procedures for comparing and combining radiocarbon age determinations: a critique. Archaeometry 20, 19–31 (1978).
Ramsey, C. B. & Lee, S. Recent and planned developments of the program OxCal. Radiocarbon 55, 720–730 (2013).
Reimer, P. J. et al. IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal bp. Radiocarbon 55, 1869–1887 (2013).
Hogg, A. G. et al. SHCal13 Southern Hemisphere calibration, 0–50,000 years cal BP. Radiocarbon 55, 1889–1903 (2013).
Marsh, E. J. et al. IntCal, SHCal, or a mixed curve? Choosing a 14C calibration curve for archaeological and paleoenvironmental records from tropical South America. Radiocarbon 60, 925–940 (2018).
Jobling, M. A. & Tyler-Smith, C. Human Y-chromosome variation in the genome-sequencing era. Nat. Rev. Genet. 18, 485–497 (2017).
Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. PLoS Genet. 2, e190 (2006).
Liu, L. T., Dobriban, E. & Singer, A. ePCA: high dimensional exponential family PCA. Preprint at https://arxiv.org/abs/1611.05550 (2016).
Patterson, N. et al. Ancient admixture in human history. Genetics 192, 1065–1093 (2012).
Lipson, M. & Reich, D. A working model of the deep relationships of diverse modern human genetic lineages outside of Africa. Mol. Biol. Evol. 34, 889–902 (2017).
Lipson, M. et al. Parallel palaeogenomic transects reveal complex genetic history of early European farmers. Nature 551, 368–372 (2017).
Moeyersons, J., Cornelissen, E., Lavachery, P. & Doutrelepont, H. L’abri sous-roche de Shum Laka (Cameroun Occidental): données climatologiques et occupation humaine depuis 30.000 ans. Geo. Eco. Trop. 20, 39–60 (1996).
Cornelissen, E. in Field Manual for African Archaeology (eds Smith, A. L. et al.) 168–173 (Royal Museum for Central Africa, 2017).
Prüfer, K. et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505, 43–49 (2014).
Acknowledgements
We thank I. Lazaridis, V. Narasimhan and K. Sirak for discussions and comments; M. Karmin for help with Y chromosome data; L. Eccles for help with radiocarbon dating; B. Erkkila for help with isotopic analysis; R. Bernardos, M. Mah and Z. Zhang for other technical assistance; J.-P. Warnier for his role in locating the site of Shum Laka; and O. Graf for proofreading, photograph editing and other figure assistance for the Supplementary Information. The Shum Laka excavations were supported by the Belgian Fund for Scientific Research (FNRS), the Université Libre de Bruxelles, the Royal Museum for Central Africa and the Leakey Foundation. The collection of samples from present-day individuals in Cameroon was supported by N. Bradman and the Melford Charitable Trust. The genotyping of the present-day individuals sampled from Cameroon was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/L009382/1). I.R. was supported by a Université de Montréal exploration grant (2018-2020). M.G.T. was supported by Wellcome Trust Senior Investigator Award Grant 100719/Z/12/Z. G.H. was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant number 098386/Z/12/Z). C.L-F. was supported by Obra Social La Caixa 328, Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya (GRC 2017 SGR 880), and a FEDER-MINECO grant (PGC2018-095931-B-100). Radiocarbon work was supported by the NSF Archaeometry program (grant BCS-1460369) to D.J.K. and B.J.C. M.E.P. was supported by a fellowship from the Radcliffe Institute for Advanced Study at Harvard University during the development of this project. D.R. was supported by the National Institutes of Health (NIGMS GM100233), by an Allen Discovery Center grant and by grant 61220 from the John Templeton Foundation, and is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
N.R., G.H., M.E.P. and D.R. supervised the study. I.R., R.N.A., H.B., E.C., I.C., P.d.M., P.L., C.M.M., R.O., E.S., P.S., W.V.N., C.L.-F., S. MacEachern and M.E.P. provided samples and assembled archaeological and anthropological materials and information. S.L., N. Bradman, F.L.M.F., M.G.T., K.R.V. and G.H. provided data from present-day populations. S. Mallick, N.R., N.A., N. Broomandkhoshbacht, A.M.L., J.O., K.S. and D.R. performed ancient DNA laboratory and data-processing work. B.J.C. and D.J.K. performed radiocarbon analysis. M.L., S. Mallick, I.O., N.P. and D.R. analysed genetic data. M.L., I.R., H.B., E.S., C.L.-F., S. MacEachern, M.E.P. and D.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks George Busby, Katerina Harvati, Stephan Schiffels, Lluis Quintana-Murci and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Overview of the site of Shum Laka.
The left column represents generalized stratigraphy, with radiocarbon dates (uncalibrated years before present) shown as red dots on the y axis, and deposits indicated by their archaeological nomenclature. P, S/Si, Pleistocene; T, A, Holocene; Ao, Holocene ochre ashy layer; Ag, Holocene grey ashy layer (after ref. 76). Columns 1–6 display the chronological extents of technological traditions: 1, microlithic quartz industry; 2, macrolithic flake and blade industry on basalt; 3, bifaces of the axe–hoe type; 4, pecked grounded adze and arrow heads; 5, pottery; and 6, iron objects. Column 7 indicates the two burial phases. Column 8 shows climatic reconstructions based on carbon stable isotopes and pollen from organic matter extracted from sediment cores at Lake Barombi Mbo in western Cameroon (more arid conditions to the left and more humid conditions to the right60,76), along with archaeological eras. IA, Iron Age; LSA, Later Stone Age; SMA, Stone to Metal Age. RMCA Collection; drawings by Y. Paquay, composition © RMCA, Tervuren; modified by E. Cornelissen77.
Extended Data Fig. 2 Kinship analysis.
Mean genome-wide allelic mismatch rates for each pair of individuals (blue), as well as intra-individual comparisons (red), are shown. We selected one read per individual at random at each targeted SNP (using all 1,233,013 targeted sites). Monozygotic twins (or intra-individual comparisons) are expected to have a value one-half as large as unrelated individuals; first-degree relatives, halfway between monozygotic twins and unrelated individuals; second-degree relatives, halfway between first-degree relatives and unrelated individuals; and so on. The presence of inbreeding also serves to reduce the rate of mismatches. For 4/A and 5/B, we can eliminate a grandparent–grandchild relationship because both died as children, and the lack of long segments with IBD sharing on both homologous chromosomes implies that they are not double cousins (the few ostensible double-IBD stretches are probably a result of inbreeding (Supplementary Information section 2)). Thus, we can conclude that they were either uncle and niece (or aunt and nephew) or half-siblings. Bars show 99% confidence intervals (computed by block jackknife).
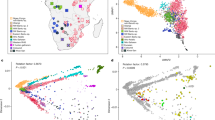
Extended Data Fig. 3 Alternative PCA and allele-sharing analyses.
a, Broad-scale PCA (differing from Fig. 2a by projecting all present-day Cameroon populations; again using 593,124 Human Origins SNPs). Groups shown in blue were projected onto axes computed using the other populations. HG, hunter-gatherers. The grouping marked W-Cent. HG consists of Aka and Cameroon hunter-gatherers (Baka, Bakola and Bedzan). The majority of the present-day Cameroon individuals fall in a tight cluster near other West Africans and Bantu-speakers. b, Relative allele sharing (mean ± s.e.m., multiplied by 10,000, computed on 538,133 SNPs, as in Fig. 3b) with the Shum Laka individuals versus East Africans (f4(X, Yoruba; Shum Laka, Somali); x axis) and versus Aka (f4(X, Yoruba; Shum Laka, Aka); y axis) for present-day populations from Cameroon (blue points) and southern and eastern Bantu-speakers (Herero in red and Chewa in orange). The Mada and Fulani share more alleles with the Shum Laka individuals than they do with the Aka, but this is probably a secondary consequence of admixture from East or North African sources (as reflected in greater allele sharing with Somali individuals) (Supplementary Information section 3). Bars show one s.e.m. in each direction.
Extended Data Fig. 4 Primary inferred admixture graph with full parameters.
Of the approximately 1,200,000 targeted SNPs, 932,000 are used for fitting (that is, are covered by all populations in the model). Branch lengths (in units of squared allele frequency divergence) are rounded to the nearest integer. All f-statistics relating the populations are predicted to within 2.3 standard errors of their observed values.
Extended Data Fig. 5 Schematic of first alternative admixture graph.
Results are shown including ancient individuals from Taforalt in Morocco associated with the Iberomaurusian culture, with the Shum Laka individuals modelled as having a mixture of ancestry related to western Central African hunter-gatherers plus two additional components: one from within the main portion of the West African clade, and one that splits at nearly the same point as one of the sources that contributes ancestry to the Taforalt individuals. Branch lengths are not drawn to scale. Points at which multiple lineages are shown diverging simultaneously indicate splits that occur in short succession (the order of which we cannot confidently assess) but are not meant to represent exact multifurcations. *Proportion not well-constrained (for Mbuti, the sum of the two indicated proportions is well-constrained but not the separate values). Supplementary Information section 3 provides the full parameters of the inferred model.
Extended Data Fig. 6 Deep ancestry correlation from the West African clade.
An allele-sharing statistic sensitive to ancestry that splits more deeply than southern African hunter-gatherers (f4(X, Mursi; chimpanzee, ancient South African hunter-gatherers), mean ± 2 s.e.m. from block jackknife, computed on 1,121,119 SNPs, as in Fig. 3a) is shown as a function of ancestry related to the West African clade (from admixture graph results; the Mota individual, Yoruba and Lemande are shifted slightly away from the boundaries for legibility). The (relative) allele-sharing rate for Mursi is zero according to the definition of the statistic.
Extended Data Fig. 7 Schematic of the second alternative admixture graph.
Results are shown with a single-component deep source for West Africans. Branch lengths are not drawn to scale. Points at which multiple lineages are shown diverging simultaneously indicate splits that occur in short succession (the order of which we cannot confidently assess) but are not meant to represent exact multifurcations. *Proportion is not well-constrained (for Mbuti, the sum of the two indicated proportions is well-constrained but not the separate values). Supplementary Information section 3 provides the full parameters of the inferred model.
Supplementary information
Supplementary Information
This file contains supplementary information sections 1-3.
Supplementary Tables
This file contains Supplementary Tables 1-5.
Rights and permissions
About this article
Cite this article
Lipson, M., Ribot, I., Mallick, S. et al. Ancient West African foragers in the context of African population history. Nature 577, 665–670 (2020). https://doi.org/10.1038/s41586-020-1929-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-1929-1
This article is cited by
-
The Allen Ancient DNA Resource (AADR) a curated compendium of ancient human genomes
Scientific Data (2024)
-
The genetic legacy of the expansion of Bantu-speaking peoples in Africa
Nature (2024)
-
Medieval DNA from Soqotra points to Eurasian origins of an isolated population at the crossroads of Africa and Arabia
Nature Ecology & Evolution (2024)
-
Whole genomes from Angola and Mozambique inform about the origins and dispersals of major African migrations
Nature Communications (2023)
-
Imputation of ancient human genomes
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.