Abstract
Since 2006, five penis transplants have been performed worldwide. Mixed outcomes have been reported, and two of the five penile transplants have required explantation. However, the long-term outcomes have been encouraging when compliance is implemented, whether standard induction and triple therapy maintenance, or single therapy maintenance. Follow-up monitoring of transplant recipients has enabled a synthesis of technical considerations for surgical success and has shown stable leukocyte counts and renal function after a donor bone-marrow-based immunomodulatory regimen followed by tacrolimus monotherapy as long as 3 years post-transplant, as well as continuous nerve regeneration of penile allografts 3 years post-transplant. Areas of uncertainty include the ethics of donor–recipient colour mismatch, surveillance for sexually transmitted infections and how to optimize patient compliance. Questions also remain with respect to the long-term immunological sequelae of penile tissue, functional outcomes, psychosocial implications and patient selection. Patient counselling should be modified to mention the possibility of long-term improvement in nerve regeneration and sufficient renal function with single-therapy maintenance, and to build a longitudinal dialogue and partnership between the patient and the multidisciplinary care team regarding the risks of sexually transmitted infection instead of surveillance.
Key points
-
Since 2006, five penile transplantations have been completed worldwide. Results are mixed: allograft wound-healing problems are common, all transplants have required subsequent surgical intervention and two have required explantation.
-
Various surgical techniques have been used to reestablish continuity of the urethra and muscular corpora. Transplants forgoing direct tunica albuginea and corpus spongiosum repair maintained good urethral and ejaculatory function; one case that excluded direct corpora cavernosa repair was explanted before restoration of erectile function.
-
Data suggest that the dorsal penile artery alone is adequate for adequate penile allograft perfusion; however, five unique vascular anastomotic techniques have been described.
-
Lifelong immunosuppression remains a challenge, with acute rejection events occurring in all long-term transplant recipients. Conversely, nerve regeneration seems to keep improving as long as 3 years postoperatively in this setting.
-
Patient compliance, periodic screening and longitudinal holistic care are critical factors in optimizing long-term outcomes. Telemedicine and committed resource allocation to penile vascularized composite allotransplantation (VCA) patients were essential during the COVID-19 pandemic and could be used in future.
-
The outcomes of the existing cases have enabled evidence-based modifications to be made to the Baltimore Criteria for ethical penile transplantation.
Similar content being viewed by others
Introduction
The first penis transplant was reported in 2006 and a total of five penis transplants have been performed since that time1,2,3,4,5,6. The unique, challenging surgical considerations required for successful transplantation have been mostly elucidated, but questions remain in terms of the long-term immunological sequelae of penile tissue viability, functional outcomes, psychosocial implications, long-term transplant care, patient satisfaction and patient selection. Indeed, these considerations have contributed to mixed outcomes in some cases: two of the five penile transplants have required explantation, all have reported vascular congestion and/or haematoma requiring surgical intervention, and all but one patient have reported some degree of allograft tissue necrosis3,4,7. Substantial advances have been made in the first 15 years of this field, but many questions remain.
Penile loss is devastating for patient quality of life, with severe psychosocial and physical consequences8,9,10,11. The inability to partake in acts such as voiding urine while standing and sexual intercourse adversely affects patient self-esteem and their ability to maintain social relationships8,9,10,12. To address this unique challenge, traditional reconstructive options use the principles of free-tissue transfer, which involves taking a patient’s native tissues from another part of their body, harvesting these tissues with a distinct artery and vein to perfuse the entire tissue segment adequately, safely reshaping this tissue segment to appear and function as a neophallus, and using microvascular surgical techniques to connect this newly fashioned penile substitute to recipient arteries, veins, nerves and the urethra for perfusion and functional restoration13,14. These traditional reconstructive principles have yielded satisfactory results, but limitations persist, including donor site morbidity, neo-urethral fistulae formation at the neophallus and neo-urethral stricture15,16. Furthermore, no substitute tissue is capable of truly replacing native penile tissue and its unique properties — reconstructed tissue offers poor erogenous sensation and the unique erectile capacity of penile corpora tissue can only be mimicked using a prosthesis17. In some patients, even traditional reconstruction is not feasible owing to severe injury patterns that leave limited tissue to borrow from the rest of the body to create the neophallus18,19,20. These limitations have been the impetus for advancing penile transplantation as an alternative approach to reconstruction.
Penile transplantation is a type of vascularized composite allotransplantation (VCA), whereby transplantation of tissues from donor to recipient is made as a composite unit (such as a limb, face or penis), including tissue components such as skin, subcutaneous tissue, muscle, fat and bone5,21,22. VCA offers patients the unique benefit of receiving a transplant that cannot be convincingly reconstructed by other means, such as a face, arm or penis. By replacing ‘like-with-like’, reconstructive transplantation can achieve a level of restored normalcy that cannot be matched by other means, and which functions as originally designed23. This principle has been demonstrated over the past 20 years through face, arm, uterine and penile transplantation5,24,25,26.
Although >100 upper extremity and almost 50 face transplants have been performed to date, only five penis transplants have been performed since the first was completed just over 15 years ago: one in Guangzhou (Fig. 1), Baltimore (Fig. 2) and Boston (Fig. 3), and two in Cape Town (Fig. 4). The limited number of procedures performed, along with the paucity of longitudinal outcomes reported for these patients, have made it challenging to elaborate on the indications that currently exist27, to optimize technical considerations for surgical success, and to monitor postoperative health functionally, immunologically and psychosocially.
The patient originally presented with all native vascular structures intact. In this procedure, the dorsal penile arteries, superficial dorsal vein, and deep dorsal vein of the transplant were anastomosed to the equivalent recipient vessels. The transplant was explanted after 2 weeks owing to psychological rejection. DDV, deep dorsal vein; DPA, dorsal penile artery; SDV, superficial dorsal vein.
The patient originally presented with obliterated native deep penile arteries and cavernous arteries owing to an improvised explosive device detonation. The transplant included bilateral external pudendal arteries and veins. The left and right dorsal penile arteries of the transplant were anastomosed to the recipient left and right inferior epigastric arteries, respectively. The superficial dorsal vein of the transplant was anastomosed to the recipient’s right inferior epigastric vein, whereas the deep dorsal vein was anastomosed to the recipient’s left inferior epigastric vein. The transplant left external pudendal artery was supplied by an anastomosis between the supplying left transplant femoral artery and the recipient femoral artery. Likewise, the transplant left external pudendal vein drains to the left greater saphenous vein, which was anastomosed to the left recipient greater saphenous vein. This transplant maintains good long-term outcomes. DDV, deep dorsal vein; DPA, dorsal penile artery; EPA, external pudendal artery; EPV, external pudendal vein; FA, femoral artery; FV, femoral vein; GSV, greater saphenous vein; IEA, inferior epigastric artery; IEV, inferior epigastric vein; SDV, superficial dorsal vein.
The patient originally presented with obliteration of the native dorsal penile arteries. The transplant cavernous arteries were anastomosed to the equivalent recipient vessels. The right external pudendal artery of the transplant was anastomosed to the recipient right femoral artery, although this failed. The right dorsal penile artery of the transplant was successfully anastomosed to the recipient superficial femoral artery via a reversed distal leg vein graft. Finally, the deep dorsal vein of the transplant was anastomosed to its equivalent recipient vessel. This transplant maintains good long-term outcomes. CA, cavernous artery; DDV, deep dorsal vein; DPA, dorsal penile artery; FA, femoral artery.
The patient originally presented with obliteration of the native dorsal penile arteries, superficial dorsal vein and deep dorsal vein, owing to botched circumcision. The left and right dorsal penile arteries of the transplant were anastomosed to the recipient left external pudendal artery and left inferior epigastric artery, respectively. The deep dorsal vein of the transplant was anastomosed to the recipient inferior epigastric vein. After 2 years and 8 months, half of the flap was lost owing to rejection, which was later reversed and reconstructed with skin grafts. DDV, deep dorsal vein; DPA, dorsal penile artery; EPA, external pudendal artery; IEA, inferior epigastric artery; IEV, inferior epigastric vein.
In this Review, we consider patient selection, surgical methods and technical outcomes to optimize and reproduce surgical, sexual, urinary and psychological outcomes, as well as discuss ethical dilemmas and the future of penile transplantation. Furthermore, analysis of the techniques and outcomes of these five procedures enables us to suggest updates to the Baltimore criteria for successful penile transplantation.
Patient selection and Baltimore Criteria
Patient selection criteria for penile transplantations remain poorly defined. Although the global experience has offered considerable insight for success, additive findings offering guidance on rigorous patient selection were limited in the early clinical reports of penile transplantation1,2,3,4,5.
To address these limitations, our group previously produced ethical guidelines for penile allotransplantation, dubbed the Baltimore Criteria28 (Box 1). These criteria did not inform the worldwide experience of penile transplantation, but rather considered previous challenges and successes to suggest best practices. They offer guidance in terms of indications for transplant, consent and privacy, postoperative concerns and institutional requirements. However, the worldwide experience diverges from the original Baltimore Criteria and so evidence-based modifications to the Baltimore Criteria are offered here, based on these experiences (Box 1).
The first penis transplant, performed in Guangzhou, China, has elicited criticism for the paucity of psychological screening provided, which ultimately resulted in explantation of the graft on postoperative day 14 at the request of the patient’s wife1,29,30. Rigorous screening for psychological well-being and social support is a key facet of the original Baltimore Criteria. With regard to optimized graft cosmesis, the second patient from Cape Town is notable for being the only recipient of a colour-mismatched allograft — the donor was white and the recipient was Black6,7. Although this patient ultimately required explantation owing to non-compliance with immunosuppressive regimens, skin-tone mismatch was not determined to be a factor in this decision6,7.
Several procedures have demonstrated divergence from the recipient–donor age guidelines of the Baltimore criteria, which recommend a maximum 5-year age discrepancy between donor and recipient. The first Cape Town patient was 21 years old at the time he received an allograft from a 36-year-old, brain-dead donor — a much larger age differential than the maximum 5 years recommended by the original Baltimore criteria3. Although the Baltimore criteria do not offer an explanation for this age differential, it encourages the selection of a physiologically matched graft, with further consideration of ethical and biological barriers to using an immature graft from a donor under 16 years of age28. The recipient from Boston was 64 years old with a history of penile cancer treated with partial penectomy, who underwent rigorous psychosocial screening and received an allograft from a 27-year-old brain-dead donor with no known genitourinary history 4 years later. The age discrepancy was 37 years rather than the 5 years recommended by the Baltimore criteria, but the patient has had an excellent clinical course4,31. The Baltimore recipient was a 30-year-old man with a history of genitourinary trauma caused by a wartime improvised explosive device (IED) 8 years before transplantation. He was also screened rigorously for psychosocial and clinical clearance, and received a donor allograft from a donor within 5 years of his age with an excellent clinical course.
Given these findings, we propose evidence-based modifications to the Baltimore Criteria for patient selection (Box 1). First, recipients should be adults suffering substantial penile loss secondary to traumatic or oncological aetiologies or from congenital anomalies such as ambiguous genitalia or severe micropenis, as is often seen in individuals with exstrophy–epispadias complex. In the setting of trauma, at least 6 months of recovery time should be allowed before intervention, mirroring published recommendations for face and hand transplantation32,33. In settings of prior malignancy, transplant-sustaining immunosuppression may cause cancer recurrence, and a prolonged remission period consistent with very low risk based on the most current evidence available should be required34,35. No waiting period is needed for treatment of congenital ambiguous genitalia in adults.
Second, recipients should be seeking a reconstructive outcome that can only be provided by penile tissue: that is, spontaneous erections, capacity for penetrative intercourse without an implant, and penile function and cosmesis that parallels a native penis. These patients should not be candidates for traditional reconstructive modalities owing to substantial tissue loss at potential donor sites (such as forearm skin and neurovasculature used for radial forearm free flap) or other reasons such as previous failed attempts at reconstruction (such as graft-, flap-, or prosthesis-based approaches)36,37.
Third, patients and their support systems (for example, their spouse or partner) must undergo rigorous assessment and education and meet their VCA team’s psychological, social and clinical inclusion criteria. Consideration of cultural differences in societal regard for penile loss and subsequent availability of social support are crucial considerations. For example, whereas patients who have experienced penile loss can be ostracized following botched circumcision in South Africa, an American veteran might instead be appreciated as a hero who made a great personal sacrifice for their country6. Thus, cultural interpretations of penile loss are likely to exert different influences on a patient’s self-image and suicide risk.
Finally, the deceased donor must have a viable, functional graft, but does not require strict age proximity to the recipient. Although skin-tone matching considerations are strongly recommended, if the patient and VCA team feels that a mismatch is outweighed by the potential benefits of transplantation to the individual, proceeding with transplantation should be considered.
Surgical approach and technical outcomes
Technical considerations, operative decisions, and perioperative outcomes have differed across previously performed penile VCAs (Table 1). All penile transplants included the following steps: urethral anastomosis, dorsal penile nerve coaptation, penile soft tissue reapproximation and vascular anastomosis.
Penile soft tissue and urethra
All cases created the anastomosis of the urethra around a Foley catheter for structural support during healing. Although the Boston group’s anastomosis included spatulation to attenuate urethral stricture risk38,39, no other group performed spatulation, and no strictures have been reported to date in any of the patients. Normal voiding was reported as early as postoperative day (POD) 10 by the Guangzhou team, postoperative week (POW) 3 by the Boston team, POW 5 by the Baltimore team after an unsuccessful attempt at Foley removal due to urinary retention on POW 3, and for the first Cape Town patient by postoperative month (POM) 3, after formation of a urethrocutaneous fistula on POD 8.
The tunica albuginea is a fibrous layer of connective tissue that envelops both the corpus spongiosum and the corpora cavernosa40. Penile erection is achieved when corpora cavernosal arteries dilate, causing the corpora cavernosa to expand and compress the penile veins against the tunica albuginea40. The corpus spongiosum surrounding the urethra achieves comparatively less tumescence owing to a thinner surrounding layer of tunica albuginea, and undergoes compression by the ischiocavernosus and bulbocavernosus muscles just prior to ejaculation40. In all cases, the penile corpora were reapproximated via direct repair of the corpora muscle and/or the tunica albuginea. The Cape Town group and Baltimore group repaired all corpora muscle in addition to the tunica albuginea, whereas the Boston group excluded tunica albuginea repair. None of these groups has reported urethral or ejaculatory complications. All transplants that addressed direct corpora cavernosa muscle repair demonstrate good long-term erectile function, whereas the only transplant that did not, performed by the Guangzhou group, was explanted before restoration of erectile capacity could be assessed. The Cape Town, Boston and Baltimore groups used tadalafil differently to facilitate the return of erections. The Cape Town group began tadalafil treatment 1 week postoperatively and administered 5 mg for 3 months, the Boston group began tadalafil treatment 1 week postoperatively and administered 2.5 mg for an unknown duration, and the Baltimore group began tadalafil treatment 2 weeks postoperatively, which is still ongoing3,4,5. Although the rate of return of erectile function was inconsistently reported between groups, the influence of tunica albuginea repair, tadalafil treatment and the psychological state of the patients cannot be definitively stated based on the currently available evidence.
Penile blood supply and innervation
The penile blood supply is robust and characterized by many intrinsic collaterals — three pairs of arteries originate from the internal pudendal artery: the dorsal penile arteries (DPAs), cavernous arteries (CAs) and the bulbourethral arteries (BAs)41. The DPA supplies the penile glans and shaft, and has small, proximal branches that also supply the urethra and corpus spongiosum, with vascular redundancy from the BA41. The CA supplies the corpora cavernosa41. In addition to these vessels, the external pudendal artery (EPA) supplies the proximal penile skin and the lower half of the scrotum41. The deep dorsal vein (DDV) provides essential drainage of the penis41,42.
For transplantation, the DPAs and DPVs are essential for inflow and drainage; all transplant groups included anastomosis of these vessels. However, the need for arterial anastomoses beyond the DPAs is unclear — the penile replantation literature suggests that the DPA alone is adequate for functional restoration43, and that some necrosis of the penile skin is to be expected44.
The dorsal penile nerve branches from the pudendal nerve to innervate the skin of the penis42. All transplants were coapted directly from recipient to donor nerve except for the Boston group’s, which included a cadaveric acellular nerve graft that was needed to make up a nerve length deficit. All cases (except the Guangzhou group’s transplant, which was explanted by POD 14), reported a successful return of normal erogenous and tactile sensation at varied times. The first Cape Town and Baltimore patients reported normal penile sensation at POM 7 and 12, respectively, although the Baltimore patient continued to have diminished sensation in the lower half of the scrotum and part of the abdominal allograft tissue, and the Boston patient reported sensation at the proximal half of the penis by POM 6, with complete return of sensation by 2 years after the operation.
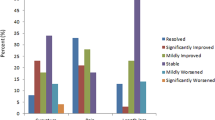
With respect to erection and sexual function, the Baltimore, Boston and the first Cape Town patients reported some degree of function by POM 12, with the first Cape Town patient reporting some function as early as POW 3 and penetrative intercourse by POW 5. The Baltimore patient had partial spontaneous erections at POM 6 and full erections by POM 12, and the Boston patient had partial erections by 6 months and continued, improved erection and sexual function as late as 3 years after surgery. Although the second Cape Town patient did not report initial erection data, sexual function returned and was present for 2 years before the onset of complications requiring explantation.
Synthesis of technical outcomes
Overall, combined outcomes from all groups suggest that urethral spatulation is not required to avoid stricture-related sequelae; the dorsal penile nerve is a reliable coaptation site for achieving erogenous and tactile sensation of the penis and, in the setting of immunosuppressed patients, regeneration can be seen as late as 3 years postoperatively; the corpus spongiosum does not need to be reapproximated for a penile transplant to function normally, but the corpora cavernosa are needed to restore erectile function; and finally, that the DPA is adequate for general perfusion, but including the EPA might protect against even partial skin necrosis. Available follow-up reports to date suggest that assessment of vessel patency is reassuring as late as 3 years after surgery.
Immunosuppression
The limited number of penile transplants performed make it challenging to draw conclusions about an ideal immunosuppressive regimen. The Cape Town team has not reported the immunological regimen of their second penile transplant, and the transplant performed by the Guangzhou team lacks long-term data owing to graft removal on POD 14. However, the long-term outcomes of the remaining three patients are encouraging when compliance is implemented.
Induction and maintenance
Several induction and maintenance protocols have been used for patients who have received a penile transplant (Fig. 5). Various combinations of drugs have been reported for immunosuppressive induction, including antithymocyte globulin (ATG) or a monoclonal antibody, ATG with steroids, or this combination with or without mycophenolate mofetil (MMF). ATG is a mixture of nonspecific anti-lymphocyte immunoglobulins and is the most widely used T cell-depleting therapy in the USA45. ATG has been associated with reduced solid organ graft rejection rates, permitting earlier steroid withdrawal and shorter hospital admission46, and is widely used in hand and face VCA47. Maintenance after solid organ transplantation often includes a calcineurin inhibitor and mycophenolate mofetil, with or without a steroid48. This approach arose from results of the Symphony trial, which investigated renal transplant function and rejection in the setting of various immunosuppression regimens and drug dosages. Symphony concluded that an tacrolimus-based triple therapy regimen offered the highest graft survival rate (90%) and lowest acute rejection rate (14%) at 3 years, while preserving glomerular filtration rate (GFR) with minimal drug dosages49,50. These results gave rise to the standardized triple therapy for VCA maintenance using tacrolimus, MMF and steroids51.
Triple therapy maintenance has led to varied outcomes in penile VCA. Only the first South African patient presented with renal toxicity, which arose 7 months after transplant and was managed with tacrolimus dose adjustment. However, 1 month later the patient contracted two infections secondary to supratherapeutic immunosuppression: an infected suprapatellar bursa and a foot lesion infected with phaeohyphomycosis. The patient from Boston experienced grade I rejection on POD 28, which was managed using intravenous methylprednisolone, and who was admitted on POD 32 with grade III rejection, which was successfully managed with steroids and an ATG taper, then on POD 44 for tacrolimus toxicity with hyperkalaemia. No other rejection episodes have been reported in any of the transplant recipients.
Mixed haematopoietic chimerism
Mixed haematopoietic chimerism is a state in which haematopoietic stem cells from two individuals can coexist in the bone marrow, resulting in tolerance of both host and donor tissues52,53. The Baltimore approach uses a donor bone marrow-based immunomodulatory regimen followed by tacrolimus monotherapy instead of conventional triple therapy protocol in all of their VCA recipients54. In this protocol, bone marrow was harvested from the penile donor and infused into the recipient 14 days after the transplant to introduce a second donor antigen stimulus and cause exhaustion and deletion of anti-donor T cells within the recipient55. As expected with this regimen, mixed haematopoietic chimerism was low and transient, peaking at 8% in POW 4, with undetectable levels in later assays5. At the time of writing, this patient continues successful maintenance immunosuppression with tacrolimus monotherapy, with stable leukocyte counts and renal function (Fig. 6). However, acute rejections have been observed in the Baltimore penis transplant patient despite good compliance, characterized by inflammatory cell infiltration and minor local cellular destruction of the transplanted tissue (Fig. 7). The cause of these episodes is unclear; however, the patient has since recovered and has been doing well to date.
a, White blood cell count and absolute neutrophil count during initial postoperative admission. Counts were highest in the early postoperative weeks, followed by resolution to normal ranges. Early peaks were associated with a haematoma evacuation on postoperative day 1 and a rejection-like episode on postoperative day 8. The peak on postoperative day 22 is associated with local inflammation at the time of biopsy; however, no evidence of rejection on histology was observed. b, White blood cell count and absolute neutrophil count after initial discharge. The peak at 19.6 months coinicides with treatment of a blotchy rash on a suprapubic portion of the transplant with clobetasol. Skin biopsy at 27.4 months revealed Banff grade III rejection requiring solumedrol 500 mg IV for 2 days. Peaks at 30.7 months and 36.7 months occurred at the time of two osteointegration sessions. c, Blood urea nitrogen and creatinine during initial postoperative admission. Blood urea nitrogen fluctuates at or above the normal range and creatinine is maintained within the normal range; this holds true even during a period of urinary retention during postoperative week 3. d, Blood urea nitrogen and creatinine after initial discharge. Creatinine grossly remains within normal limits. Blood urea nitrogen varies widely despite good urinary function throughout the long-term postoperative course. e, Glomerular filtration rate (GFR) during initial postoperative admission, showing maintenance within normal range with occasional fluctuations. f, GFR after initial discharge, showing maintenance within normal range with occasional fluctuations.
a, March 2018: 4× magnification shows Banff grade I/II rejection. b, March 2018: 10× magnification demonstrates mild perivascular infiltrate of lymphocytes and vacuolar change to basilar keratinocyte cytoplasm. c, March 2019: 4× magnification shows no signs of rejection and normal biopsy appearance. d, March 2019: 10× magnification shows no signs of rejection and normal biopsy appearance. e, June 2020: 4× magnification demonstrates multifocal areas of moderate perivascular and periadnexal lymphocytic inflammation. f, June 2020: 40× magnification demonstrating focal regions of dyskeratosis (circled).
Long-term outcomes, compliance and ethics
As experience with penile transplantation grows, new questions and uncertainties arise. As the current number of transplants performed remains very small, long-term data are sparse.
The first penile transplant was performed in Guangzhou in 2006, but no long-term data are available as it was explanted 14 days later owing to psychological rejection from both the patient and his wife, as well as patient agitation from considerable postoperative swelling and disfigurement of the penis2.
The first Cape Town patient received a transplant in December 2014 and was able to impregnate his partner 3–4 months after surgery. The team did not report any issues of rejection until 2018 (ref. 7). Non-compliance with the immunosuppressive regimen was reported, although a second pregnancy was reported in 2020 (ref. 7). However, in 2021 the patient acquired several sexually transmitted infections (STIs), which, in combination with non-compliance, were complicated by urethral stricture and partial allograft necrosis and loss. Even so, the transplant remains attached to date.
The second Cape Town patient was a Black male who received a transplant from a white donor in April 2017 — the only skin-tone mismatch of the penile transplants to date6,7. We note that the patient reported no concerns about the mismatch and declined tattooing for skin-tone matching, citing such poor quality of life after penile loss that he stated in a published interview that he would be content with a penile transplant that was “white, or…pink or purple. I will settle for anything”6. The team reported good sexual function until the end of 2019, at which point he began to experience episodes of rejection accompanied by severe pain that was refractory to plasma exchange, followed by graft necrosis and, ultimately, explantation in October 2021 (ref. 7). The Cape Town experience brought to light the issues surrounding rigorous patient screening to optimize compliance, skin-tone matching and longitudinal screening for STIs.
The Boston patient received a transplant in 2016, and had one acute rejection episode at 28 days after surgery, but has otherwise been stable on triple therapy. The Baltimore patient received a transplant in 2018 and has had intermittent episodes of acute rejection (Fig. 2). Even so, both patients maintain excellent form and function currently31,56, and emphasis has been placed on the surprising degree of nerve regeneration that has been observed as late as 3 years after transplant in the Boston patient. This response has also been demonstrated in upper extremity transplant surgery, suggesting that nerve healing in the setting of immunomodulation is promising57. The Baltimore patient’s renal function continues to be good on single therapy, although close, continual monitoring is warranted.
The future of penile transplantation
The American Society of Reconstructive Transplantation met in November 2021 to discuss global updates on VCA and provide expert opinion on future directions58. With respect to penile VCA, the most promising realization is that penile VCA is unequivocally the gold standard for penile reconstruction when considering functional and aesthetic outcomes and — as is the case in other forms of VCA being treated with calcineurin inhibitors — nerve function continues to improve in patients as late as 3 years after surgery, warranting patient counselling that some nerve regeneration in this setting could be expected years later. The subjects of greatest concern included the inability to biopsy penile tissue owing to limited tissue availability, which means that skin changes must be relied upon to monitor rejection, and the concern for STI prevalence. Given the immunosuppressive regimen VCA patients required to prevent transplant rejection, STIs have the potential to become severe before being detected and can have devastating sequelae to the transplant and patient. As such, close monitoring and patient counselling are essential.
Key considerations for optimal penile transplant outcomes include resources to expedite sexual function and satisfaction following transplantation, with special consideration of erogenous sensation, erection and ejaculation. Such considerations might include early initiation of PDE5 inhibitors (within 1 month of surgery), counselling to manage expectations and psychological sequelae and, potentially, sex therapy for the patient and their respective partners59. Men often display avoidance of sexual behaviours or resistance to penile rehabilitation treatments, and several studies have found psychological counselling and couples-based therapy to improve expectation management, treatment compliance and sexual satisfaction60,61,62,63,64,65.
VCA and the media
Interactions between VCA teams and the media must always be conscientious and thoughtful. VCA is an exciting, novel field and hence media coverage of patient outcomes and technical progress can be sensationalized and/or misinterpreted. Multidisciplinary VCA teams and patients must avoid being drawn into communications where controversial statements can be made or misquoted and, crucially, these parties must not be misrepresented or exploited. The privacy of the family and patient should be prioritized despite their participation in widely publicized advances in VCA. However, advancing the field of VCA will require educating the public about what is possible via transparent communications about advances and short-term and long-term results, and the media will undoubtedly have a key role in this process. These communications should focus on what modern medicine can offer patients, rather than individual accolades for medical teams.
COVID-19 and penile transplantation
Reallocation of health-care resources has been a common theme during the initial and sustained responses to the COVID-19 pandemic. The conversion of entire sections of hospitals to exclusively manage patients with COVID-19 and the concomitant redeployment of health-care staff was a common practice at many institutions. Considering these pressing needs, many VCA programmes, including our own in Baltimore, were temporarily shut down to address resource limitations. For penile transplantation, this translated into substantially longer periods of time taken to identify and screen potential donors, and no VCA of any kind has been performed at our institution since the beginning of the COVID-19 pandemic, although at the time of writing, our resources have been reallocated and our VCA programme reinstated.
Importantly, performing any kind of VCA — including a penis transplant — requires tremendous amounts of resources even before the actual transplantation is performed. The pool of available penile donors remains very small, as VCA donation requires specific permission that is separate from donor consent as designated by a driver’s licence or donor authorization card, instead requiring the approval of the deceased donor’s authorized representative66. From willing organ donors, genitourinary organs are among the least likely to be donated; only 61% of men were willing to donate their penis, versus 81% willing to donate a limb67. Identifying a donor with a healthy penis who is HLA-matched to the recipient and has a similar skin tone can be challenging, as about 86% of prior VCA donors from the USA were white28,68. As such, patient screening and identification of an optimal donor often take very long periods of time and can be frustrating for both the patient and VCA team. However, attempting to expedite this process is potentially disastrous and jeopardizes the transplant success for all parties involved, as shown by preventable measures that resulted in penis transplant failure in the published cases (such as lack of rigorous preoperative psychosocial screening in the explanted Guangzhou case). Thus, even considering the diminished resources and prolonged waiting periods consequent to COVID-19, our institution has not modified our stringent screening criteria for penile transplantation.
With respect to the Baltimore penis transplant patient’s follow-up care, although the COVID-19 pandemic resulted in shutdown of outpatient clinic services for a brief period, the rise of telemedicine has been especially valuable for remote monitoring the penile allograft. Skin changes that are appreciable on gross examination are characteristic of early acute rejection in VCA69; thus, any suggestion of an acute rejection episode can be easily triaged remotely and addressed quickly. Indeed, the Baltimore patient had several telemedicine visits during the first two waves of the COVID-19 pandemic in the USA, some of which were reassuring and one that demonstrated some mild erythema. On the basis of telemedicine triage, a clinic visit for biopsy and admission for intravenous steroid treatment was initiated with total resolution of symptoms (R.J.R., unpublished work).
Any centre that aspires to offer penile transplantation as a reconstructive option for patients requires substantial institutional commitment and support. Such support is especially pertinent in a post-COVID-19 world, where resource allocation is a valid concern. In our experience, an institutional commitment to support an agreed-upon number of VCA procedures as well as all aspects of postoperative care is an essential part of establishing a penile transplantation programme. Without such a commitment, we do not believe that establishing a penile transplantation programme is feasible.
Conclusions
Penile transplantation is a reconstructive modality that offers the gold-standard option for penile reconstructions, as it cannot be matched by conventional approaches. However, questions remain about patient selection, immunosuppressive regimens and optimizing compliance. From an operative standpoint, inclusion of the external pudendal artery for transplant perfusion seems to be uniquely protective of soft tissue viability and is essential to the success of the procedure. Finally, when considering the need for STI surveillance, we do not believe that surveillance is required, but rather an ongoing dialogue with patients about the unique aspects of STI presentation in VCA and the unique consequences of transplant failure. Thus, for the first time, we are able to provide comprehensive evidence-based recommendations to guide future penile transplantation in the form of an updated Baltimore Criteria.
References
Hu, W. et al. A preliminary report of penile transplantation. Eur. Urol. 50, 851–853 (2006).
Hu, W. et al. A preliminary report of penile transplantation: part 2. Eur. Urol. 50, 1115–1116 (2006).
van der Merwe, A. et al. Penile allotransplantation for penis amputation following ritual circumcision: a case report with 24 months of follow-up. Lancet 390, 1038–1047 (2017).
Cetrulo, C. L. J. et al. Penis transplantation: first US experience. Ann. Surg. 267, 983–988 (2018).
Redett, R. J. et al. Total penis, scrotum, and lower abdominal wall transplantation. N. Engl. J. Med. 381, 1876–1878 (2019).
van der Merwe, A., Toefy, Y., Moosa, M. R., van Deventer, H. & Scott, C. J. Living with someone else’s penis: the lived experiences of two South African penile allograft recipients: a descriptive phenomenological study. Ann. Med. Surg. 69, 102794 (2021).
van der Merwe, A. Plenary Session II – Clinical Speed Update from Stellenbosch University and Tygerberg Hospital, Cape Town. Presented at the American Society for Reconstructive Transplantation 2021 One-Day “Virtual” Meeting (2021).
Kepe, T. ‘Secrets’ that kill: crisis, custodianship and responsibility in ritual male circumcision in the Eastern Cape Province, South Africa. Soc. Sci. Med. 70, 729–735 (2010).
Serkin, F. B. et al. Combat urologic trauma in US military overseas contingency operations. J. Trauma 69, S175–S178 (2010).
Katz, A. Postcombat sexual problems. Am. J. Nurs. 108, 35–39 (2008).
De Castro, R., Merlini, E., Rigamonti, W. & Macedo, A. Jr. Phalloplasty and urethroplasty in children with penile agenesis: preliminary report. J. Urol. 177, 1112–1116 (2007).
Bullen, K., Edwards, S., Marke, V. & Matthews, S. Looking past the obvious: experiences of altered masculinity in penile cancer. Psychooncology 19, 933–940 (2010).
Monstrey, S. et al. Penile reconstruction: is the radial forearm flap really the standard technique? Plast. Reconstr. Surg. 124, 510–518 (2009).
Doornaert, M. et al. Penile reconstruction with the radial forearm flap: an update. Handchir. Mikrochir. Plast. Chir. 43, 208–214 (2011).
Garaffa, G., Raheem, A. A., Christopher, N. A. & Ralph, D. J. Total phallic reconstruction after penile amputation for carcinoma. BJU Int. 104, 852–856 (2009).
Morrison, S. D. et al. Phalloplasty: a review of techniques and outcomes. Plast. Reconstr. Surg. 138, 594–615 (2016).
Ma, S., Cheng, K. & Liu, Y. Sensibility following innervated free radial forearm flap for penile reconstruction. Plast. Reconstr. Surg. 127, 235–241 (2011).
DePalma, R. G., Burris, D. G., Champion, H. R. & Hodgson, M. J. Blast injuries. N. Engl. J. Med. 352, 1335–1342 (2005).
Landecker, A. & Macieira, L. Jr Penile and upper extremity amputation following high-voltage electrical trauma: case report. Burns 28, 806–810 (2002).
Gomez, R. G., Castanheira, A. C. & McAninch, J. W. Gunshot wounds to the male external genitalia. J. Urol. 150, 1147–1149 (1993).
Dubernard, J. M. et al. Functional results of the first human double-hand transplantation. Ann. Surg. 238, 128–136 (2003).
Barret, J. P. et al. Full face transplant: the first case report. Ann. Surg. 254, 252–256 (2011).
FitzGibbon, G. M. The commandments of Gillies. Br. J. Plast. Surg. 21, 226–239 (1968).
Khalifian, S. et al. Facial transplantation: the first 9 years. Lancet 384, 2153–2163 (2014).
Shores, J. T., Brandacher, G. & Lee, W. P. A. Hand and upper extremity transplantation: an update of outcomes in the worldwide experience. Plast. Reconstr. Surg. 135, 351e–360e (2015).
Brännström, M., Enskog, A., Kvarnström, N., Ayoubi, J. M. & Dahm-Kähler, P. Global results of human uterus transplantation and strategies for pre-transplantation screening of donors. Fertil. Steril. 112, 3–10 (2019).
Tuffaha, S. H. et al. Penile transplantation: an emerging option for genitourinary reconstruction. Transpl. Int. 30, 441–450 (2017).
Ngaage, L. M. et al. The Baltimore Criteria for an ethical approach to penile transplantation: a clinical guideline. Transpl. Int. 33, 471–482 (2020).
Patel, H. D. Human penile transplantation: an unjustified ethical dilemma. Eur. Urol. 74, 246–247 (2018).
Caplan, A. L., Kimberly, L. L., Parent, B., Sosin, M. & Rodriguez, E. D. The ethics of penile transplantation: preliminary recommendations. Transplantation 101, 1200–1205 (2017).
Cetrulo, C. L. J. Plenary Session II – Clinical Speed Update from Massachusetts General Hospital. Presented at the American Society for Reconstructive Transplantation 2021 One-Day “Virtual” Meeting (2021).
Lawson, S., Wang, L., Fries, C. A., Davis, M. & Gorantla, V. S. Development of a clinical vascularized composite allotransplantation program: requirements and recommendations. in From Auto-to Allotransplantation Vol. 5, 67–78 (Karger Publishers, 2016).
Gordon, C. R. & Siemionow, M. Requirements for the development of a hand transplantation program. Ann. Plast. Surg. 63, 262–273 (2009).
Penn, I. The effect of immunosuppression on pre-existing cancers. Transplantation 55, 742–747 (1993).
Girndt, M. & Köhler, H. Waiting time for patients with history of malignant disease before listing for organ transplantation. Transplantation 80, S167–S170 (2005).
Bryk, D. J., Yamaguchi, Y. & Zhao, L. C. Tissue transfer techniques in reconstructive urology. Korean J. Urol. 56, 478–486 (2015).
Khavanin, N. & Redett, R. J. Conventional surgical techniques and emerging transplantation in complex penile reconstruction. Plast. Aesthetic Res. 7, 47 (2020).
Jezior, J. R., Brady, J. D. & Schlossberg, S. M. Management of penile amputation injuries. World J. Surg. 25, 1602–1609 (2001).
Biswas, G. Technical considerations and outcomes in penile replantation. Semin. Plast. Surg. 27, 205–210 (2013).
MacDonald, S. M. & Burnett, A. L. Physiology of erection and pathophysiology of erectile dysfunction. Urol. Clin. North. Am. 48, 513–525 (2021).
Tuffaha, S. H. et al. Using the dorsal, cavernosal, and external pudendal arteries for penile transplantation: technical considerations and perfusion territories. Plast. Reconstr. Surg. 134, 111e–119e (2014).
Yiee, J. H. & Baskin, L. S. Penile embryology and anatomy. Sci. World J. 10, 1174–1179 (2010).
Landstrom, J. T., Schuyler, R. W. & Macris, G. P. Microsurgical penile replantation facilitated by postoperative HBO treatment. Microsurgery 24, 49–55 (2004).
Tuffaha, S. H., Budihardjo, J. D., Sarhane, K. A., Azoury, S. C. & Redett, R. J. Expect skin necrosis following penile replantation. Plast. Reconstr. Surg. 134, 1000e–1004e (2014).
Kueckelhaus, M. et al. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl. Int. 29, 655–662 (2016).
Bamoulid, J. et al. Anti-thymocyte globulins in kidney transplantation: focus on current indications and long-term immunological side effects. Nephrol. Dial. Transpl. 32, 1601–1608 (2017).
Sarhane, K. A. et al. Minimization of immunosuppression and tolerance induction in reconstructive transplantation. Curr. Surg. Rep. 1, 40–46 (2013).
Wojciechowski, D. & Wiseman, A. Long-term immunosuppression management: opportunities and uncertainties. Clin. J. Am. Soc. Nephrol. 16, 1264–1271 (2021).
Ekberg, H. et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N. Engl. J. Med. 357, 2562–2575 (2007).
Knight, S. R., Russell, N. K., Barcena, L. & Morris, P. J. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation 87, 785–794 (2009).
Fischer, S. et al. Acute rejection in vascularized composite allotransplantation. Curr. Opin. Organ. Transpl. 19, 531–544 (2014).
Pham, S. M. et al. Mixed hematopoietic chimerism induces donor-specific tolerance for lung allografts in rodents. Am. J. Respir. Crit. Care Med. 159, 199–205 (1999).
Li, Z., Czechowicz, A., Scheck, A., Rossi, D. J. & Murphy, P. M. Hematopoietic chimerism and donor-specific skin allograft tolerance after non-genotoxic CD117 antibody-drug-conjugate conditioning in MHC-mismatched allotransplantation. Nat. Commun. 10, 616 (2019).
Schneeberger, S. et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann. Surg. 257, 345–351 (2013).
Sachs, D. H., Kawai, T. & Sykes, M. Induction of tolerance through mixed chimerism. Cold Spring Harb. Perspect. Med. 4, a015529 (2014).
Redett, R. J. Plenary Session II – Clinical Speed Update from Johns Hopkins University. Presented at the American Society for Reconstructive Transplantation 2021 One-Day “Virtual” Meeting (2021).
Mackinnon, S. E., Doolabh, V. B., Novak, C. B. & Trulock, E. P. Clinical outcome following nerve allograft transplantation. Plast. Reconstr. Surg. 107, 1419–1429 (2001).
Gelb, B. E. et al. Allograft procurement in the first successful combined face and bilateral hand transplant: timing and sequence [abstract]. SAGE Open Med. https://doi.org/10.1177/20503121221074750 (2022).
Girard, A. O. et al. Vascularized composite allotransplantation of the penis: current status and future perspectives. Int. J. Impot. Res. 34, 383–391 (2022).
Nelson, C. J. et al. Men’s experience with penile rehabilitation following radical prostatectomy: a qualitative study with the goal of informing a therapeutic intervention. Psychooncology 24, 1646–1654 (2015).
Nelson, C. J. et al. Acceptance and commitment therapy to increase adherence to penile injection therapy-based rehabilitation after radical prostatectomy: pilot randomized controlled trial. J. Sex. Med. 16, 1398–1408 (2019).
Canada, A. L., Neese, L. E., Sui, D. & Schover, L. R. Pilot intervention to enhance sexual rehabilitation for couples after treatment for localized prostate carcinoma. Cancer 104, 2689–2700 (2005).
Chambers, S. K. et al. ProsCan for Couples: randomised controlled trial of a couples-based sexuality intervention for men with localised prostate cancer who receive radical prostatectomy. BMC Cancer 8, 226 (2008).
Narang, G. L., Figler, B. D. & Coward, R. M. Preoperative counseling and expectation management for inflatable penile prosthesis implantation. Transl Androl. Urol. 6, S869–S880 (2017).
Schover, L. R. & von Eschenbach, A. C. Sex therapy and the penile prosthesis: a synthesis. J. Sex. Marital. Ther. 11, 57–66 (1985).
[No authors listed.] Guidance on optimizing VCA recovery from deceased donors. Organ Procurement and Transplantation Network https://optn.transplant.hrsa.gov/policies-bylaws/public-comment/guidance-on-optimizing-vca-recovery-from-deceased-donors/ (2018).
Ward, S. et al. Attitudes toward organ, tissue, and vascularized composite allograft (VCA) donation and transplantation: a survey of united states military veterans. Transplantation 105, 1116–1124 (2021).
Wainright, J. L. et al. VCA deceased donors in the United States. Transplantation 103, 990–997 (2019).
Sarhane, K. A. et al. A critical analysis of rejection in vascularized composite allotransplantation: clinical, cellular and molecular aspects, current challenges, and novel concepts. Front. Immunol. 4, 406 (2013).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made a substantial contribution to discussion of content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks André Van der Merwe, Wayne Hellstrom and Curtis Cetrulo for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lopez, C.D., Girard, A.O., Lake, I.V. et al. Lessons learned from the first 15 years of penile transplantation and updates to the Baltimore Criteria. Nat Rev Urol 20, 294–307 (2023). https://doi.org/10.1038/s41585-022-00699-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00699-7