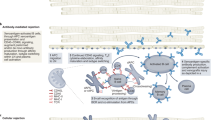
Abstract
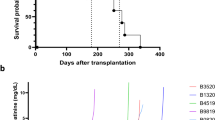
Heart transplantation is the only cure for patients with terminal cardiac failure, but the supply of allogeneic donor organs falls far short of the clinical need1,2,3. Xenotransplantation of genetically modified pig hearts has been discussed as a potential alternative4. Genetically multi-modified pig hearts that lack galactose-α1,3-galactose epitopes (α1,3-galactosyltransferase knockout) and express a human membrane cofactor protein (CD46) and human thrombomodulin have survived for up to 945 days after heterotopic abdominal transplantation in baboons5. This model demonstrated long-term acceptance of discordant xenografts with safe immunosuppression but did not predict their life-supporting function. Despite 25 years of extensive research, the maximum survival of a baboon after heart replacement with a porcine xenograft was only 57 days and this was achieved, to our knowledge, only once6. Here we show that α1,3-galactosyltransferase-knockout pig hearts that express human CD46 and thrombomodulin require non-ischaemic preservation with continuous perfusion and control of post-transplantation growth to ensure long-term orthotopic function of the xenograft in baboons, the most stringent preclinical xenotransplantation model. Consistent life-supporting function of xenografted hearts for up to 195 days is a milestone on the way to clinical cardiac xenotransplantation7.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
03 April 2019
In this Letter, Mayuko Kurome and Valeri Zakhartchenko have been added to the author list (affiliated with Institute of Molecular Animal Breeding and Biotechnology, Gene Center, LMU Munich, Munich, Germany). The author list and ‘Author contributions’ section have been corrected online; see accompanying Amendment.
References
Lund, L. H. et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult heart transplantation report—2017; focus theme: allograft ischemic time. J. Heart Lung Transplant. 36, 1037–1046 (2017).
Rossano, J. W. et al. The Registry of the International Society for Heart and Lung Transplantation: twentieth pediatric heart transplantation report—2017; focus theme: allograft ischemic time. J. Heart Lung Transplant. 36, 1060–1069 (2017).
Eurotransplant. Annual Report 2016. 103 (Eurotransplant International Foundation, 2017).
Mohiuddin, M. M., Reichart, B., Byrne, G. W. & McGregor, C. G. A. Current status of pig heart xenotransplantation. Int. J. Surg. 23, 234–239 (2015).
Mohiuddin, M. M. et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat. Commun. 7, 11138 (2016).
Byrne, G. W., Du, Z., Sun, Z., Asmann, Y. W. & McGregor, C. G. Changes in cardiac gene expression after pig-to-primate orthotopic xenotransplantation. Xenotransplantation 18, 14–27 (2011).
Cooper, D. K. et al. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J. Heart Lung Transplant. 19, 1125–1165 (2000).
Lower, R. R. & Shumway, N. E. Studies on orthotopic homotransplantation of the canine heart. Surg. Forum 11, 18–19 (1960).
Lowe, M. et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am. J. Transplant. 12, 2079–2087 (2012).
Binder, U. & Skerra, A. PASylation®: A versatile technology to extend drug delivery. Curr. Opin. Colloid Interface Sci. 31, 10–17 (2017).
Mayr, T. et al. Hemodynamic and perioperative management in two different preclinical pig-to-baboon cardiac xenotransplantation models. Xenotransplantation 24, e12295 (2017).
Byrne, G. W. & McGregor, C. G. Cardiac xenotransplantation: progress and challenges. Curr. Opin. Organ Transplant. 17, 148–154 (2012).
Steen, S., Paskevicius, A., Liao, Q. & Sjöberg, T. Safe orthotopic transplantation of hearts harvested 24 hours after brain death and preserved for 24 hours. Scand. Cardiovasc. J. 50, 193–200 (2016).
Devereux, R. B. et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am. J. Cardiol. 57, 450–458 (1986).
Lang, R. M. et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18, 1440–1463 (2005).
Kuwaki, K. et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat. Med. 11, 29–31 (2005).
Azimzadeh, A. M. et al. Development of a consensus protocol to quantify primate anti-non-Gal xenoreactive antibodies using pig aortic endothelial cells. Xenotransplantation 21, 555–566 (2014).
Tanabe, T. et al. Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am. J. Transplant. 17, 1778–1790 (2017).
Lesnik, J. J., Singh, G. K., Balfour, I. C. & Wall, D. A. Steroid-induced hypertrophic cardiomyopathy following stem cell transplantation in a neonate: a case report. Bone Marrow Transplant. 27, 1105–1108 (2001).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Imamura, T. et al. Everolimus attenuates myocardial hypertrophy and improves diastolic function in heart transplant recipients. Int. Heart J. 57, 204–210 (2016).
Paoletti, E. mTOR inhibition and cardiovascular diseases: cardiac hypertrophy. Transplantation 102, S41–S43 (2018).
Manning, B. D. Game of TOR — the target of rapamycin rules four kingdoms. N. Engl. J. Med. 377, 1297–1299 (2017).
Wuensch, A. et al. Regulatory sequences of the porcine THBD gene facilitate endothelial-specific expression of bioactive human thrombomodulin in single- and multitransgenic pigs. Transplantation 97, 138–147 (2014).
Aslan, J. E., Tormoen, G. W., Loren, C. P., Pang, J. & McCarty, O. J. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood 118, 3129–3136 (2011).
Acknowledgements
We thank the Walter Brendel Centre of Experimental Medicine, Munich for support and provision of facilities, especially U. Pohl, M. Shakarami and all animal caretakers. Financial support was provided by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) TRR 127. We acknowledge K. Reimann for providing the CD40 monoclonal antibody for the experiments.
Reviewer information
Nature thanks C. Knosalla and J. Madsen for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
B.R., P.B., T.M., M.L. and J.-M.A. conceived and led the study; M.L., T.M., B.R., R.E., R. Herzog, S.G., S.B., S.M., A.D., A. Bauer, F.W., M. Mihalj, A. Panelli, L.I., J.Y., A.K.F., L.Q., C.H., I.L., I.B., M. Mokelke, J.R., P.B. and J.-M.A. performed the experiments and collected samples; S.S., T.S., A. Paskevicius and L.Q. performed non-ischaemic heart preservation experiments; M.L., A. Panelli, A. Bauer and J.-M.A. performed haemodynamic and echocardiographic analyses; R.S. and R.R. provided immunological analyses; E.K., K.K., R. Hinkel and C.K. performed histochemical analyses; M.D. and E.W. provided protein analysis; A.F. and S.R. performed necropsy and histological analyses with contributions from T.M. and A. Panelli; M.L., T.M., B.R., R.S., R.R., R. Hinkel, M.D. and J.-M.A. analysed the data; A. Baehr, S.E., B.K., M.K., V.Z., D.A., E.W. and N.K. provided genetically multi-modified donor pigs; U.S. and F.-J.K. provided non-human primates; U.B., G.W. and A.S. developed PASylated anti-CD40L Fab; B.R., M.L., T.M. and J.-M.A. wrote the manuscript; A.K., A.S., R.R., S.S., R. Hinkel, P.B. and E.W. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.A. is chief executive officer and chief scientific officer of Revivicor. Inc. A.S. and U.B. are cofounders of XL-protein GmbH, Germany. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Haemodynamic data, measured by transpulmonary thermodilution and post-operative catecholamine support.
Measurements were taken after induction of anaesthesia (before) and 60 min after termination of CPB (after). Donor hearts of group I (black) received crystalloid cardioplegia, donor hearts of groups II (red) and III (magenta) were preserved with continuous cold hyperoncotic perfusion; data are presented as scatter plots with mean ± s.d. with individuals shown as dots; n = 14 animals, two-sided paired and unpaired t-tests, P values as indicated. a, Stroke volume index. b, Cardiac index before and after CPB. Both parameters decreased in group I and were lower in group I after CPB than in group II and III. c, Dosages of catecholamines 60 min after termination of CPB. d, Durations of post-operative vasopressive and inotropic support. Animals in group I required more noradrenaline and adrenaline than those in group II and III. Animals in group I required inotropic support with adrenaline for a longer time.
Extended Data Fig. 2 Graphics of left ventricular sizes during diastole and systole that were derived from transthoracic echocardiography.
Left, diastole; right, systole. a, Animal 9 (group II, survival 40 days): left ventricular mass had increased by 303% on day 38, left ventricular function was severely impaired because of myocardial hypertrophy and decreased left ventricular filling volume. Left ventricular fractional shortening measurements were 32% and 14% on day 1 and 38. b, Animal 11 (group III, survival 90 days): in contrast to animal 9, left ventricular mass had increased by only 22% on day 82, left ventricular function was preserved. Left ventricular fractional shortening measurements were 27% and 34% on day 1 and 82. c, Pig 5157 (control, donor sibling of the pig whose heart was transplanted in animal 9): left ventricular mass had increased by 187% on day 33, left ventricular function was preserved. Left ventricular fractional shortening measurements were 32% and 41% on day 1 and 33. Compared to 9 (a), the left ventricle had grown less in size and showed no hypertrophy.
Extended Data Fig. 3 Additional laboratory parameters.
a, b, Serum concentrations of lactate dehydrogenase (a) and platelet counts (b) in animals of groups I (black), II (red) and III (magenta). At the end of experiments in groups I and II, platelet counts decreased whereas LDH increased. Group III animals did not show these alterations.
Extended Data Fig. 4 Immunofluorescence staining of myocardial tissue.
a–d, Immunofluorescence staining of myocardial sections from group I (3; left row), group II (9; middle row) and group III (11, right row) for IgM (a), IgG (b), C3b/c (c; red), C4b/c (c; green) and fibrin (d); nuclei were stained with DAPI (blue). Scale bars, 25 μm. n = 1, group I; n = 3, group II; n = 5, group III; one representative biological sample per group is shown.
Extended Data Fig. 5 Immunohistochemistry of post-mortem myocardial specimens.
a, b, Expression of human membrane cofactor protein (hCD46) (a) and human thrombomodulin (hTM) (b) was consistent in all donor organs (1–14). Scale bars, 50 μm. n = 14 GTKO/hCD46/hTM pigs; n = 1 wild-type pig (control). Biological samples from all animals are shown.
Supplementary information
Supplementary Figure 1
This file contains gel source data.
Video 1
Transthoracic echocardiographic midpapillary short axis view of porcine graft after cardiac xenotransplantation. Experiment 9 (group II, day 30): increased LV wall thickness and reduced LV filling volume indicating myocardial hypertrophy. LV function was impaired.
Video 2
Transthoracic echocardiographic midpapillary short axis view of porcine graft after cardiac xenotransplantation. Experiment 11 (group III, day 57): normal LV wall thickness and normal LV filling volume. LV function was preserved.
Video 3
Transthoracic echocardiographic midpapillary short axis view of porcine graft after cardiac xenotransplantation. Experiment 14 (group III, day 180): increased LV wall thickness, but normal LV filling volume. LV function was preserved.
Rights and permissions
About this article
Cite this article
Längin, M., Mayr, T., Reichart, B. et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 564, 430–433 (2018). https://doi.org/10.1038/s41586-018-0765-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0765-z
Keywords
This article is cited by
-
Mapping and functional characterization of structural variation in 1060 pig genomes
Genome Biology (2024)
-
Kidney xenotransplantation edges closer to the clinic
Nature Reviews Nephrology (2024)
-
Preparation and characterization of monoclonal antibodies against porcine gasdermin D protein
Applied Microbiology and Biotechnology (2024)
-
Current status and challenges of pig-to-human organ xenotransplantation
Science China Life Sciences (2024)
-
Kardiale Xenotransplantation in Deutschland
Zeitschrift für Herz-,Thorax- und Gefäßchirurgie (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.