Abstract
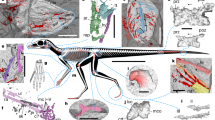
Powered flight evolved independently in vertebrates in the pterosaurs, birds and bats, each of which has a different configuration of the bony elements and epidermal structures that form the wings1,2. Whereas the early fossil records of pterosaurs and bats are sparse, mounting evidence (primarily from China) of feathered non-avian dinosaurs and stemward avians that derive primarily from the Middle–Upper Jurassic and Lower Cretaceous periods has enabled the slow piecing together of the origins of avian flight3,4. These fossils demonstrate that, close to the origin of flight, dinosaurs closely related to birds were experimenting with a diversity of wing structures3,5. One of the most surprising of these is that of the scansoriopterygid (Theropoda, Maniraptora) Yi qi, which has membranous wings—a flight apparatus that was previously unknown among theropods but that is used by both the pterosaur and bat lineages6. This observation was not universally accepted7. Here we describe a newly identified scansoriopterygid—which we name Ambopteryx longibrachium, gen. et sp. nov.—from the Upper Jurassic period. This specimen provides support for the widespread existence of membranous wings and the styliform element in the Scansoriopterygidae, as well as evidence for the diet of this enigmatic theropod clade. Our analyses show that marked changes in wing architecture evolved near the split between the Scansoriopterygidae and the avian lineage, as the two clades travelled along very different paths to becoming volant. The membranous wings supported by elongate forelimbs that are present in scansoriopterygids probably represent a short-lived experimentation with volant behaviour, and feathered wings were ultimately favoured during the later evolution of Paraves.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data—including the measurements, source data for morphometric analysis and phylogenetic data matrix—that support the findings of this research are included as Supplementary Information. The specimen (IVPP V24192) described in this study is archived and available on request from the IVPP. A Life Science Identifier for the newly described species has been registered at ZooBank (http://zoobank.org/): urn:lsid:zoobank.org:act:0A2DE2F0-CE78-4149-B0BD-A0DE91FC1328. Any other relevant data are available from the corresponding author upon reasonable request.
Change history
11 June 2019
Change history: In this Letter, it should have been acknowledged that the silhouettes of Scansoriopterygidae in Fig. 3a were modified from a sketch by Jaime Headden. The original Letter has been corrected online.
References
Gatesy, S. M. & Middleton, K. M. in Fins Into Limbs: Evolution, Development, and Transformation (ed. Hall, B. K.) 269–283 (Univ. Chicago Press, Chicago, 2007).
Norberg, U. Vertebrate Flight: Mechanics, Physiology, Morphology, Ecology and Evolution (Springer, Berlin, 1990).
Xu, X. et al. An integrative approach to understanding bird origins. Science 346, 1253293 (2014).
Gatesy, S. M. & Middleton, K. M. Bipedalism, flight, and the evolution of theropod locomotor diversity. J. Vertebr. Paleontol. 17, 308–329 (1997).
Chatterjee, S. & Templin, R. J. Biplane wing planform and flight performance of the feathered dinosaur Microraptor gui. Proc. Natl Acad. Sci. USA 104, 1576–1580 (2007).
Xu, X. et al. A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature 521, 70–73 (2015).
Padian, K. Dinosaur up in the air. Nature 521, 40–41 (2015).
Liu, Y., Liu, Y., Ji, S. A. & Yang, Z. U–Pb zircon age for the Daohugou Biota at Ningcheng of Inner Mongolia and comments on related issues. Chin. Sci. Bull. 51, 2634–2644 (2006).
Huang, D. Yanliao biota and Yanshan movement (in Chinese). Acta Palaeontologica Sin. 54, 501–546 (2015).
Campione, N. E., Evans, D. C., Brown, C. M. & Carrano, M. T. Body mass estimation in non-avian bipeds using a theoretical conversion to quadruped stylopodial proportions. Methods Ecol. Evol. 5, 913–923 (2014).
Persons, W. S., Currie, P. J. & Norell, M. A. Oviraptorosaur tail forms and functions. Acta Palaeontol. Pol. 59, 553–567 (2013).
O’Connor, J. K. & Sullivan, C. Reinterpretation of the Early Cretaceous maniraptoran (Dinosauria: Theropoda) Zhongornis haoae as a scansoriopterygid-like non-avian, and morphological resemblances between scansoriopterygids and basal oviraptorosaurs. Vertebr. Palasiat. 52, 3–30 (2014).
Gatesy, S. M. & Thomason, J. in Functional Morphology in Vertebrate Paleontology (ed. Thomason, J. J.) 219–234 (Cambridge Univ. Press, Cambridge, 1995).
Zhang, F., Zhou, Z., Xu, X., Wang, X. & Sullivan, C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 455, 1105–1108 (2008).
Zhang, F., Zhou, Z., Xu, X. & Wang, X. A juvenile coelurosaurian theropod from China indicates arboreal habits. Naturwissenschaften 89, 394–398 (2002).
Turner, A. H., Makovicky, P. J. & Norell, M. A. A review of dromaeosaurid systematics and paravian phylogeny. Bull. Am. Mus. Nat. Hist. 371, 1–206 (2012).
Xu, X. et al. A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chin. Sci. Bull. 54, 430–435 (2009).
Balanoff, A. M. & Norell, M. A. Osteology of Khaan mckennai (Oviraptorosauria: Theropoda). Bull. Am. Mus. Nat. Hist. 372, 1–77 (2012).
Hutchinson, J. R. The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes). Zool. J. Linn. Soc. 131, 123–168 (2001).
Zhang, F. et al. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463, 1075–1078 (2010).
Thorington, J. R. W., Darrow, K. & Anderson, C. G. Wing tip anatomy and aerodynamics in flying squirrels. J. Mamm. 79, 245–250 (1998).
Oshida, T., Hiraga, H., Nojima, T. & Yoshida, M. C. Anatomical and histological notes on the origin of the long accessory styliform cartilage of the Russian flying squirrel, Pteromys volans orii. Mammal Study 25, 41–48 (2000).
Dial, K. P. Wing-assisted incline running and the evolution of flight. Science 299, 402–404 (2003).
Lovette, I. J. & Fitzpatrick, J. W. Handbook of Bird Biology 3rd edn (John Wiley & Sons, Hoboken, 2016).
Sullivan, C. et al. The vertebrates of the Jurassic Daohugou biota of northeastern China. J. Vertebr. Paleontol. 34, 243–280 (2014).
Liu, Y., Liu, Y. & Zhang, H. LA-ICPMS zircon U-Pb dating in the Jurassic Daohugou beds and correlative strata in Ningcheng of Inner Mongolia. Acta Geol. Sin. 80, 733–742 (2006).
Liu, Y.-Q. et al. Timing of the earliest known feathered dinosaurs and transitional pterosaurs older than the Jehol biota. Palaeogeogr. Palaeoclimatol. Palaeoecol. 323–325, 1–12 (2012).
Chu, Z. et al. High-precision U-Pb geochronology of the Jurassic Yanliao biota from Jianchang (western Liaoning Province, China): age constraints on the rise of feathered dinosaurs and eutherian mammals. Geochem. Geophys. Geosyst. 17, 3983–3992 (2016).
Xu, X., Zhou, Z., Sullivan, C., Wang, Y. & Ren, D. An updated review of the Middle–Late Jurassic Yanliao Biota: chronology, taphonomy, paleontology and paleoecology. Acta Geol. Sin. 90, 2229–2243 (2016).
Benson, R. B. J. et al. Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 12, e1001853 (2014).
Christiansen, P. & Fariña, R. A. Mass prediction in theropod dinosaurs. Hist. Biol. 16, 85–92 (2004).
Serrano, F. J., Palmqvist, P. & Sanz, J. L. Multivariate analysis of neognath skeletal measurements: implications for body mass estimation in Mesozoic birds. Zool. J. Linn. Soc. 173, 929–955 (2015).
Campione, N. E. & Evans, D. C. A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biol. 10, 60 (2012).
Goloboff, P. A. & Catalano, S. A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238 (2016).
Brusatte, S. L. in Computational Paleontology (ed. Elewa, A. M. T.) 53–74 (Springer, Heidelberg, 2011).
Laurin, M. The evolution of body size, Cope’s rule and the origin of amniotes. Syst. Biol. 53, 594–622 (2004).
Wang, M. & Lloyd, G. T. Rates of morphological evolution are heterogeneous in Early Cretaceous birds. Proc. R. Soc. Lond. B 283, 20160214 (2016).
Bapst, D. W. paleotree: an R package for paleontological and phylogenetic analyses of evolution. Methods Ecol. Evol. 3, 803–807 (2012).
Revell, L. J. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268 (2009).
Benson, R. B. J. & Choiniere, J. N. Rates of dinosaur limb evolution provide evidence for exceptional radiation in Mesozoic birds. Proc. R. Soc. Lond. B 280, 20131780 (2013).
Hu, D. et al. A bony-crested Jurassic dinosaur with evidence of iridescent plumage highlights complexity in early paravian evolution. Nat. Commun. 9, 217 (2018).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 20, 289–290 (2004).
Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1985).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Acknowledgements
We thank S.-X. Jiang, D.-Y. Huang, Y.-H. Pan and Z.-Q. Yu for discussion, Q.-R. Meng for help in the field, T. Zhao for taking scanning electron microscopy photographs, D.-H. Li for specimen preparation and W. Gao for photographing. We thank J. Headden for providing the original sketch of the Scansoriopterygidae, which was modified in Fig. 3a. This research was supported by the National Natural Science Foundation of China (41688103; 41722202), Youth Innovation Promotion Association CAS (2016073) and the State Key Laboratory of Lithospheric Evolution (Z201604).
Reviewer information
Nature thanks Thomas Richard Holtz, Peter Makovicky and Kevin Padian for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Z.Z. and M.W. designed the research project; Z.Z. and M.W. conducted the fieldwork; M.W. performed the phylogenetic, histological and phylogenetic principal component analyses; and M.W., J.K.O., X.X. and Z.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Additional photographs of Ambopteryx, IVPP V24192.
a, Counter slab. b, Skeletal reconstruction based on preserved bones. c, Skull. d, Gastroliths and the unidentified bony stomach content. Abbreviations as in Fig. 1, except for: fe, feather associated with the neck; lil, left ilium;; lti, left tibia; pd, pedal digits; and ub, unidentified bony element. The white box indicates the position from which the sample was taken for histological analysis. Scale bars, 10 mm (a, c, d), 20 mm (b).
Extended Data Fig. 2 Bone histology of Ambopteryx.
a–d, Thin cross-section of the left humerus (a, b) and the unidentified bony stomach content (c, d). The arrowheads indicate the osteocyte lacunae. Scale bars, 100 μm (a–c), 200 μm (d).
Extended Data Fig. 3 Anatomy of Ambopteryx, IVPP V24192.
a–d, Interpretative drawings of the neck and pectoral girdle (a), caudal vertebrae and pygostyle (b), left forelimb (c) and pelvis and left hindlimb (d). Abbreviations as in Figs. 1, 2, except for: ca, caudal vertebrae; cm, calcaneum; de, deltopectoral crest of humerus; do, dorsal vertebrae; I–III, metacarpals I–III; ip, iliac peduncle of ilium; mtII–IV, metatarsals II–IV; p1–4, pedal digits I to IV; and st, styliform element. Scale bars, 10 mm (a–d).
Extended Data Fig. 4 Forelimb comparisons between Ambopteryx and Yi.
a, Left forelimb of Ambopteryx (IVPP V24192). b–d, Left forelimb (b), right humerus (c) and the right styliform element (d) of Yi (STM 31-2). The proximal margins of the humeri are marked in white dashed lines to show the differences between these two taxa. Abbreviations as in Figs. 1, 2, except for: st, styliform element. Scale bars, 10 mm (a), 20 mm (b–d).
Extended Data Fig. 5 Comparisons of hand morphology among Scansoriopterygidae.
a–c, Line drawings of the hands of Ambopteryx (a), Epidendrosaurus (b) and Yi (c). Scale bars, 10 mm (a, b), 20 mm (c).
Extended Data Fig. 6 Scanning electron microscopy photographs of the soft tissues that are preserved in Ambopteryx.
a–d, Feather samples associated with the neck. e–f, Samples of membranous tissues taken from the area between the left femur and left manual digits. Arrows denote the positions of the samples. Scale bars, 2 μm.
Extended Data Fig. 7 Time-scaled recovered strict-consensus tree of Mesozoic coelurosaurians.
Bremer and bootstrap values are labelled near the corresponding node in bold italic and upright non-bold font, respectively.
Extended Data Fig. 8 Compiled super-tree of the sampled Mesozoic coelurosaurians used in morphometric analyses.
Complete tree for the coelurosaurians used in generating the PPCA morphospaces shown in Fig. 3b, c.
Supplementary information
Supplementary Information
This file contains Supplementary Text Sections 1–5; which include additional anatomical description, stomach contents and diet of Ambopteryx longibrachium, Supplementary Tables 1, 2, 4 and 5, and the data used in the phylogenetic analysis.
Supplementary Table 3
This file contains appendicular limb bone measurements of Mesozoic coelurosaurians used in the phylogenetic principal components analysis.
Rights and permissions
About this article
Cite this article
Wang, M., O’Connor, J.K., Xu, X. et al. A new Jurassic scansoriopterygid and the loss of membranous wings in theropod dinosaurs. Nature 569, 256–259 (2019). https://doi.org/10.1038/s41586-019-1137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1137-z
This article is cited by
-
Escape behaviors in prey and the evolution of pennaceous plumage in dinosaurs
Scientific Reports (2024)
-
Low morphological disparity and decelerated rate of limb size evolution close to the origin of birds
Nature Ecology & Evolution (2023)
-
A new avialan theropod from an emerging Jurassic terrestrial fauna
Nature (2023)
-
A new confuciusornithid bird with a secondary epiphyseal ossification reveals phylogenetic changes in confuciusornithid flight mode
Communications Biology (2022)
-
Exceptional preservation and foot structure reveal ecological transitions and lifestyles of early theropod flyers
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.