Abstract
Patients with prostate cancer frequently show resistance to androgen-deprivation therapy, a condition known as castration-resistant prostate cancer (CRPC). Acquiring a better understanding of the mechanisms that control the development of CRPC remains an unmet clinical need. The well-established dependency of cancer cells on the tumour microenvironment indicates that the microenvironment might control the emergence of CRPC. Here we identify IL-23 produced by myeloid-derived suppressor cells (MDSCs) as a driver of CRPC in mice and patients with CRPC. Mechanistically, IL-23 secreted by MDSCs can activate the androgen receptor pathway in prostate tumour cells, promoting cell survival and proliferation in androgen-deprived conditions. Intra-tumour MDSC infiltration and IL-23 concentration are increased in blood and tumour samples from patients with CRPC. Antibody-mediated inactivation of IL-23 restored sensitivity to androgen-deprivation therapy in mice. Taken together, these results reveal that MDSCs promote CRPC by acting in a non-cell autonomous manner. Treatments that block IL-23 can oppose MDSC-mediated resistance to castration in prostate cancer and synergize with standard therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
Watson, P. A., Arora, V. K. & Sawyers, C. L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 15, 701–711 (2015).
Bianchini, D. et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur. J. Cancer 50, 78–84 (2014).
Zhang, T. et al. Exploring the clinical benefit of docetaxel or enzalutamide after disease progression during abiraterone acetate and prednisone treatment in men with metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 13, 392–399 (2015).
Badrising, S. et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer 120, 968–975 (2014).
Noonan, K. L. et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann. Oncol. 24, 1802–1807 (2013).
Schrader, A. J. et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur. Urol. 65, 30–36 (2014).
Sartor, O. & de Bono, J. S. Metastatic prostate cancer. N. Engl. J. Med. 378, 645–657 (2018).
Karantanos, T., Corn, P. G. & Thompson, T. C. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 32, 5501–5511 (2013).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305 (2010).
Lee, G. T. et al. Bone morphogenetic protein-6 induces castration resistance in prostate cancer cells through tumor infiltrating macrophages. Cancer Sci. 104, 1027–1032 (2013).
Toso, A. et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9, 75–89 (2014).
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Lu, X. et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732 (2017).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016).
Hossain, D. M. et al. TLR9-targeted STAT3 silencing abrogates immunosuppressive activity of myeloid-derived suppressor cells from prostate cancer patients. Clin. Cancer Res. 21, 3771–3782 (2015).
Mehra, N. et al. Myeloid-derived suppressor cells (MDSCs) in metastatic castration-resistant prostate cancer (CRPC) patients (PTS). Ann. Oncol. 27, 757P (2016).
Murdoch, C., Muthana, M., Coffelt, S. B. & Lewis, C. E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 8, 618–631 (2008).
Lunardi, A. et al. A co-clinical approach identifies mechanisms and potential therapies for androgen deprivation resistance in prostate cancer. Nat. Genet. 45, 747–755 (2013).
Bezzi, M. et al. Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nat. Med. 24, 165–175 (2018).
Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017).
Durant, L. et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 32, 605–615 (2010).
Kastelein, R. A., Hunter, C. A. & Cua, D. J. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25, 221–242 (2007).
Zhou, L. et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
Chen, T., Wang, L. H. & Farrar, W. L. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 60, 2132–2135 (2000).
Wang, J. J. et al. ROR-γ drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat. Med. 22, 488–496 (2016).
Campa, M., Mansouri, B., Warren, R. B. & Menter, A. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis. Dermatol. Ther. (Heidelb.) 6, 1–12 (2016).
Gordon, K. B. et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N. Engl. J. Med. 373, 136–144 (2015).
Moreira, R. B. et al. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget 8, 84572–84578 (2017).
Gangemi, S. et al. Clinical significance of circulating interleukin-23 as a prognostic factor in breast cancer patients. J. Cell. Biochem. 113, 2122–2125 (2012).
Grivennikov, S. I. et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258 (2012).
Teng, M. W. L. et al. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc. Natl Acad. Sci. USA 107, 8328–8333 (2010).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Graff, J. N. et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 7, 52810–52817 (2016).
Marigo, I. et al. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity 32, 790–802 (2010).
Lechner, M. G., Liebertz, D. J. & Epstein, A. L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 185, 2273–2284 (2010).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Drost, J. et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11, 347–358 (2016).
Acknowledgements
We acknowledge all members of the A.A. laboratory and M. Bellone for scientific discussions. R. D’Antuono helped with immunofluorescence acquisition. We thank F. Sallusto and R. Marzi for providing Il23aKO mice and all the members of the IRB animal core facility and E. Mira Catò for technical assistance and the animal work; T. Canu and A. Napolitano (Preclinical Imaging Facility of the San Raffaele Scientific Institute) for helping with MRI acquisition and analysis; and M. Montopoli (University of Padova) for helping with sample logistics. This work was supported by ERC consolidator (683136) and Swiss Cancer League (KFS4267-08-2017) grant, Dr. Josef Steiner Foundation, Swiss Card-Onco-Grant of Alfred and Annemarie von Sick grant, and Helmut Horten Foundation, SNSF (310030_176045), PCUK (RIA15-ST2-018) and IBSA Foundation. Work in the de Bono laboratory was supported by the Movember Foundation, Prostate Cancer UK, the US Department of Defense, the Prostate Cancer Foundation, Stand Up To Cancer, Cancer Research UK, and the UK Department of Health through an Experimental Cancer Medicine Centre grant. A.S. is supported by the Medical Research Council, the Academy of Medical Sciences and Prostate Cancer UK.
Reviewer information
Nature thanks N. Brennen, D. Gabrilovich, M. Galsky and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.A. and A.C. conceived and designed the project. A.C., C.S., E.Z. and M.M. performed experiments. V.G. performed experiments with human organoids. D.D.M., M.C. and A.N. established and carried out fluorescence microscopy. A.R. checked the quality of the RNA and performed NanoString. D.D. performed statistical analyses of multiplex immunofluorescence in human tissue sections. W.Y. performed bioinformatic analyses. G.D.B. performed surgical castration or sham operation of all animals. M.L. performed immunohistochemical experiments and analysis. A.S., M.B.L., S.S., A.F., A.B., R.L., C.A.B. and T.P.-G. selected and provided human samples. D.N.-R. reviewed tissue segmentation, cell segmentation and phenotype determination maps of tissue sections. E.P. took care of genotyping the animals. S.B. provided the AZD5069 compound. A.E. performed MRI analyses and analysed the results. J.d.B. supervised human experiments and interpreted the data. A.C. and A.A. interpreted the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
S.B. is affiliated to IMED Oncology AstraZeneca, Li Ka Shing Centre, Cambridge, UK and provided the AZD5069 compound. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
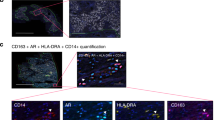
Extended Data Fig. 1 Multispectral images of PMN-MDSCs in human biopsies and set-up of the different CRPC mouse models.
a, Multispectral microscopy images (EpCAM, yellow; CD15, green; CD33, red; CD11b, pink) of castration-sensitive and castration-resistant prostate cancers. n = 3 biological independent patients. Scale bars, 20 μm. b, Quantification of the number of CD11b+CD15− cells in the tumour of castration-sensitive and castration-resistant prostate cancers (CSPC, n = 51; CRPC, n = 51 biological independent patients). Cells were counterstained with the nuclear marker DAPI (blue). Statistical analyses (negative binomial regression model): P = 0.062. c, MRIs of one representative sham-operated (Sham) or surgically castrated (CTX) PtenPC−/− mouse of the three analysed at different time points. d, Waterfall plot depicting proportional change in tumour response for sham (n = 3) and CTX (n = 3) PtenPC−/− mice. e, Prostate PMN-MDSC frequencies determined by flow cytometry in sham (n = 3) and CTX (n = 3) Ptenpc+/+ mice (12 weeks after castration). Statistical analyses (two-sided unpaired Student’s t-test): P = 0.85. f, Schematic representation of the experiment. Six-week-old C57BL/6 males were challenged subcutaneously with TRAMP-C1 cells. When tumours reached approximately 100 mm3, mice were sham-operated (sham, n = 9) or surgically castrated (CTX, n = 5). g, Tumour PMN-MDSC frequencies were determined by flow cytometry during castration-sensitive and castration-resistant phases. Sham CSPC, n = 5; CTX CSPC, n = 4; sham CRPC, n = 8; CTX CRPC n = 6. h, Schematic representation of the experiment. Six-week-old FVB males were challenged subcutaneously with MyC-CaP cells. When tumours reached approximately 100 mm3, mice were sham-operated (sham, n = 3) or surgically castrated (CTX, n = 3). i, Tumour PMN-MDSC frequencies were determined by flow cytometry during castration-sensitive and castration-resistant phases. Sham CSPC, n = 3; CTX CSPC, n = 4; sham CRPC, n = 4; CTX CRPC, n = 3. b, d, e, g, i, Data are mean ± s.e.m. d, g, i, Statistical analyses (unpaired two-sided Student’s t-test): ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. f, h, Statistical analyses (two-sided unpaired Student’s t-test followed by Wilcoxon signed-rank test): *P < 0.05.
Extended Data Fig. 2 Multiparametric flow cytometry analysis of myeloid populations in the tumours of different CRPC mouse models.
a, Gating strategy relative to the quantification of PMN-MDSCs in the tumour of sham- and CTX-operated mice for PtenPC−/−, TRAMP-C1 and MyC-CaP models. b, Representative dot plot reporting the Ly6G+ and Ly6C+ cells in the tumour of sham- and CTX-operated mice for PtenPC−/−, TRAMP-C1 and MyC-CaP models. Data were validated in at least three biological independent mice. c, Representative dot plot and quantification of CD11b+F4/80+ cells and CD11c+CD206− (M1-like) or CD11c−CD206+ (M2-like) macrophages in the tumour of sham- and CTX-operated mice for PtenPC−/− (sham, n = 5; CTX, n = 9), TRAMP-C1 (sham, n = 7; CTX, n = 6) and MyC-CaP (sham, n = 4; CTX, n = 4) models. Data are mean ± s.e.m. Statistical analyses (unpaired two-sided Student’s t-test): **P < 0.01.
Extended Data Fig. 3 Factors secreted by MDSCs promote insensitivity to ADT in androgen-dependent mouse and human prostate cancer cell lines and the CXCR2 antagonist impairs tumour recruitment of MDSCs in PtenPC−/− mice.
a, Representative dot plot reporting the BM-MDSCs after in vitro differentiation. Data were validated in two biological independent experiments. b, Experimental scheme. c, Cell proliferation of MyC-CaP cells (none, n = 5; conditioned medium (C.M.) from BM-MDSCs, n = 3; FAD, n = 13; FAD and BM-MDSCs, n = 13 biological independent samples). d, Percentage of annexin V− and 7AAD− MyC-CaP cells. e, Percentage of annexin V+ and 7AAD+ MyC-CaP cells. f, qRT–PCR analyses of the indicated genes in MyC-CaP cells. g, h, i, Cell proliferation of VCaP (none, n = 4; conditioned medium from human BM-MDSCs, n = 4; FAD, n = 7; FAD and human BM-MDSCs, n = 8 biological independent samples), 22Rv1 (none, n = 4; conditioned medium from human BM-MDSCs, n = 8; FAD, n = 8; FAD and human BM-MDSCs, n = 4 biological independent samples) and PC3 (none, n = 4; conditioned medium from human BM-MDSCs, n = 8; FAD, n = 4; FAD and human BM-MDSCs, n = 8 biological independent samples) prostate cancer cells. j, Tumour MDSC frequencies determined by flow cytometry of prostate tumours of CTX PtenPC−/− mice treated or not with CXCR2 antagonist (αCXCR2) at completion of the study (12 weeks after CTX). CTX castration-sensitive, n = 3; CTX and CXCR2 antagonist castration-sensitive, n = 3; CTX castration-resistant, n = 4; CTX and CXCR2 antagonist castration-resistant, n = 7 biological independent mice. k, Cell proliferation of TRAMP-C1 cells after 72 h of treatment with CXCR2 antagonist. l, qRT–PCR analyses of the indicated genes in TRAMP-C1 cells after 24 h of treatment (fold change compared to the FAD condition). k, l, Aggregated data from three independent experiments are reported, fold change compared to the FAD condition. c–l, Data are mean ± s.e.m. c–f, n = 3 biological independent samples. d, e, g–j, Statistical analyses (unpaired two-sided Student’s t-test): ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. c, f, Statistical analyses (two-sided unpaired Student’s t-test followed by Wilcoxon signed-rank test): *P < 0.05.
Extended Data Fig. 4 Impaired tumour recruitment of MDSCs enhances response to surgical castration in different allograft models of prostate cancers.
a, Schematic representation of the experiment. Six-week-old C57BL/6 males were challenged subcutaneously with TRAMP-C1 cells. When tumours reached approximately 100 mm3, mice were surgically castrated and left untreated (CTX, n = 8) or treated with CXCR2 antagonist (CTX and CXCR2 antagonist, n = 9). Representative flow cytometry plots of PMN-MDSCs (CD11b+Ly6G+ cells, gated on CD45+ cells) in tumours for each experimental condition. b, qRT–PCR analyses of the indicated genes in the prostate tumours after CTX or CTX and CXCR2 antagonist treatment (n = 3 per group). Data are mean ± sem. Statistical analyses (unpaired two-sided Student’s t-test): *P < 0.05; ***P < 0.001. c, Mean tumour volume (±s.e.m.) for each experimental group. Statistical analyses (unpaired two-sided Student’s t-test followed by Wilcoxon signed-rank test): ***P < 0.001. d, Survival curves are reported in Kaplan–Meier plot. Statistical analyses (two-sided log-rank test): ***P < 0.001. e, Schematic representation of the experiment. Six-week-old FVB males were challenged subcutaneously with MyC-CaP cells. When tumours reached approximately 100 mm3, mice were surgically castrated and left untreated (CTX, n = 5) or treated with CXCR2 antagonist (CTX and CXCR2 antagonist, n = 5). Representative flow cytometry plots of PMN-MDSCs (CD11b+Ly6G+ cells, gated on CD45+ cells) in tumours for each experimental condition. f, qRT–PCR analyses of the indicated genes in the prostate tumours after CTX or CTX and CXCR2 antagonist treatment (n = 3 per group). Data are mean ± s.e.m. Statistical analyses (unpaired two-sided Student’s t-test): **P < 0.01; ***P < 0.001. g, Average tumour volume (±s.e.m.) for each experimental group. Statistical analyses (two-sided unpaired Student’s t-test followed by Wilcoxon signed-rank test): *P < 0.05. h, Survival curves reported as Kaplan–Meier plot. Statistical analyses (two-sided log-rank test): **P < 0.01. i, Schematic representation of the experiment. Six-week-old NOD/SCID males were challenged subcutaneously with LNCaP cells or with LNCaP cells and human BM-MDSCs. When tumours reached approximately 70 mm3, mice were sham-operated (sham, n = 5) or sham-operated and injected every three days intraperitoneally with 3 × 106 human BM-MDSCs (sham and human BM-MDSCs, n = 5) or surgically castrated and left untreated (CTX, n = 8) or treated with human BM-MDSCs (CTX and human BM-MDSCs, n = 5). j, Average tumour volume (±s.e.m.) for each experimental group. Statistical analyses (unpaired two-sided Student’s t-test followed by Wilcoxon signed-rank test): **P < 0.01.
Extended Data Fig. 5 IL-23 pathway is the most upregulated in the tumour after castration.
a, Gene expression of selected genes determined by NanoString nCounter gene expression assay in sham PtenPC−/− and CTX PtenPC−/− tumours. Data are shown as pool of n = 5. b, Analyses of the conditioned medium of bone marrow-derived MDSCs tested for the indicated soluble molecules by Mouse CytokineMAP B version 1.0. The graph shows the concentration of the indicated soluble molecules as log10 of the concentration found in the conditioned medium of BM-MDSCs, the values were subtracted of the background (culture medium). Data are shown as pool of n = 10. c, qRT–PCR analyses of the indicated genes in sham (n = 6) and CTX (n = 6) PtenPC−/− tumours. Data are mean ± s.e.m. of biological independent animals. Statistical analyses (unpaired two-sided Student’s t-test): *P < 0.05. d, Protein level of CXCL1, CXCL2 and CXCL5 in CTX PtenPC−/− tumours. Data are analysed as ratio between CTX (pool of three samples) and sham (pool of three samples) PtenPC−/− tumours and reported as fold increase in protein level. e, f, IL-23R protein level analysed by flow cytometry and western blot on TRAMP-C1 cells under normal culture conditions (FBS) or androgen-deprivation culture conditions (charcoal-stripped FBS). n = 4 biological independent samples per group. f, Numbers indicate fold change in protein level. Loading control: anti-β-actin antibody. The western blot was validated at least twice. g, Protein profile of the plasma of patients with CSPC and CRPC. Data are analysed as ratio between CRPC (pool of 18 samples) and CSPC (pool of 17 samples) and reported as fold increase in protein level.
Extended Data Fig. 6 Characterization of IL-23+ cells in the tumour of CTX PtenPC−/− mice and patients with CRPC.
a, Multispectral microscopy images (EpCAM, yellow; CD15, green; IL-23, red) of three patients with CRPC. b, Correlation analyses of the numbers of CD15−CD11b+ cells in the tumour and IL-23 levels in the plasma of patients of CRPC (n = 28). Statistical analyses (negative binomial regression model): P = 0.63. c, d, IL23A and IL23R mRNA expression in the tumour of CSPCs (n = 549) and mCRPCs (n = 116). e, f, Expression of IL-23 in PMN-MDSC marker-positive (CD11b+CD33+CD15+) tumours from patients with CSPC or mCRPC. c–f, Statistical analyses (unpaired two-sided Student’s t-test) are reported. g, Representative plots of IL-23+, CD45+ and CD45–, Ly6GbrightCD11b+ and Ly6GintCD11bint, CD11b+F4/80+ cells pregated on the reported population in the tumour of CTX PtenPC−/− mice. IL-23 gate was decided based on isotype control panel (insert). Pie chart shows the percentage of the different subsets gated on IL-23+ cells in the tumour of PtenPC−/− mice (mean, n = 9). h, qRT–PCR analyses of IL-23 in the prostate tumours of castrated (CTX; n = 6) or castrated and treated with CXCR2 antagonist (CTX + CXCR2 antagonist; n = 7) PtenPC−/− mice. Data are mean ± s.e.m. i, PMN-MDSC and TAM frequencies determined by flow cytometry in the tumour of castrated NSG TRAMP-C1 allografts upon treatment with isotype, CSF1R antibody, CXCR2 antagonist. Data are mean ± s.e.m. (n = 3 per group). j, qRT–PCR analyses of IL-23 in the tumour of castrated NSG TRAMP-C1 allografts upon treatment with isotype (n = 4), CSF1R antibody (n = 5), CXCR2 antagonist (n = 5). Data are mean ± s.e.m. Each dot represents a biological independent animal. h–j, Statistical analyses (unpaired two-sided Student’s t-test): *P < 0.05; **P < 0.01; ***P < 0.01.
Extended Data Fig. 7 Il23aWT and Il23aKO BM-MDSCs have equal secretome and equal immunosuppressive capability.
a, Correlation analyses of the gene expression of selected genes determined by NanoString nCounter gene expression assay in Il23aWT and Il23aKO BM-MDSCs shown as scatter plot analyses. Data are shown as pool of n = 10. R2 values were determined using linear regression analyses. b, Representative flow cytometry plots of CFSE dilution (gated on CD8+ cells) and quantification of proliferating CD8+ T cells exposed to conditioned medium from Il23aWT and Il23aKO BM-MDSCs. Data are mean ± s.e.m. of three independent biological samples. Statistical analyses (unpaired two-sided Student’s t-test): **P < 0.01; ***P < 0.01.
Extended Data Fig. 8 Genetic inhibition of IL-23 limits resistance to castration in prostate cancer in PtenPC−/− mice.
a, Experimental set-up. Sham-operated (sham) or castrated (CTX) PtenPC−/− mice were lethally irradiated and transplanted with bone marrow precursors depleted of T, B and NK cells from Il23aWT and Il23aKO mice. The mice were then monitored by MRI for tumour progression. b, Representative dot plot of bone marrow precursors pre- and post-depletion of T, B, and NK cells. Data were validated in two biological independent experiments. c, Quantification of the tumour size of sham-operated PtenPC−/−Il23aWT (n = 4) and PtenPC−/−Il23aKO (n = 4), and castrated PtenPC−/−Il23aWT (n = 4) and PtenPC−/−Il23aKO (n = 7) mice at the completion of the study is reported as fold increase of the prostate anterior lobe (AL) volume (fold change compared to the CTX PtenPC−/−Il23aWT group). Data are mean ± s.e.m. Statistical analyses (unpaired two-sided Student’s t-test): **P < 0.01, ***P < 0.001. d, Haematoxylin and eosin, Ki-67 and pSTAT3(Y705) immunohistochemical staining (Ki-67 and pSTAT3(Y705), brown; nuclei, blue) of one representative PtenPC−/−Il23aWT and PtenPC−/−Il23aKO mouse of at least three mice analysed at completion of the study. Scale bars, 50 μm (left) and 25 μm (right).
Extended Data Fig. 9 Genetic inhibition of IL-23 limits resistance to castration in prostate cancer in TRAMP-C1 allograft model in vivo.
a, Schematic representation of the experiment. Six-week-old C57BL/6 males were lethally irradiated and transplanted with bone marrow precursors from Il23aWT and Il23aKO mice. After the bone marrow engraftment, the animals were challenged subcutaneously with TRAMP-C1 cells. When tumours reached approximately 100 mm3, mice were surgically castrated and monitored for tumour progression. b, Haematoxylin and eosin, Ki-67 and pSTAT3(Y705) immunohistochemical staining (Ki-67 and pSTAT3(Y705), brown; nuclei, blue) of representative TRAMP-C1 Il23aWT and TRAMP-C1 Il23aKO mice. Scale bars, 25 μm. c, Quantification of Ki-67+ cells is reported as a percentage of the total number of cells. TRAMP-C1 Il23aWT (n = 8) and TRAMP-C1 Il23aKO (n = 4), one tumour per mouse, mean of three sections per tumour, ≥3 fields per section. Data are mean ± s.e.m. of biologically independent mice. Statistical analyses (unpaired two-sided Student’s t-test): **P < 0.01. d, e, PMN-MDSC frequencies determined by flow cytometry in the tumour and in the spleen of TRAMP-C1 Il23aWT (n = 3) and TRAMP-C1 Il23aKO (n = 3) mice 10 days after castration. Data are mean ± s.e.m. f, Quantification of pSTAT3(Y705) reported as a percentage of the total number of cells. TRAMP-C1 Il23aWT (n = 8) and TRAMP-C1 Il23aKO (n = 4), one tumour per mouse, mean of three sections per tumour, ≥3 fields per section. Statistical analyses (unpaired two-sided Student’s t-test): ***P < 0.001. g, Western blot for RORγ, pSTAT3(Y705) and total STAT3 levels in prostate tumours of TRAMP-C1 Il23aWT and TRAMP-C1 Il23aKO mice. Loading control: HSP90 antibody or total ERK antibody. The western blot was validated at least twice. h, Quantification is reported as mean ± s.e.m. of biological independent experiments: TRAMP-C1 Il23aWT RORγ, n = 9; and TRAMP-C1 Il23aKO RORγ, n = 9; TRAMP-C1 Il23aWT pSTAT3(Y705), n = 4; and TRAMP-C1 Il23aKO pSTAT3(Y705), n = 3. Statistical analyses (unpaired two-sided Student’s t-test): *P < 0.05.
Extended Data Fig. 10 Pharmacological inhibition of IL-23 in association with ADT delays disease progression in prostate cancer.
a, Haematoxylin and eosin and Ki-67 immunohistochemical staining (Ki-67, brown; nuclei, blue) of representative castrated PtenPC−/− mice treated with IL-23, ENZA or both. Scale bars, 50 μm. b, Quantification of Ki-67 reported as a percentage of total within the glands. One tumour per mouse, three sections per tumour, ≥3 fields per section. Data are mean ± s.e.m. of biologically independent animals. Untreated, n = 6; IL-23, n = 6; ENZA, n = 5 or both, n = 6. Statistical analyses (unpaired two-sided Student’s t-test): **P < 0.01; ***P < 0.001. c, After castration, PMN-MDSCs progressively infiltrate the tumour bed mainly recruited by CXCL5. Within the tumour, PMN-MDSCs start to produce higher amount of IL-23, thus establishing a positive-feedback loop that induces the overexpression of IL-23R on the tumour epithelial cells and confer resistance to castration in prostate cancer by activating the STAT3–RORγ pathway. ENZA treatment can block the AR, inducing sensitiveness of prostate cancer cells to androgen deprivation, but the persistent presence of PMN-MDSC-derived IL-23 rescues the drug sensitiveness leading to ADT resistance. Anti-IL-23 treatment reinstates sensitivity to castration in prostate cancer enhancing the efficacy of ENZA.
Supplementary information
Supplementary Information
This file contains the uncropped gels and Supplementary Table 1
Rights and permissions
About this article
Cite this article
Calcinotto, A., Spataro, C., Zagato, E. et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559, 363–369 (2018). https://doi.org/10.1038/s41586-018-0266-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0266-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.