Abstract
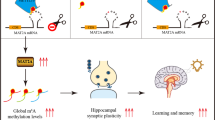
N6-methyladenosine (m6A), the most prevalent internal RNA modification on mammalian messenger RNAs, regulates the fates and functions of modified transcripts through m6A-specific binding proteins1,2,3,4,5. In the nervous system, m6A is abundant and modulates various neural functions6,7,8,9,10,11. Whereas m6A marks groups of mRNAs for coordinated degradation in various physiological processes12,13,14,15, the relevance of m6A for mRNA translation in vivo remains largely unknown. Here we show that, through its binding protein YTHDF1, m6A promotes protein translation of target transcripts in response to neuronal stimuli in the adult mouse hippocampus, thereby facilitating learning and memory. Mice with genetic deletion of Ythdf1 show learning and memory defects as well as impaired hippocampal synaptic transmission and long-term potentiation. Re-expression of YTHDF1 in the hippocampus of adult Ythdf1-knockout mice rescues the behavioural and synaptic defects, whereas hippocampus-specific acute knockdown of Ythdf1 or Mettl3, which encodes the catalytic component of the m6A methyltransferase complex, recapitulates the hippocampal deficiency. Transcriptome-wide mapping of YTHDF1-binding sites and m6A sites on hippocampal mRNAs identified key neuronal genes. Nascent protein labelling and tether reporter assays in hippocampal neurons showed that YTHDF1 enhances protein synthesis in a neuronal-stimulus-dependent manner. In summary, YTHDF1 facilitates translation of m6A-methylated neuronal mRNAs in response to neuronal stimulation, and this process contributes to learning and memory.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
High-throughput sequencing data can be accessed in the Gene Expression Omnibus under accession number GSE106607. Source data for bar graphs and box-plots in Figures and Extended Data Figures are provided in separate excel files.
References
Roundtree, I. A., Evans, M. E., Pan, T. & He, C. Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200 (2017).
Wang, X. et al. N 6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 (2014).
Wang, X. et al. N 6-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 (2015).
Xiao, W. et al. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol. Cell 61, 507–519 (2016).
Roundtree, I. A. et al. YTHDC1 mediates nuclear export of N 6-methyladenosine methylated mRNAs. eLife 6, e31311 (2017).
Hess, M. E. et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 16, 1042–1048 (2013).
Lence, T. et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature 540, 242–247 (2016).
Li, L. et al. Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Hum. Mol. Genet. 26, 2398–2411 (2017).
Weng, Y.-L. et al. Epitranscriptomic m6A regulation of axon regeneration in the adult mammalian nervous system. Neuron 97, 313–325.e6 (2018).
Widagdo, J. et al. Experience-dependent accumulation of N 6-methyladenosine in the prefrontal cortex is associated with memory processes in mice. J. Neurosci. 36, 6771–6777 (2016).
Walters, B. J. et al. The role of the RNA demethylase FTO (Fat Mass and Obesity-Associated) and mRNA methylation in hippocampal memory formation. Neuropsychopharmacology 42, 1502–1510 (2017).
Zhao, B. S. et al. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 542, 475–478 (2017).
Ivanova, I. et al. The RNA m6A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol. Cell 67, 1059–1067.e4 (2017).
Li, H.-B. et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548, 338–342 (2017).
Yoon, K. J. et al. Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell 171, 877–889.e17 (2017).
Jia, G. et al. N 6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887 (2011).
Zheng, G. et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 (2013).
Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012).
Meyer, K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012).
Slobodin, B. et al. Transcription impacts the efficiency of mRNA translation via co-transcriptional N 6-adenosine methylation. Cell 169, 326–337.e12 (2017).
Sutton, M. A. & Schuman, E. M. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127, 49–58 (2006).
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Shen, B. et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat. Methods 11, 399–402 (2014).
Morris, R. G. M., Garrud, P., Rawlins, J. N. P. & O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683 (1982).
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Liu, J., Xu, Y., Stoleru, D. & Salic, A. Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc. Natl Acad. Sci. USA 109, 413–418 (2012).
Ma, D. K. et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077 (2009).
Guo, J. U., Su, Y., Zhong, C., Ming, G. L. & Song, H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434 (2011).
Franklin, K. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates 2nd edn (Academic, San Diego, 2004).
Li, K. et al. βCaMKII in lateral habenula mediates core symptoms of depression. Science 341, 1016–1020 (2013).
Huang, W. et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat. Neurosci. 16, 441–448 (2013).
Chiu, S. L. et al. GRASP1 regulates synaptic plasticity and learning through endosomal recycling of AMPA receptors. Neuron 93, 1405–1419.e8 (2017).
Shioda, N. et al. Aberrant calcium/calmodulin-dependent protein kinase II (CaMKII) activity is associated with abnormal dendritic spine morphology in the ATRX mutant mouse brain. J. Neurosci. 31, 346–358 (2011).
Su, Y. et al. Neuronal activity modifies the chromatin accessibility landscape in the adult brain. Nat. Neurosci. 20, 476–483 (2017).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protocols 9, 171–181 (2014).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Trapnell, C. et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17, 10–12 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Corcoran, D. L. et al. PARalyzer: definition of RNA binding sites from PAR-CLIP short-read sequence data. Genome Biol. 12, R79 (2011).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Huang, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protocols 4, 44–57 (2009).
Huang, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Acknowledgements
This study was supported by the National Key R&D Program of China (2016YFA0500903 to X.H.), the National Institute of Health (HG008935 and GM113194 to C.H.; DA043361 to X. Zhuang), the National Natural Science Foundation of China (31500866 to T.Z. and 31471077 to X.C.), the Simons Foundation Autism Research Initiative (SFARI) to H. Song, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF) to G.-l.M. C.H. is an investigator of the Howard Hughes Medical Institute. T.Z. is sponsored by the Shanghai Rising-Star Program. X.H. is sponsored by the Startup Foundation of ShanghaiTech University. X. Zhang is sponsored by Zhejiang Public Welfare Technology Application Research Project (2018C37118). We thank P. Cao, and X. Wang for discussions; Z. Qiu and T. Cheng for help with primary neuron culture; M. Wu for suggestions on proteomics data analyses; and J. Tauler, P. J. Hsu, and A. C. Zhu for editing help.
Author information
Authors and Affiliations
Contributions
C.H. and T.Z. conceived the study. H. Shi, X. Zhang., Y-L.W., C.H., H. Song, and T.Z. designed experiments. X. Zhang and T.Z. performed animal and cell biology experiments. H. Shi, X. Zhang, and T.Z. performed biochemistry experiments. H. Shi, Zhike Lu, and Y-L.W. analysed sequencing data. Zongyang Lu helped with primary neuron culture and virus packaging. J.L. helped with plasmid construction. P.H. performed mass spectrometry experiments and data analysis. X.C. helped with behavioural tests and electrophysiological recordings. Y-L.W., F.Z., Y.S., G.-l.M., and H. Song performed in vivo ECT stimulation. X.H. and B.S. helped with generation of knockout mice. Y.L. and Y.Z. helped with cell culture and RNAi testing. Y.W. helped with CLIP-seq experiments. J.Y.D., M.J.P., and X. Zhuang helped with mouse tissue dissection. H. Shi, X. Zhang, C.H., and T.Z. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
C.H. is a scientific founder of Accent Therapeutics, Inc. and a shareholder of Epican Genetech.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Generation and evaluation of Ythdf1-KO mice.
a, Schematic diagram of the targeting strategy for generating Ythdf1-KO mice using CRISPR–Cas9. Two sgRNAs (red) were designed to target the fourth exon (E4) of Ythdf1. PAM sequence, underlined, green; F1 and R1, genotyping primers. b, Genotyping PCR products of the seven founders co-injected with 20 ng Cas9 mRNA and the two sgRNAs (5 ng each). c, Genotypes of sequenced mice. PCR products were cloned and sequenced. Founder #4 with a 179-bp deletion was crossed with C57BL/6 wild-type mice for further analysis. d, Representative genotyping PCR products of offspring mice with different genotypes. e, Validation of Ythdf1 knockout by western blot using mouse hippocampal tissues. For gel source data, see Supplementary Fig. 1. f, Representative images of YTHDF1 immunostaining in the mouse basal lateral amygdala (BLA) and the cortex.
Extended Data Fig. 2 Ythdf1-KO mice are normal in hippocampal neurogenesis, cortical morphology, motor activities, anxiety-like behaviour, and depressive-like behaviour.
a, b, Representative images of Doublecortin (DCX, a marker of neurogenesis) immunostaining (a) and quantification of the number of DCX+ cells (b) in the dentate gyrus (DG) region of Ythdf1-KO and wild-type control mice at different postnatal development stages. Scale bar, 100 µm. c, Representative images of cortical morphology staining using Hoechst in adult control and Ythdf1-KO mice. Scale bar, 200 µm. d, Representative confocal immunostaining of CTIP2 (a marker for deep layer cortical neurons) and SATB2 (a marker for upper layer cortical neurons) in the cortex of adult control and Ythdf1-KO mice. Scale bar, 200 µm. e–h, Motor activities measured by various parameters as listed in the open-field test. i–l, Anxiety-like behaviour measured by the light–dark box transition test (i, j) and the elevated-plus maze test (k, l). m, Depressive-like behaviour measured by tail suspension test. P values, two tailed t-test. Numbers in bars, numbers of mice. Data shown as mean ± s.e.m.
Extended Data Fig. 3 MWM tests and fear conditioning tests in Ythdf1-KO mice.
a, Schematics of procedure of MWM training and MWM probe tests. b, c, Number of crossings over previous platform location (b) and swimming velocity (c) of control and Ythdf1-KO mice in MWM probe tests. d, Schematics of the fear conditioning procedures (left) and freezing responses measured at different stages (right). e, Titration curves of the freezing level of wild-type mice 24 h after training with different foot shock intensities. The conditioning protocols used in later experiments (moderate protocols) are indicated by arrows. f, Learning curves for auditory fear conditioning under moderate (left) or strong (right) training protocols. The training sessions were separated into two parts: baseline (base) and tone periods (tone). g, h, Auditory fear memory of control and Ythdf1-KO mice assessed 24 h (g) and 2 h (h) after the indicated training sessions. P values, two-way repeated measures ANOVA (d) and two tailed t-test (b, c, f–h). Numbers in bars, numbers of mice. Data shown as mean ± s.e.m.
Extended Data Fig. 4 PPRs, spine morphology, and total protein levels of various LTP-related genes in Ythdf1-KO mouse hippocampus.
Related to Fig. 2. a, b, PPR with different inter-stimulus intervals in CA1 neurons from wild-type control and Ythdf1-KO mice. c, d, Representative images of Lucifer yellow staining (c) and statistical analyses of spine density (d, left) and spine size (d, right) in CA1 neurons from adult control and Ythdf1-KO brains. e, Uncropped western blot images for Fig. 2g. f, Total protein levels of a set of LTP-related genes in control and Ythdf1-KO mouse hippocampus. For gel source data, see Supplementary Fig. 1. P values, two-way repeated measures ANOVA with post hoc two-tailed t-test (a) and two tailed t-test (b, d, f). Numbers in bars, numbers of slices (b), neurons/mice (d, left), spines (d, right), or mice (f). Data shown as mean ± s.e.m.
Extended Data Fig. 5 Viral targeting in Ythdf1-KO mouse hippocampus and behavioural analyses of Ythdf1-KO mice injected with AAV virus. Related to Fig. 3.
a, Representative fluorescence images of brain slices from rostral to caudal positions dissected from a mouse injected with AAV-YTHDF1 virus. Hoechst, blue; YTHDF1 co-expressed with mCherry, red. b, Representative images of virus expression (mCherry, red) and YTHDF1 immunostaining (green) in the mouse hippocampus after AAV-control or AAV-YTHDF1 infection. Hoechst, blue. c, YTHDF1 protein overexpression level indicated by immunofluorescent signal intensity in the CA1 and DG regions. d, Number of crossings over the previous platform location for Ythdf1-KO mice injected with AAV-YTHDF1 or AAV-control in MWM probe tests. e, Anxiety-like behaviour of the injected mice measured as open-arm durations in elevated-plus maze. f–h, Motor activities of the injected mice measured as total distance (f), number of moves (g), and average velocity (h) in the open-field test. P values, two tailed t-test (c-h). Numbers in bars, numbers of mice. Data shown as mean ± s.e.m.
Extended Data Fig. 6 Impaired spatial learning and memory after selective knockdown of YTHDF1 in the hippocampus of wild-type mice.
a, Schematics of the AAV construct expressing YTHDF1 shRNA. b, Western blot and quantification of protein expression level of YTH proteins in N2A cells after YTHDF1-shRNA (RNAi) or control vector (Ctrl) transfection. For gel source data, see Supplementary Fig. 1. c, Spatial learning curves in the hidden-platform MWM training sessions for RNAi (red) and control (grey) mice. d–f, Spatial memory performances measured by quadrant time (%) (d) and number of platform crossings (e), and motor activities (f) of RNAi (red) and control (grey) mice in MWM probe tests. g, i, Contextual (g) and auditory (i) fear memories assessed 24 h after fear conditioning in RNAi and control mice. h, Anxiety level of mice assessed by open-arm durations in elevated-plus maze. P values, two-way repeated measures ANOVA with post hoc two-tailed t-test (c), two-way ANOVA with two-tailed t-test (comparison between group or to ‘Target’) (d), and two tailed t-test (b, e–i). Numbers in bars, numbers of biologically independent samples (b) and mice (d–i). Data shown as mean ± s.e.m.
Extended Data Fig. 7 Impaired spatial learning and memory after acute knockdown of METTL3 in the hippocampus of wild-type mice.
a, Representative western blot (left) and quantification (right) of METTL3 protein level in N2A cells transfected with METTL3-shRNA (RNAi) or control vector (Ctrl). For gel source data, see Supplementary Fig. 1. b–d, Spatial learning curves in the hidden-platform MWM training sessions (b), and spatial memory performance measured by quadrant time (per cent) (c) and the number of platform crossings (d) in MWM probe tests, for METTL3-RNAi and control mice. e, Contextual (left) and auditory (right) fear memories measured by freezing levels 24 h after fear conditioning in METTL3-RNAi and control mice. f, Motor activities of mice accessed in the open-field test. P values, two-way repeated measures ANOVA with post hoc two-tailed t-test (b), two-way ANOVA with two-tailed t-test (comparison between groups or to ‘Target’) (c), and two tailed t-test (a, d–f), Numbers in bars, numbers of biologically independent samples (a) and mice (c–f). Data shown as mean ± s.e.m.
Extended Data Fig. 8 YTHDF1 binding sites and m6A sites in the hippocampus of adult mice, and YTHDF1-mediated effects of m6A on hippocampal transcriptome and proteome.
a, Peak overlap among three biological replicates of YTHDF1-CLIP-seq. b, Validation of immunoprecipitation efficiency for YTHDF1-CLIP-seq. The position of the gel slice cut during the step of protein–RNA complex size selection is indicated in red (see Methods). c, Consensus motif and its P value generated by HOMER40 of the three sets of hippocampal m6A sites from biological replicates of m6A-CLIP-seq. d, e, Distribution of m6A-CLIP peaks along the different regions of transcripts (d) and genome (e). f, Functional annotation of m6A-modified transcripts in the adult mouse hippocampus (number of mutations in m6A-CLIP-seq ≥ 5, n = 2,922). g, Peak overlap between high-confidence YTHDF1-CLIP peaks and high-confidence m6A-CLIP peaks. The percentage of YTHDF1-CLIP peaks overlapped is indicated. h, Integrative Genomics Viewer (IGV) screenshots of the piled mutated reads for the each of the biological triplicates of YTHDF1-CLIP-seq (red) and m6A-CLIP-seq (blue). Three examples of synaptic plasticity transcripts were presented; the overlapped peak regions are highlighted in orange. i, j, Box-plots of mRNA abundance (i) and protein abundance (j) log2 fold changes (Δ) between Ythdf1-KO hippocampus and wild-type control for all expressed genes (black), non-YTHDF1-CLIP transcripts (grey), YTHDF1-CLIP targets (red), transcripts with overlapped YTHDF1-CLIP peaks and m6A-CLIP peaks (pink), and m6A-modified transcripts (blue). Box-plot elements: centre line, median; box limits, upper and lower quartiles, whiskers, 1–99%; P values, two-sided unpaired Kolmogorov–Smirnov test; number of genes and 95% confidence interval of mean are indicated for each box (i, j).
Extended Data Fig. 9 Effects of YTHDF1 on nascent protein synthesis in cultured hippocampal neurons in response to KCl stimulus.
a, Additional representative images of nascent protein (Nascent-P) synthesis in cultured wild-type control and Ythdf1-KO hippocampal neurons before (sham) and 2 h after KCl depolarization, related to Fig. 4e, f. b, c, Representative images (b) and quantification (c) of Nascent-P in wild-type control and Ythdf1-KO hippocampal neurons before (sham) and 4 h after KCl depolarization. d, e, Representative images (d) and quantification (e) of Nascent-P in AAV-control and AAV-YTHDF1-RNAi treated hippocampal neurons before (sham), 2 h, and 4 h after KCl depolarization. Intensities of Nascent-P were normalized to that of wild-type control (c) or AAV-control (e) neurons under the sham condition. P values, two-tailed t-test (c, e). Numbers in bars, numbers of images/biologically independent samples. Data shown as mean ± s.e.m.
Extended Data Fig. 10 Neuronal-stimulus-dependent functions of YTHDF1 in the mouse hippocampus and potential underlying mechanisms.
a, Representative western blot (left) and quantification (right) of the protein levels of BSN (top) and CAMK2A (bottom), in the whole hippocampus and the PSD fraction, respectively, before (Mock) and 2 h after fear conditioning (FC). The protein quantification was normalized to the Mock condition for each genotype separately. For gel source data, see Supplementary Fig. 1. b, c, Representative western blot (b) and quantification of YTHDF1 protein level in the hippocampal postsynaptic density (PSD) fraction (c, left) and the whole hippocampus (c, right) before (Mock) and 2 h after fear conditioning. For gel source data, see Supplementary Fig. 1. d, Schemes of the experimental design to quantify the change in the extent of m6A methylation for each transcript in the dentate gyrus region before (Mock) and 1 h after ECT. e, A proposed mechanism for how YTHDF1 contributes to memory formation: YTHDF1 promotes translation of m6A-modified target transcripts, including transcripts related to synaptic transmission and LTP, in response to learning stimulus, thus facilitating synapse strength adequately for a memory to occur. P values, two-tailed t-test (a, c). Numbers in bars, numbers of biologically independent samples. Data shown as mean ± s.e.m.
Supplementary information
Supplementary Information
The Supplementary Information file contains Supplementary Discussion and a section titled “Statistics and Reproducibility” providing the numbers of experiments that were repeated independently with similar results shown in the representative images/traces in main and extended data figures.
Supplementary Figure 1
This figure contains uncropped scans of western blots displayed in main and extended data figures in the paper.
Supplementary Table 1
Summary of the sequencing samples in this study. This table contains information on the mice (tissue, strain, genotype, age, and gender) used in each sequencing experiments, as well as sequencing depth and library preparation methods.
Supplementary Table 2
Sequencing data summary of YTHDF1-CLIP-seq. Sheet 1 | List of high-confidence YTHDF1-CLIP peaks in the adult mouse hippocampus, including Gene ID, gene symbol, gene description, chromosome number, genomic coordinates of the peak (start and end, mm10), strand information, number of mutations detected under the peak, location of the peak on the transcript, and RefSeq number. Sheet 2 | List of YTHDF1-CLIP targets in the adult mouse hippocampus and their numbers of CLIP peaks and mutations identified in YTHDF1-CLIP-seq. Sheet 3 | Functional annotation summary of YTHDF1-CLIP targets (n = 1,032) in the adult mouse hippocampus (FDR < 0.05) generated by DAVID45,46. FDR values were calculated by modified Fisher’s exact test followed by adjustments for multiple comparisons.
Supplementary Table 3
Sequencing data summary for m6A-CLIP-seq. Sheet 1 | List of high-confidence m6A-CLIP sites on the adult mouse hippocampal poly(A)+ RNA, including Gene ID, gene symbol, gene description, chromosome number, genomic coordinates of the peak (start and end, mm10), strand information, number of mutations detected under the peak, and location of the peak on the transcript. Sheet 2 | List of m6A-modified transcripts in the adult mouse hippocampus and their numbers of peaks and mutations identified in m6A-CLIP-seq. Sheet 3 | Functional annotation of the m6A-modified transcripts in the adult mouse hippocampus (number of mutations >= 5) (FDR < 0.05) generated by DAVID45,46. FDR values were calculated by modified Fisher’s exact test followed by adjustments for multiple comparisons. Sheet 4 | Summary of percentages of peak overlap between YTHDF1-CLIP-seq and m6A-CLIP-seq. Sheet 5 | List of high-confidence YTHDF1-CLIP peaks that overlap with high-confidence m6A-CLIP peaks (>1 nt in peak location), including Gene ID, gene symbol, gene description, chromosome number, genomic coordinates of the peak (start and end, mm10), strand information, number of mutations detected under the peak, location of the peak on the transcript, and RefSeq number.
Supplementary Table 4
RNA-seq of adult WT control and Ythdf1-KO mouse hippocampus. This table contains rpkm value of WT and Ythdf1-KO hippocampal transcriptome (rpkm of WT samples >1).
Supplementary Table 5
Proteome of adult WT control and Ythdf1-KO mouse hippocampus. This table contains proteome data summary from WT (n = 3) and Ythdf1-KO (n = 2) hippocampus (Q-value < 1%). Protein name, number of unique peptides, and normalized intensities for each sample are highlighted. The P value adjusted using an optimized FDR approach, namely, the Q-value, was calculated by target-decoy approach with MaxQuant.
Supplementary Table 6
m6A-RIP-seq of poly(A)+ RNA from the dentate gyrus (DG) of adult WT mice before (Mock) and after electroconvulsive treatment (ECT). This table contains rpkm value of transcriptome and m6A-modified transcriptome of mouse DG before and after ECT respectively (rpkm > 1).
Source data
Rights and permissions
About this article
Cite this article
Shi, H., Zhang, X., Weng, YL. et al. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 563, 249–253 (2018). https://doi.org/10.1038/s41586-018-0666-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0666-1
Keywords
This article is cited by
-
Multigenerational paternal obesity enhances the susceptibility to male subfertility in offspring via Wt1 N6-methyladenosine modification
Nature Communications (2024)
-
Usf2 Deficiency Promotes Autophagy to Alleviate Cerebral Ischemia-Reperfusion Injury Through Suppressing YTHDF1-m6A-Mediated Cdc25A Translation
Molecular Neurobiology (2024)
-
Utilities of Isolated Nerve Terminals in Ex Vivo Analyses of Protein Translation in (Patho)physiological Brain States: Focus on Alzheimer’s Disease
Molecular Neurobiology (2024)
-
The mechanism underlying redundant functions of the YTHDF proteins
Genome Biology (2023)
-
The function and clinical implication of YTHDF1 in the human system development and cancer
Biomarker Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.