Abstract
On 11 March 2020, the WHO declared the coronavirus disease 2019 (COVID-19) outbreak a pandemic and COVID-19 emerged as one of the biggest challenges in public health and economy in the twenty-first century. The respiratory tract has been the centre of attention, but COVID-19-associated complications affecting the genitourinary tract are reported frequently, raising concerns about possible long-term damage in these organs. The angiotensin-converting enzyme 2 (ACE2) receptor, which has a central role in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) invasion, is highly expressed in the genitourinary tract, indicating that these organs could be at a high risk of cell damage. The detection of SARS-CoV-2 in urine and semen is very rare; however, COVID-19 can manifest through urological symptoms and complications, including acute kidney injury (AKI), which is associated with poor survival, severe structural changes in testes and impairment of spermatogenesis, and hormonal imbalances (mostly secondary hypogonadism). The effect of altered total testosterone levels or androgen deprivation therapy on survival of patients with COVID-19 was intensively debated at the beginning of the pandemic; however, androgen inhibition did not show any effect in preventing or treating COVID-19 in a clinical study. Thus, urologists have a crucial role in detecting and managing damage of the genitourinary tract caused by COVID-19.
Key points
-
High expression levels of the angiotensin-converting enzyme 2 (ACE2) receptor, which has a central role in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) invasion, can be found in the genitourinary tract, especially in kidney proximal tubule cells, bladder urothelial cells and testes.
-
Acute kidney injury (AKI) occurs frequently in patients with coronavirus disease 2019 (COVID-19) and is the most common complication of COVID-19. Cytokine-storm-induced systemic inflammatory response and direct cytopathic effects are possible pathophysiological mechanisms of AKI.
-
The detection of SARS-CoV-2 in urine is rare, although the virus can be detected in urine up to 52 days after disease onset. SARS-CoV-2 is rarely detected in semen.
-
Results of autopsy studies have shown severe structural changes in testes of deceased patients with COVID-19. An acute impairment of spermatogenesis in patients with COVID-19 is probably caused by fever; however, the existence of long-term effects of COVID-19 on fertility, as observed in other viral orchitides, is still conceivable.
-
Low levels of total testosterone in patients with COVID-19 are reported frequently, and hypogonadism is often secondary. The effect of androgen deprivation therapy on COVID-19 survival is still debated.
-
COVID-19-associated coagulopathy can cause endothelial damage and vasculitis, potentially leading to thromboembolism, which also affects urogenital organs.
Similar content being viewed by others
Introduction
A pneumonia outbreak of unknown aetiology was reported in Wuhan at the end of 2019 (ref.1). The previously unknown virus responsible for this pneumonia was identified and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)2, and the new disease was termed coronavirus disease 2019 (COVID-19) in the WHO situation report3. On 11 March 2020, the WHO declared the COVID-19 outbreak a pandemic4. As the virus spread, new findings were published on a daily basis and, although the respiratory tract seemed to be the main field of interest, attention soon focused on extra-pulmonary manifestations of the infection5. Results from autopsy studies on patients deceased of COVID-19-associated causes reported severe tissue alterations in various organs6,7.
In gastroenterology, diarrhoea is a frequently reported symptom of SARS-CoV-2 infection8, and COVID-19 has also been associated with liver damage and dysfunction9,10, as well as with acute pancreatitis11. Cardiologists report that COVID-19-induced myocarditis can lead to fulminant myocardial dysfunction and is associated with poor overall prognosis12. In neurology, systemic inflammatory responses induced by COVID-19 can result in devastating hypoxic and metabolic changes that can lead to encephalopathy and encephalitis13. Experience with long-term complications of COVID-19 is still limited. After the acute phase of SARS-CoV-2 infection, patients might still suffer from fatigue, breathing difficulty and fever14. In patients with so-called long COVID (a complex condition characterized by prolonged heterogeneous symptoms following SARS-CoV-2 infection, such as weakness, general malaise or fatigue15), mounting evidence of impairment or scarring of multiple organs has been reported16. In urology, evidence suggests that kidneys and testes are at a particularly high risk of severe damage following COVID-19 infection17,18.
In this Review, data concerning the effects of COVID-19 on the urogenital system are discussed to provide further information for the medical treatment of patients with COVID-19 and evaluate the risk of possible long-term damage. Current findings on urological symptoms of COVID-19 and damage to organs of the genitourinary tract induced by SARS-CoV-2 infection are also presented. Furthermore, the effect of COVID-19 on male sexual health and the risk of viral transmission through urine or semen are discussed.
SARS-CoV-2 viral entry into human cells
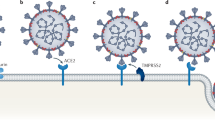
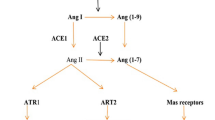
Spike proteins of SARS-CoV-2 are membrane proteins that give the virus its crown-like appearance and bind to angiotensin-converting enzyme 2 (ACE2) receptors on the cellular surface, enabling the entry of the virus19. At this point, transmembrane protease serine 2 (TMPRSS2) is responsible for the cleavage of spike proteins (defined as ‘priming’), which is required for fusion of cellular and viral membranes20 (Fig. 1). Interestingly, TMPRSS2 is a crucial factor in the pathogenesis of SARS-CoV-2, and also an important regulator in prostate cancer21,22. TMPRSS2 is, in fact, predominantly expressed in prostate epithelium23 and is upregulated in prostate cancer with high Gleason grades24. Moreover, the risk of viral invasion in different tissues was hypothesized to be dependent on the expression level of ACE2 (ref.25). Results from a single-cell RNA-sequencing study in which datasets from different organs were analysed showed that the average proportion of ACE2+ type II alveolar cells in the lungs was ~1%, with 1% standard deviation17. On this basis, cell types with >1% of ACE2+ cells were considered at a ‘high risk’ of SARS-CoV-2 infection. High ACE2 expression levels were found in kidney proximal tubule cells (4% positivity) and in bladder urothelial cells (2.4% positivity)17. However, the investigators emphasized that gene expression variation between individuals should also be considered and that SARS-CoV-2 invasion does not exclusively depend on ACE2. Thus, the existence, in kidney and bladder, of a higher risk of cell damage than other organs remains unclear17 (Fig. 2). In another study in which transcriptome data from published single-cell RNA-sequencing studies was analysed, high expression levels of ACE2 and TMPRSS2 in kidney and testis tissue were reported18. However, to date, no clear proof is available to support the original hypothesis that high ACE2 expression levels increase the risk of viral invasion. In fact, high activity of ACE2 has also been postulated to be potentially beneficial for patients with COVID-19 by diminishing lung injury and inflammation26. ACE2 is, indeed, also a major component of the renin–angiotensin–aldosterone system (RAAS)27, a crucial regulatory system for fluid and electrolyte balance, systemic vascular resistance and, thereby, blood pressure28. ACE2 converts angiotensin II, which is a main perpetrator of inflammation, into angiotensin 1–7, which has anti-inflammatory properties27. Thus, ACE2 might have a double role in COVID-19: pro-infection, acting as a cellular receptor for SARS-CoV-2, and protective during SARS-CoV-2 infection, by mitigating inflammation26,29.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds to angiotensin-converting enzyme 2 (ACE2; step 1 in the left and right panels) on the host-cell membrane and undergoes a conformational change in the spike protein subunit 1 (S1) leading to exposure of the S2′ cleavage site in the S2 subunit of the virus; these cleavage events in the two spike proteins of the virus are necessary for the virus entry process. Two viral entry pathways (endosomal and cell surface) are available. Left: in the case of insufficient transmembrane protease serine 2 (TMPRSS2) levels on the host cell, or if a virus–ACE2 complex does not encounter TMPRSS2, the virus–ACE2 complex is internalized via clathrin-mediated endocytosis (step 2) into the endolysosomal compartment, where S2′ is cleaved by the enzymes cathepsins, working in an acidic environment (steps 3 and 4). Right: in the presence of TMPRSS2, S2′ is cleaved at the cell surface (step 2). In both entry pathways, cleavage of the S2′ site exposes the fusion peptide (FP) and induces dissociation of S1 from S2. A dramatic conformational change in the S2 subunit moves the FP forward into the target membrane, initiating membrane fusion (step 5 on the left and step 3 on the right). After the fusion between viral and cellular membranes, viral RNA is released into the host-cell cytoplasm through a fusion pore and viral RNA uncoating occurs (step 6 on the left and step 4 on the right). Several agents disrupt the interaction between the S proteins and ACE2, such as ACE2 mimetics, therapeutic antibodies (targeting S protein) and vaccine-elicited antibodies (blocking the virus binding to ACE2). Other strategies target post-receptor-binding steps: the serine protease inhibitor camostat mesylate acts on TMPRSS2, blocking the TMPRSS2-mediated entry pathway; hydroxychloroquine and chloroquine block endosomal acidification, which is necessary for cathepsin activity, acting on the cathepsin-mediated entry pathway. Reprinted from ref.19, Springer Nature Limited.
Results from a single-cell RNA-sequencing study showed that the average proportion of angiotensin-converting enzyme 2 (ACE2)-positive type II alveolar cells in the lungs was ~1%, with 1% standard deviation17. On this basis, cell types with >1% of ACE2+ cells were defined as being at a ‘high risk’ of SARS-CoV-2 infection17. High ACE2 expression levels were found in kidney proximal tubule cells (4% ACE2+ cells) and in bladder urothelial cells (2.4% ACE2+ cells); the percentage of ACE2+ cells in these compartments was even higher than that observed in respiratory epithelial cells (2.0% ACE2+ cells)17.
Thus, the discussion on the effects of RAAS blockers on the outcome of patients with COVID-19 has been quite controversial26; however, drugs targeting the RAAS have shown no relevant improvements in the risk of COVID-19 infection and disease outcome30.
Acute kidney injury in COVID-19
The reported incidence of acute kidney injury (AKI) in patients with COVID-19 is heterogeneous and ranges from 1% to 46%31. Results from a big retrospective, observational study analysing the incidence of AKI in 3,993 hospitalized patients with COVID-19 showed that AKI occurred in 46% of patients32; 19% of whom required kidney replacement therapy32. The mortality was 50% in patients with AKI and 8% in patients without AKI32. Moreover, only ~30% of patients who survived showed a recovery of kidney function at the time of discharge32. Thus, AKI in patients with COVID-19 is common and associated with high mortality; recovery of kidney function is delayed or absent in many patients with AKI. AKI was reported to be the most common complication of COVID-19 in a multicentre cohort study including 80,388 patients with COVID-19 (ref.33). However, in another prospective multicentre cohort study including 85,687 patients, only 2,198 patients (2.6%) received kidney replacement therapy34, indicating that the need for this therapy in patients with COVID-19 and AKI might be lower than previously suggested32. In a prospective cohort study including 701 patients with COVID-19, 43.9% had proteinuria and 26.7% had haematuria on admission, indicating severe damage of kidney tissue35. Patients with highly prevalent risk factors, such as diabetes, obesity or hypertension, are at an increased risk of developing SARS-CoV-2-mediated AKI (adjusted odds ratio (OR): 1.76 for diabetes, 1.11 for obesity and 1.25 for hypertension)36,37. The reported mortality in hospitalized patients with COVID-19 and AKI varies tremendously (34–80%)36, but might still be considered substantially higher than the average mortality of all hospitalized patients with COVID-19 (including patients without AKI), reported to be between 9.3% and 19.7% in a cohort study including 503,409 patients38. The rate of AKI correlates with the classification of COVID-19 disease severity by the WHO, as AKI has been observed in 3.2%, 15.2% and 24.3% of patients with moderate COVID-19, severe COVID-19 and acute respiratory distress syndrome, respectively, in a prospective assessment of 333 patients with COVID-19 (ref.39). AKI in patients with COVID-19 also correlates with patients’ history of chronic kidney disease (CKD)39. In a case series of 7,624 patients with COVID-19, the reported mortality was 23.1% in patients with CKD and 10.2% in patients without CKD (P < 0.001), with odds of mortality 1.51 times higher (95% CI 1.19–1.90) in patients with CKD than in the non-CKD group40.
Pathophysiological mechanisms leading to AKI development in patients with COVID-19 have not yet been fully elucidated and are probably multifactorial. Severe structural changes can be detected in kidney biopsy samples from patients with COVID-19 (refs41,42). In a study analysing kidney biopsy samples from 14 patients with COVID-19, 5 patients had collapsing glomerulopathy, 1 had minimal change disease, 2 had membranous glomerulopathy, 1 had crescentic transformation of lupus nephritis, 1 had anti-glomerular basement membrane nephritis and 4 had isolated acute tubular injury41. In another study, post-mortem kidneys from 42 patients who died of COVID-19 were examined, and acute tubular injury was the most common kidney alteration (detected in 45% of patients)42. Two instances of acute necrotizing glomerulonephritis associated with COVID-19 have also been described in children43. One of the biggest studies in which histopathological changes in kidney tissue in patients with COVID-19 have been evaluated is an international multicentre retrospective cohort study, including 284 patients with COVID-19 and kidney symptoms (AKI, acute on CKD or proteinuria)44. Overall, 240 native biopsy samples and 44 allograft biopsy samples were compared with biopsy samples from a pre-COVID-19 database and a wide range of histopathological changes were found44; the most common observation in patients with COVID-19 and kidney symptoms was acute tubular injury (detected in 78.3% of native biopsy samples and 88.6% of allograft biopsy samples). However, acute tubular injury as the sole or predominant finding was detected in only 13.3% of native biopsy samples and 27.3% of the allograft biopsy samples, not significantly different from what was detected in pre-COVID-19 biopsy samples (11.9%, P = 0.52 for native biopsy samples and 17.7%, P = 0.09 for allograft biopsy samples)44. The most common finding in native biopsy samples (25.8% of patients) was collapsing glomerulopathy, also known as COVID-19-associated nephropathy (COVAN)44. The G1 and G2 alleles of the apolipoprotein L1 (APOL1), which are very frequent in men with African ancestry and greatly increase the risk of multiple types of kidney disease45, were found in 91.7% of the tested kidney biopsy samples from African American and Hispanic patients with COVAN44. Thus, whether the glomerular changes observed in this population (African American and Hispanic patients with COVID-19 who express the G1 and G2 risk alleles of APOL1) are a direct consequence of SARS-CoV-2 infection or emerge as a form of a ‘second hit’ additional to a pre-existing risk factor is still unclear46. Analysis of biopsy samples from patients with kidney allograft showed that allograft rejection disproportionately affected patients with COVID-19 (61.4% versus 27.1% in allograft biopsy samples from the pre-COVID-19 database, P < 0.001)44. Taken together, these results show that a wide spectrum of pathological changes in kidney could be detected in patients with COVID-19, with COVAN being the most common diagnosis in native biopsy samples44; moreover, patients with COVID-19 who have a kidney transplant seem to be at a considerable risk of developing an allograft rejection44. In this study, no evidence of direct SARS-CoV-2 infection was found in the examined kidney biopsy samples44, whereas, in other studies, the virus could be detected in some kidney samples, although this event seems to be rare and depends on the method of detection. However, understanding the viral load in kidney tissue might help to clarify whether the alterations in kidney observed in patients with COVID-19 are caused by the viral infection or occur in response to systemic inflammation.
One possible pathophysiological mechanism of AKI in COVID-19 is a cytokine-storm-induced systemic inflammatory response31,41. A cytokine storm is characterized by an excessive and maladaptive release of pro-inflammatory cytokines during an inflammatory response, which can lead to excessive organ dysfunction47. SARS-CoV-2 might cause inflammatory cytokine storms, which lead to acute respiratory distress syndrome or multiple-organ failure and could be associated with disease severity48,49,50. Results from a study including 41 hospitalized patients with COVID-19 showed higher plasma concentration of multiple cytokines, such as IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF) and tumour necrosis factor (TNF) in patients treated in the intensive care unit (ICU)51 than in patients in the non-ICU group48. In another study, the concentration of numerous cytokines was significantly higher in patients with COVID-19 (n = 44) than in healthy individuals (n = 66, P < 0.05) and cytokine levels were shown to be associated with different extents of COVID-19 severity52. However, no single definition for the term cytokine storm is widely accepted and the line between a normal and a dysregulated response to a severe infection is blurred47.
Another mechanism of COVID-19-mediated AKI might be the induction of structural changes in host cells31. ACE2 is highly expressed in renal tubular cells, and SARS-CoV-2 has been shown to be able to bind to ACE2 on the surface of these cells in in vitro studies53,54. Results from a single-cell transcriptome analysis of 15 kidney samples from healthy individuals showed co-expression of ACE2 and TMPRSS in the kidney, especially in proximal tubular cells and podocytes55. The co-expression level of ACE2 and TMPRSS in these cells was similar to that observed in the lung, supporting the hypothesis that the kidney might be a main target organ for SARS-CoV-2 (ref.55). Podocytes have a crucial role in urine filtration, and proteinuria has been observed in patients with COVID-19 (44% of patients (n = 701) in a prospective study)35. Thus, based on the high level of ACE2 and TMPRSS co-expression in podocytes, the investigators concluded that proteinuria in patients with COVID-19 might be caused by a direct cytopathic effect of SARS-CoV-2 on podocytes55. Evidence for ACE2-independent cell damage in kidney cells has also been reported. In kidney epithelial cells, a novel route of viral invasion for SARS-CoV-2 mediated by the interaction of spike proteins with CD147 (a transmembrane glycoprotein also known as basigin) has been described56,57, suggesting that other ACE2-independent mechanisms of viral invasion in the kidney might exist and warrant further investigation.
In summary, AKI is a serious and frequently reported complication in patients with COVID-19 (ref.31), but the pathophysiological background of AKI is not fully understood. A wide range of severe pathological changes can be found in kidney tissue samples from patients with COVID-19 (refs41,42,44), but whether this damage is caused by an exuberant systemic inflammatory response31,41,48,49,50,52 or by a direct cytopathic effect of SARS-CoV-2 (refs31,53,54,55) is still unclear. However, physicians should consider COVID-19 as a potential diagnosis for patients showing fever and signs of AKI.
SARS-CoV-2 in urine and semen
The detection of SARS-CoV-2 in urine is very rare. In a meta-analysis of 93 studies in which urine samples from 533 patients with COVID-19 were analysed, the presence of SARS-CoV-2 in urine was reported in only 14 studies, in 24 patients in total (4.5%)58. In most studies, the urinary SARS-CoV-2 viral load was lower than that observed in oropharyngeal or rectal samples58. However, SARS-CoV-2 was detected in urine up to 52 days after disease onset, indicating that the virus might be detectable in urine in the acute stage of COVID-19 and also after recovery58. In adults, SARS-CoV-2 was more likely to be found in the urine samples of patients with moderate or severe disease58. These observations could suggest either that the viral load of SARS-CoV-2 in the urogenital tract is low or that the viral excretion via the urogenital tract is highly restricted. SARS-CoV-2 could also be unstable in urine, but a PCR test should still detect it.
The detection of SARS-CoV-2 in mucous membranes seems to be dependent on the region tested59,60. SARS-CoV-2 can be detected in anal swabs and in some patients, the test remained positive even when throat swabs and sputum swabs were negative59. Thus, this method has been also discussed as a possible tool to evaluate hospital discharge of patients with COVID-19 (ref.59). The situation seems to be different for urethral swabs: in a case series, all ten of the patients tested had a negative urethral swab for SARS-CoV-2 RNA, despite having a positive rhino-oropharyngeal test60.
In a meta-analysis of eight studies including 160 semen samples from patients with COVID-19, SARS-CoV-2 was only detected in six patients from one study61. The presence of SARS-Cov-2 viral RNA in ejaculate has only been reported in four studies to date62,63,64,65 (6.66%62, 15.8%63, 2.3%64 and 14.3%65 of patients). During sexual intercourse, COVID-19 infection through respiratory droplets is of course possible, but, according to the available data, a sexual transmission of SARS-Cov-2 via semen seems to be very unlikely66
Overall, these results indicate that detection of SARS-CoV-2 in urine and semen is possible, but is a rare event58,61,62,63,64,65.
Cystitis and lower urinary tract symptoms
Viral cystitides dependent on SARS-CoV-2 infection have been observed since the beginning of the pandemic, with a high urinary frequency observed in 7 of 57 men with COVID-19, who had no signs of AKI, bacterial infection or prostatitis67. De novo urinary symptoms associated with COVID-19, such as increased urinary frequency and nocturia, were also reported in another study including SARS-CoV-2-positive outpatients (n = 39)68. A bacterial urinary tract infection in these patients was excluded; therefore, the urinary symptoms were assumed to be caused by SARS-CoV-2, although no viral detection was conducted68. In another study, the average International Prostate Symptom Score (IPSS), which is used as a validated questionnaire to quantify lower urinary tract symptoms (LUTS)69, was assessed in patients with COVID-19 aged >50 years (n = 62) in the acute stage of the disease and surveyed retrospectively for the time before COVID-19 infection70. In the acute stage of COVID-19, the IPSS was significantly higher than before (9 ± 6.4 versus 5.1 ± 4.1, P < 0.0001); no correlation was found between IPSS and radiological degree of pulmonary infiltrates, and no significant differences in IPSS before and during COVID-19 were reported in a group of patients <50 years old (P = 0.053)70. Taken together, these results provide clinical evidence that SARS-CoV-2 infection might trigger LUTS, especially in elderly men70. The absence of statistically significant differences in IPSS before and during COVID-19 in patients <50 years might be explained by the small sample size of this age group in this study (n = 32)70.
Urinary symptoms reported in patients with COVID-19 overlap with common diseases such as benign prostatic hyperplasia; therefore, proving SARS-CoV-2 is the underlying cause is difficult71. Searching for possible mechanisms explaining the high prevalence of LUTS in patients with COVID-19, the levels of pro-inflammatory cytokines in urine was assessed in a small study including eight participants (four patients with COVID-19 and de novo urinary tract symptoms and four age-matched participants without COVID-19 and with no history of urinary tract diseases)72. The investigators hypothesized that what they called COVID-19-associated cystitis is caused by an increased inflammatory cytokine release into the urine and/or expression in the bladder72. Thus, urine samples from patients and age-matched individuals were collected72; all patients positive for COVID-19 (n = 4) had significantly higher levels of IL-6 (P = 0.046) and IL-8 (P = 0.002) in urine than individuals in the COVID-19-negative group (n = 4)72. Urinary tract infections can lead to intermittent secretion of IL-6 and IL-8 into the urine73; however, increased interleukin levels in urine are not specific for urinary tract infections at all and might only depend on renal excretion of plasma interleukins.
The detection of SARS-CoV-2 in urine and semen is very rare, but the high prevalence of LUTS in the acute stage of COVID-19 reasonably suggests that the lower urinary tract might still be affected by the virus67,68,70. Whether the inflammation in the urinary tract of patients with COVID-19 directly depends on SARS-CoV-2 or is secondary to renal excretion of plasma interleukins is still unclear.
SARS-CoV-2 and male reproductive health
Long-term effects of viral orchitis caused by viruses such as mumps, HIV or hepatitis B virus on fertility have been extensively studied74. Viral infection of the testes can result in a reduction of sperm quality and endocrine function, and possible long-term effects include oncogenic outcomes or vertical transmission of virus-induced mutations75. Understanding how SARS-CoV-2 will affect male fertility has received widespread attention. A survey in multiple European countries showed that 38–58% of individuals who had planned to have a baby in 2020 were going to postpone this decision76, although expected financial challenges caused by the pandemic was the main reason for this choice.
ACE2 expression levels in testes are amongst the highest in the body77; ACE2 can be found in Sertoli cells, Leydig cells and cells of the seminiferous ducts78. In vitro cell-binding assays showed that SARS-CoV-2 can also bind to ACE2 on the surface of these cells53,78. Results from histopathological assessment of testicular and epididymal specimens of six deceased men with COVID-19 showed the presence of interstitial oedema, congestion and red blood cell exudation, which were absent in the group of deceased men without COVID-19 (ref.79). Furthermore, thinning of seminiferous epithelium was observed in deceased patients with COVID-19, and the proportion of apoptotic cells in testes of these patients was significantly higher (2.95-fold, P = 0.018) than the average of deceased men without COVID-19. However, no data on histopathological changes in patients with non-fatal COVID-19 are available; therefore, whether the alterations in testes are a general consequence of COVID-19 or exclusively occur in patients with fatal COVID-19 is still unclear.
In another study, SARS-CoV-2 could be detected in testis tissue samples from deceased patients with COVID-19 (n = 6) by transmission electron microscopy, and the density of ACE2 receptors (quantified through immunofluorescence) was reported to be inversely proportional to the level of spermatogenesis, defined as impaired if full spermatogenesis was not visualized in all observed tubules80. The direct association between increased ACE2 levels and impairment of spermatogenesis supports the hypothesis that organs with high ACE2 levels are at a high risk of cell damage and suggests that the testes might be target organs of SARS-CoV-2. In another study, results from ten autopsies performed on deceased men with COVID-19 (median age: 49.5 years) revealed morphological testes alterations in 70% of patients, attributable to oxidative stress, as well as sloughing of spermatocytes, elongation of spermatids, swelling of Sertoli cells and multifocal microthrombi81.
Testes seem to also be affected in in men with non-fatal COVID-19. In a cross-sectional testicular ultrasonography study, incidental epididymitis was found in 42% of patients with mild-to-moderate COVID-19, all of whom had no scrotal complaints and no clinical signs of orchitis82. In another study evaluating the potential association between COVID-19 and testicular pain or epididymo-orchitis in 91 patients with COVID-19, 11% of patients reported scrotal pain83. These results suggest that COVID-19 can affect testis and epididymis in the acute stage of the infection, although these effects might be clinically inapparent in many instances.
Effects of SARS-CoV-2 infection on sperm quality also seem to be of concern. Results from a study including 23 patients with COVID-19 and 22 age-matched healthy men showed oligozoospermia (mean ratio: 0.29 (95% CI 0.12–0.71, P = 0.008)) and a significant increase in leukocytes (P = 0.006) in semen of patients with COVID-19 compared with the healthy participants group79. In another study, impaired sperm quality in patients with COVID-19 was attributed to recurrent fever81, which has been shown to have a detrimental effect on sperm quality84, potentially owing to the thermal dependence of spermatogenesis85,86. In a prospective, observational study, sperm quality after recovery from COVID-19 was assessed using the WHO criteria. Sperm parameters were evaluated at three different time points after the infection: short (0–31 days, n = 35); intermediate (32–62 days, n = 51); and long (≥63 days, n = 34)87. Mean progressive sperm motility was reduced in 60%, 37% and 28% of men in the short, intermediate and long follow-up time groups, respectively, whereas mean sperm count was reduced in 37%, 29% and 6% of patients in the three groups, respectively87. The authors concluded that the estimated recovery time of sperm quality after COVID-19 infection is 3 months, but further follow-up studies are needed to determine whether a minority of men might suffer from permanent testicular damage87. The severity of COVID-19 infection (for example, the need to be hospitalized) and the presence of fever did not correlate with sperm characteristics in this study87. The effect of COVID-19 on fertility potential in the acute stage of the infection was assessed in a multicentre study including 69 patients aged 20–45 years who previously had COVID-19 but had recovered at least 3 months earlier88. Sperm motility and vitality significantly decreased (P ≤ 0.03) in these patients after COVID-19 infection compared with the values from a spermiogram analysis performed in the same patients within the year before SARS-CoV-2 onset. These results could indicate that the estimated recovery time of sperm quality after COVID-19 infection might be longer than the 3 months previously suggested87. In another study, men recovering from COVID-19 (n = 30, median age: 40 years) showed a lower total sperm number than age-matched healthy men89. In a follow-up analysis (median time: 3 months), the total sperm number in 5 of these 30 men was similar to that reported in the acute phase of COVID-19 (ref.89). No data on the sperm quality in these men before COVID-19 infection are available; therefore, the possibility of a low sperm count before COVID-19 infection exists. In another study, sperm quality was assessed in 74 men aged between 20 and 50 years, who were recovering from COVID-19 (two consecutive negative pharyngeal swab PCR tests were required for inclusion)90. Urine, expressed prostatic secretion and semen were analysed for SARS-CoV-2 detection, and the PCR was negative in all the samples collected in all patients. Semen characteristics, including semen volume, total sperm number, sperm concentration, vitality, progressive motility and total (progressive plus non-progressive) motility, were compared with the WHO reference values (based on data from a fertile population and generated from men whose partners had a time to pregnancy ≤12 months)91. The total sperm count and total sperm motility in patients with COVID-19 were lower than age-matched healthy men (total sperm count: 197 million versus 261 million, P = 0.0211; total motility: 48.9% versus 56.4%, P < 0.0001), but still above the lower reference limit released by the WHO (total sperm count: 39 million per ejaculate (95% CI 33–46), total sperm motility: 40% (95% CI 38-42))90. Furthermore, patients with a recovery time from COVID-19 of ≥90 days had a significantly lower total sperm count than patients who recovered in less than 90 days (152 million versus 224 million, P = 0.0369)90. These results suggest that no substantial long-term threats on male fertility after recovery from COVID-19 exist, but the effect of COVID-19 on fertility might be worse for men with a long recovery time than for men who recover within a short time frame.
COVID-19 infection is also associated with endocrine imbalances92,93. In a cohort study including 286 patients with COVID-19 and 281 healthy individuals, significantly lower levels of total testosterone were found in men with COVID-19 at hospital admission than in healthy participants (2.5 nmol/l versus 10.4 nmol/l, P < 0.0001)92. In detail, hypogonadism (defined as total testosterone <9.2 nmol/l) was observed in 89.8% of patients with COVID-19, and in only 14.9% of healthy individuals92. Most patients (85%), showed secondary hypogonadism (hypogonadotropic hypogonadism, total testosterone <9.2 nmol/l and luteinizing hormone (LH) ≤9.4 mUI/ml)92 (Fig. 3). These results indicate that, although the testes might be heavily affected by a SARS-CoV-2 infection under other circumstances79,81, in these patients, the cause of the imbalances in total testosterone levels lies in the central nervous system, as secondary hypogonadism indicates a problem in the pituitary gland or hypothalamus94. Results from the univariate analysis showed that total testosterone levels were inversely associated with increased risk of ICU admission (OR 0.54, P < 0.0001) or death (OR 0.68, P < 0.002)92. Thus, in these patients, SARS-CoV-2 infection is associated with low total testosterone levels, mostly secondary hypogonadism, and total testosterone correlates with COVID-19 severity92. Male hormone levels vary tremendously through acute illness or stress95; therefore, these results should be interpreted with caution. Moreover, in a prospective cohort study in which patients with COVID-19 (n = 89) were compared with patients with non-COVID-19 respiratory tract infection (n = 30) and age-matched healthy individuals (n = 143), total testosterone levels were decreased in patients with COVID-19, but also in patients with non-COVID-19 respiratory tract infections93, indicating that the observed hormonal imbalances might be a general phenomenon of critical illness, non-specific for SARS-CoV-2 infection95. In another study, the levels of follicle-stimulating hormone (FSH)96, LH, testosterone and oestradiol in 74 men aged between 20 and 50 years who were recovering from COVID-19 were reported to be within the normal range90. However, opposite results were reported from a study including 121 men who recovered from COVID-19: at a 7-month follow-up time, 55% of men still suffered from hypogonadism (total testosterone <9.2 nmol/l)97. Thus, a substantial effect of COVID-19 on the hormonal balance is observed during the acute phase of the infection, but whether this imbalance has long-term repercussions for the total testosterone balance is still unclear90,97.
In a cohort study including 286 patients with coronavirus disease 2019 (COVID-19) and 281 healthy individuals, lower levels of testosterone were found in men with COVID-19 at hospital admission than in healthy participants92. In most patients, secondary hypogonadism was observed (luteinizing hormone ≤9.2 mUI/ml).
Analysis of the effects of COVID-19 infection on male reproductive health through histopathological examinations showed that testicular tissue can be severely damaged by SARS-CoV-2 infection79,81 (Fig. 4). However, the observed reduced sperm quality in patients in the acute stage of COVID-19 could also be attributed to fever84,98, and long-term data on sperm quality after recovery from COVID-19 are still very limited. Regarding hormonal modifications induced by COVID-19, a reduction in testosterone levels can be found regularly in patients with COVID-19 (refs92,93); secondary hypogonadism is the most frequently observed form of hormonal imbalance, indicating a disturbance in the central nervous system, rather than in the testes92. However, contradictory evidence makes it difficult to understand whether or not hormonal imbalance in patients recovering from COVID-19 is only a temporary phenomenon of the acute stage of infection90,97.
In a testicular ultrasonography study, incidental epididymitis was found in 42% of patients with mild-to-moderate coronavirus disease 2019 (COVID-19)82. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can be detected in semen, but this event is rare61,62,63,64,65. Oligozoospermia, reduced sperm motility and sperm vitality, as well as leukocytospermia, are reported frequently in the acute phase of COVID-19 infection79,87,90. Testicular tissue can be severely damaged by SARS-CoV-2 infection, leading to a wide range of pathological changes in autopsy studies79,81. Angiotensin-converting enzyme 2 (ACE2) expression levels in testes are amongst the highest in the body77 and can be found in Sertoli cells, Leydig cells and cells of the seminiferous ducts78.
Testosterone and androgen deprivation therapy
A higher mortality rate for COVID-19 in men than in women has been reported in multiple studies99,100. Moreover, disease severity of COVID-19, measured by hospitalizations and ICU admissions, is higher in men than in women, a phenomenon already observed in previous coronavirus epidemics (SARS-CoV, 2002, and Middle East respiratory syndrome-related coronavirus (MERS), 2012)101. Sex disparities in the observed severity of COVID-19 are most likely multifactorial and could partly be explained by different comorbidities and behaviours101. Men might be more vulnerable to infections than women owing to biological causes (including immunological, hormonal and genetic differences) and a worse overall health status102; the higher prevalence of smoking in men than in women might also predispose to worse COVID-19 outcomes102. Testosterone has also been hypothesized to have a role in sex differences in COVID-19 outcomes, as it regulates ACE2 and TMPRSS2 expression103,104. The link between testosterone and COVID-19 symptoms could also explain why children, who generally exhibit lower expression levels of androgen receptor, show fewer COVID-19 symptoms than adults105. Furthermore, androgen-dependent prostate cancer cells highly express TMPRSS2 (a key mediator in viral invasion20) in response to androgens23. Thus, in the early stages of the pandemic, treatment with androgen deprivation therapy (ADT)32 in men with prostate cancer was hypothesized to be protective against COVID-19 infection106. In a large population-based study including 4,532 men with confirmed SARS-CoV-2 infection, patients with prostate cancer who received ADT were at a significantly lower risk of infection than patients with prostate cancer without ADT (OR 4.05, 95% CI 1.55–10.59, P = 0.0043) or patients with any other malignancy (OR 4.86; 95% CI 1.88–12.56, P = 0.0011)107. The authors attributed the protective effect of ADT to its ability to decrease TMPRSS2 levels108, which has an important role in the fusion process of cellular and viral membranes20, and to a reduction of the risk of a cytokine storm by decreasing the number and function of circulating neutrophils107,109. However, results from another study including 1,779 men with prostate cancer reported opposite findings: no significant differences in the number of SARS-CoV-2 infections were observed between patients treated with ADT (5.6%) and patients who did not receive ADT (5.8%; OR 0.93, 95% CI 0.54–1.61, P = 0.8)110. Risk factors known to increase SARS-CoV-2 infection and disease severity (such as diabetes, asthma or immune suppressive diseases)102,111,112 were equally distributed between the two cohorts, although men receiving ADT were marginally older (75.5 versus 73.8 years, P = 0.009), more likely to have smoked (68.1% versus 59.3%, P = 0.005) and had more likely taken steroids (43.8% versus 23.3%, P < 0.001) than patients with prostate cancer not treated with ADT110. These contradictory findings might be explained by the anti-inflammatory properties of testosterone113,114, which could also explain why men with COVID-19 and high levels of testosterone (≥2.9 ng/ml total testosterone at hospital admission) test positive for SARS-CoV-2 for a shorter time frame than those with total testosterone levels of <2.9 ng/ml (median time to negative PCR test: 26 days versus 18 days; P = 0.002)115. Low total testosterone levels in men have been associated with hyper-inflammatory syndrome and respiratory failure116, and total testosterone levels are inversely associated with increased risk of ICU admission or death92; thus, the (most probably transitory) hypogonadism caused by SARS-CoV-2 infection was suggested to promote severe outcomes92. In the COVIDENZA trial, including men and women >50 years old, the effect of the inhibition of testosterone signalling through enzalutamide on the outcome of hospitalized patients with COVID-19 was evaluated117. Overall, 42 patients were enrolled and randomized 2:1 to receive 5 days of treatment with enzalutamide or standard of care117. Patients treated with enzalutamide required longer hospitalization (hazard ratio for discharge from hospital: 0.43, 95% CI 0.20–0.93) than patients treated with the standard of care117. The trial was terminated early and the authors concluded that androgen inhibition should not be used for prevention or treatment of COVID-19 (ref.117).
In conclusion, evidence suggests that testosterone could be associated with higher COVID-19 disease severity in men than in women102,103,104. However, the exact effect of testosterone on COVID-19 severity and the underlying mechanisms are still unclear, as reflected in the contradictory findings about the effect of ADT on the risk of SARS-CoV-2 infection107,110
Urological thromboembolic complications
Thromboembolism is a frequent and severe complication of COVID-19 (ref.118). A study including 184 patients with COVID-19 pneumonia in the ICU reported a remarkably high incidence of thromboembolic complications in these patients (31%), including deep vein thrombosis or pulmonary embolism119. In a meta-analysis of 49 studies including 18,093 patients with COVID-19, the reported pooled incidence was 17.0% for venous thromboembolism and 7.1% for pulmonary embolism120; the incidence of venous thromboembolism was higher in studies that used systematic screening than in those relying on clinical diagnosis (33.1% versus 9.8%, P < 0.0001)120. However, in a study including 256 patients with COVID-19 pneumonia and 360 patients with non-COVID-19 pneumonia, the rate of venous thromboembolism was not significantly different between the two groups (2% versus 3.6% respectively, P = 0.229), indicating that the observed hypercoagulable state in patients with COVID-19 might not be dependent on SARS-CoV-2 (ref.121). However, the underlying mechanisms of thromboembolism in patients with COVID-19 seem to be different from COVID-19-independent thromboembolism. COVID-19-associated coagulopathy seems to be mediated by excessive inflammation, endothelial activation and injury, platelet activation, impaired or dysfunctional fibrinolysis and systemic hypercoagulability122; vascular endothelial cells are among the primary targets of SARS-CoV-2, and COVID-19 infection can result in endothelial damage and also in systemic vasculitis123. COVID-19-associated coagulopathy is probably a multifactorial combination of low-grade disseminated intravascular coagulation, thrombotic microangiopathy and released pro-inflammatory cytokines such as IL-6, which can induce tissue factor expression on mononuclear cells and subsequently initiate coagulation activation and thrombin generation124. COVID-19-associated coagulopathy is characterized by an isolated elevation of D-dimer (a degradation product from fibrin used as a biomarker of coagulation and fibrinolysis125) and is associated with disease severity and numerous thrombotic complications123. Differently from sepsis-induced disseminated intravascular coagulation, alterations in platelet counts, prothrombin time and partial thromboplastin time are uncommon in the initial presentation of patients with COVID-19-associated coagulopathy126.
Repercussions of thromboembolism in the genitourinary tract of patients with COVID-19 have also been observed, and include a patient with prostate infarction and several patients with renal infarction associated with COVID-19 (refs127,128,129,130,131). Moreover, ischaemia-related priapism was reported as a thromboembolic complication in a 62-year-old patient with COVID-19 (ref.132). However, no clear proof indicating that these thromboembolism effects are directly caused by SARS-CoV-2 is currently available.
In summary, thromboembolism is a frequent complication of COVID-19 (ref.118) and also affects organs of the genitourinary tract127,128,129,130,131,132. The incidence of thromboembolism in patients with COVID-19 is similar to that observed in non-COVID-19 pneumonia121, but the pathophysiological background seems to be completely different123,124,126.
Outlook for COVID-19 pandemic
Variants of concern are steadily replacing the wild-type form of SARS-CoV-2, but vaccination programmes are in place and compete in a neck-and-neck race. People avoiding hospitals during the pandemic might be the cause of decreased overall survival in patients with uro-oncological malignancies, owing to missed diagnoses or delayed treatments.
Variants of concern
COVID-19 variants have the potential to substantially increase mortality. In a retrospective cohort study including 212,326 patients with COVID-19, patients with variants of concern (including Alpha/B1.1.17, Beta/B.1.351 and Gamma/P.1) were at a higher risk of hospitalization (OR 1.52 (95% CI 1.42–1.63)), ICU admission (OR 1.89 (95% CI 1.67–2.17)) and death (OR 1.51 (95% CI 1.30–1.78)) than patients with non-variant of concern SARS-CoV-2 strains133. Patients infected with the Delta variant showed further increased risk of hospitalization (OR 2.08 (95% CI 1.78–2.40), ICU admission (OR 3.35 (95% CI 2.60–4.31)) and death (OR 2.33 (95% CI 1.54–3.31))133. However, mounting evidence suggests that infection with the Omicron variant of SARS-CoV-2 (lineage B.1.1.529) causes a milder disease phenotype than previous variants of concern134,135. The effects of the new COVID-19 variants on the risk of developing urological complications in patients with COVID-19 still need to be assessed.
Vaccines
Vaccines for COVID-19 are considered an essential part of the strategy for ending the pandemic136, but one major reason for COVID-19 vaccine refusal is anxiety about adverse effects137,138. Regarding urology, no evidence of male or female fertility reduction following COVID-19 vaccination has been reported139. Results from an analysis of the FDA Vaccine Adverse Event Reporting System showed that <1% of vaccinated people described urological symptoms140. Many of the reported symptoms of COVID-19 vaccination (LUTS, haematuria and urinary infection) have the same prevalence in vaccinated and unvaccinated individuals; thus, a causal attribution to the vaccination seems unlikely140. In a study analysing sperm parameters before and after COVID-19 mRNA vaccination in 45 men (median age: 28 years) receiving BNT162b2 (Pfizer-BioNTech, 46.7%) or mRNA-1273 (Moderna, 53.3%), no substantial decrease in any sperm parameter was reported141. Another reason for vaccine refusal is a misunderstanding about vaccine efficacy. Results from a study including three online behavioural experiments involving 1,800 participants showed that vaccine efficacy is constantly confused with the non-incidence rate of COVID-19 in vaccinated people, which leads to an overestimation of the probability of developing COVID-19 (ref.142). The investigators suggested that educational programmes are urgently needed to increase the understanding of vaccine safety and efficacy, which would lead to increased vaccination campaign adherence.
Effect of COVID-19 on survival in uro-oncology
Patients with medical emergencies often avoid hospital emergency departments because of the fear of COVID-19 contagion143. Urologists are also noticing a delay in consultation in some instances144, which could lead to increased morbidity and mortality, particularly in the field of oncology, in which the effect of missed diagnoses and delayed treatments might be devastating145. In the UK, 45% of patients with potential cancer symptoms (such as coughing up blood) did not contact their doctor in the first wave of the pandemic146. In urology, a dramatic decline in routine prostate cancer screening was observed147,148,149,150. A median decrease of 62% in requested total PSA tests was observed during the local lockdown period in Italy (10 March to 17 May 2020)147. Results from another study in Italy showed a noticeable decline in cancer diagnoses during the COVID-19 pandemic in 2020, compared with the same period in the previous 2 years, with the greatest decrease observed in prostate cancer and bladder cancer diagnoses (75% and 66%, respectively)148. In a cancer screening study from the USA including 192,060 patients, the decrease in PSA testing during the first peak of the pandemic (2 March to 2 June 2020) was ≥60% compared with three periods of time before and after the peak149. In Sweden, the number of men diagnosed with prostate cancer decreased by one-third in spring 2020 compared with previous years, but no reduction in the number of curative treatments was observed150. A severe increase in the number of avoidable cancer deaths owing to COVID-19-induced lockdowns is still expected151.
End of the pandemic
The perspective of when the COVID-19 pandemic will end is mainly influenced by two factors: the progress of vaccine programmes and the spread of variants of concern152. The spread of the Delta variant caused a set-back for high-income countries in the race to reach herd immunity152,153. However, the Omicron variant induces a considerably milder disease at the population level and has been shown to trigger cross-protective immunity towards the Delta variant135. The transition of COVID-19 to an endemic disease might be a more realistic end point than herd immunity152, but countless unpredictable variables make any serious prediction impossible. COVID-19 might truly become endemic only when most adults have developed natural immunity by having been exposed to the virus multiple times as children154. This process might take decades; thus, until this point, a judgement call on how vulnerable people can be protected and how many deaths society is willing to tolerate is needed154. Vaccine development and also research into the mechanisms and effects of SARS-CoV-2 infection in different organs are crucial to get a realistic chance of managing this disease efficiently, thereby bringing the pandemic to an end.
Conclusions
In March 2020, the WHO declared the COVID-19 outbreak a pandemic. Since then, a considerable number of studies describing the effects of SARS-CoV-2 infection in different organs have been published. Urology soon emerged as a focus of interest, owing to a potential threat of long-term damage induced by COVID-19 to kidney function, fertility and hormonal balance. ACE2 expression in different tissues is hypothesized to correlate with the risk of viral invasion, and results from several studies reported high expression levels of ACE2 in the genitourinary tract. AKI is a severe urological complication of COVID-19 accompanied by high mortality, which is hypothesized to be caused by a cytokine-storm-induced systemic inflammatory response and direct cytopathic effects. The detection of SARS-CoV-2 in urine and semen is very rare; however, a possible risk of transmission through these body fluids has not yet been ruled out. Viral orchitides are accepted to lead to a reduction in fertility and endocrine function; therefore, the effects of SARS-CoV-2 on male fertility seem to be a main focus of interest. Results from autopsy studies on patients with COVID-19 revealed severe structural changes in testes, and, in several studies on semen samples, an impairment of spermatogenesis was reported in patients with COVID-19. However, these effects might also be attributed to fever, and long-term data on sperm quality after recovery from COVID-19 are still very limited. Low levels of testosterone were also detected frequently in men with COVID-19, and, in most instances, hypogonadism was secondary. An intense discussion on the role of testosterone in the high prevalence of severe COVID-19 complications is in place. Low total testosterone levels are associated with poor outcomes; however, whether hypogonadism is caused by SARS-CoV-2 infection or is just a general phenomenon of critical illness is still unclear. Thromboembolic complications, which are frequent in COVID-19, also affect urogenital organs.
Long-term effects of COVID-19 cannot yet be fully evaluated, but understanding how SARS-CoV-2 causes injury in genitourinary organs and defining risk factors associated with severe damage of these organs is crucial for developing strategies to protect patients with COVID-19 from urological complications. To date, high-quality evidence of these pathophysiological processes in the urogenital system is limited. Published studies on the effect of COVID-19 on the urogenital tract often have a small sample size and, in some cases, reported heterogeneous results. Moreover, many urological symptoms overlap with common diseases and, therefore, a causal attribution to SARS-CoV-2 is difficult to prove. Some clinical manifestations might not be directly caused by SARS-CoV-2, but could be secondary effects induced by an excessive immune response or cardiorespiratory impairment; thus, further exploration with prospective studies and large cohorts is urgently needed.
In summary, published data made one point clear: COVID-19 is also a urological matter (Fig. 5) and urologists must learn to detect and manage short-term and long-term damage to the genitourinary tract caused by COVID-19 for the benefit of patients.
ACE2, angiotensin-converting enzyme 2; AKI, acute kidney injury; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SIRS, systemic inflammatory response syndrome.
References
Bogoch, I. I. et al. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J. Travel. Med. https://doi.org/10.1093/jtm/taaa008 (2020).
Gorbalenya, A. E. et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5, 536–544 (2020).
WHO. Novel Coronavirus(2019-nCoV) Situation Report — 22. WHO https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2 (2020).
WHO. Virtual press conference on COVID-19 — 11 March 2020 (WHO, 2020).
Gupta, A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26, 1017–1032 (2020).
Calabrese, F. et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 477, 359–372 (2020).
Maiese, A. et al. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci. Med. Pathol. 17, 279–296 (2021).
Deidda, S. et al. Gastrointestinal coronavirus disease 2019: epidemiology, clinical features, pathogenesis, prevention, and management. Expert. Rev. Gastroenterol. Hepatol. 15, 41–50 (2021).
Amin, M. COVID-19 and the liver: overview. Eur. J. Gastroenterol. Hepatol. 33, 309–311 (2021).
Elhence, A. et al. Coronavirus disease-2019 (COVID-19) and the liver. J. Clin. Transl. Hepatol. 9, 247–255 (2021).
de-Madaria, E. & Capurso, G. COVID-19 and acute pancreatitis: examining the causality. Nat. Rev. Gastroenterol. Hepatol. 18, 3–4 (2021).
Liu, J., Deswal, A. & Khalid, U. COVID-19 myocarditis and long-term heart failure sequelae. Curr. Opin. Cardiol. 36, 234–240 (2021).
Garg, R. K., Paliwal, V. K. & Gupta, A. Encephalopathy in patients with COVID-19: a review. J. Med. Virol. 93, 206–222 (2021).
European Observatory on Health Systems and Policies. In the wake of the pandemic: Preparing for Long COVID (World Health Organization, 2021).
Michelen, M. et al. Characterising long COVID: a living systematic review. BMJ Glob. Health https://doi.org/10.1136/bmjgh-2021-005427 (2021).
Dennis, A. et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 11, e048391 (2021).
Zou, X. et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 14, 185–192 (2020).
Ren, X. et al. Multiple expression assessments of ACE2 and TMPRSS2 SARS-CoV-2 entry molecules in the urinary tract and their associations with clinical manifestations of COVID-19. Infect. Drug Resist. 13, 3977–3990 (2020).
Jackson, C. B., Farzan, M., Chen, B. & Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 23, 3–20 (2022).
Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280 e278 (2020).
Stopsack, K. H., Mucci, L. A., Antonarakis, E. S., Nelson, P. S. & Kantoff, P. W. TMPRSS2 and COVID-19: serendipity or opportunity forintervention? Cancer Discov. 10, 779–782 (2020).
Afshari, A., Janfeshan, S., Yaghobi, R., Roozbeh, J. & Azarpira, N. Covid-19 pathogenesis in prostatic cancer and TMPRSS2-ERG regulatory genetic pathway. Infect. Genet. Evol. 88, 104669 (2021).
Lin, B. et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 59, 4180–4184 (1999).
Lucas, J. M. et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 4, 1310–1325 (2014).
Beyerstedt, S., Casaro, E. B. & Rangel, É.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 40, 905–919 (2021).
Gressens, S. B. et al. Controversial roles of the renin angiotensin system and its modulators during the COVID-19 pandemic. Front. Physiol. 12, 624052 (2021).
Hamming, I. et al. The emerging role of ACE2 in physiology and disease. J. Pathol. 212, 1–11 (2007).
Fountain, J. H. & Lappin, S. L. Physiology, Renin Angiotensin System. in StatPearls [Internet] (StatPearls, 2022).
AlGhatrif, M., Cingolani, O. & Lakatta, E. G. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol. 5, 747–748 (2020).
Coto, E., Avanzas, P. & Gómez, J. The renin-angiotensin-aldosterone system and coronavirus disease 2019. Eur. Cardiol. 16, e07 (2021).
Farouk, S. S., Fiaccadori, E., Cravedi, P. & Campbell, K. N. COVID-19 and the kidney: what we think we know so far and what we don’t. J. Nephrol. 33, 1213–1218 (2020).
Chan, L. et al. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol. 32, 151–160 (2021).
Drake, T. M. et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet 398, 223–237 (2021).
Sullivan, M. K. et al. Acute kidney injury in patients hospitalized with COVID-19 from the ISARIC WHO CCP-UK Study: a prospective, multicentre cohort study. Nephrol. Dialysis Transplant. 37, 271–284 (2021).
Cheng, Y. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 97, 829–838 (2020).
Nadim, M. K. et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 16, 747–764 (2020).
Hirsch, J. S. et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 98, 209–218 (2020).
Finelli, L. et al. Mortality among US patients hospitalized with SARS-CoV-2 infection in 2020. JAMA Netw. Open. 4, e216556–e216556 (2021).
Enikeev, D. et al. Acute kidney injury in COVID-19: are kidneys the target or just collateral damage? A comprehensive assessment of viral RNA and AKI rate in patients with COVID-19. Curr. Opin. Urol. https://doi.org/10.1097/mou.0000000000000901 (2021).
Mohamed, N. E. et al. Association between chronic kidney disease and COVID-19-related mortality in New York. World J. Urol. 39, 2987–2993 (2021).
Kudose, S. et al. Kidney biopsy findings in patients with COVID-19. J. Am. Soc. Nephrol. 31, 1959–1968 (2020).
Santoriello, D. et al. Postmortem kidney pathology findings in patients with COVID-19. J. Am. Soc. Nephrol. 31, 2158–2167 (2020).
Basiratnia, M., Derakhshan, D., Yeganeh, B. S. & Derakhshan, A. Acute necrotizing glomerulonephritis associated with COVID-19 infection: report of two pediatric cases. Pediatr. Nephrol. 36, 1019–1023 (2021).
May, R. M. et al. A multi-center retrospective cohort study defines the spectrum of kidney pathology in coronavirus 2019 disease (COVID-19). Kidney Int. https://doi.org/10.1016/j.kint.2021.07.015 (2021).
Friedman, D. J. & Pollak, M. R. APOL1 and kidney disease: from genetics to biology. Annu. Rev. Physiol. 82, 323–342 (2020).
Sharma, P., Ng, J. H., Bijol, V., Jhaveri, K. D. & Wanchoo, R. Pathology of COVID-19-associated acute kidney injury. Clin. Kidney J. 14, i30–i39 (2021).
Fajgenbaum, D. C. & June, C. H. Cytokine storm. N. Engl. J. Med. 383, 2255–2273 (2020).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Ye, Q., Wang, B. & Mao, J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J. Infect. 80, 607–613 (2020).
Ragab, D., Salah Eldin, H., Taeimah, M., Khattab, R. & Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 11, 1446 (2020).
Suriano, F. et al. Bacteriuria in patients with an orthotopic ileal neobladder: urinary tract infection or asymptomatic bacteriuria? BJU Int. 101, 1576–1579 (2008).
Kleymenov, D. A. et al. A deep look into COVID-19 severity through dynamic changes in blood cytokine levels. Front. Immunol. https://doi.org/10.3389/fimmu.2021.771609 (2021).
Fan, C., Lu, W., Li, K., Ding, Y. & Wang, J. ACE2 expression in kidney and testis may cause kidney and testis infection in COVID-19 patients. Front. Med. 7, 563893 (2020).
Song, J. et al. Expression profiles revealed potential kidney injury caused by SARS-CoV-2: a systematic analysis of ACE2 and clinical lessons learned from this discovery. Aging https://doi.org/10.18632/aging.202224 (2020).
Pan, X. W. et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 46, 1114–1116 (2020).
Wang, K. et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal. Transduct. Target. Ther. 5, 283 (2020).
Liu, C., von Brunn, A. & Zhu, D. Cyclophilin A and CD147: novel therapeutic targets for the treatment of COVID-19. Med. Drug Discov. 7, 100056 (2020).
Kashi, A. H. et al. Urinary viral shedding of COVID-19 and its clinical associations: a systematic review and meta-analysis of observational studies. Urol. J. 17, 433–441 (2020).
Sun, M. et al. Anal swab as a potentially optimal specimen for SARS-CoV-2 detection to evaluate hospital discharge of COVID-19 patients. Future Microbiol. 15, 1101–1107 (2020).
Spirito, L. et al. No detection of SARS-CoV-2 RNA on urethral swab in patients with positive nasopharyngeal swab. Adv. Virol. 2020, 8826943 (2020).
Gonzalez, D. C. et al. A systematic review on the investigation of SARS-CoV-2 in semen. Res. Rep. Urol. 12, 615–621 (2020).
Machado, B. et al. Presence of SARS-CoV-2 RNA in semen — cohort study in the United States COVID-19 positive patients. Infect. Dis. Rep. 13, 96–101 (2021).
Li, D., Jin, M., Bao, P., Zhao, W. & Zhang, S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw. Open 3, e208292 (2020).
Gacci, M. et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum. Reprod. 36, 1520–1529 (2021).
Purpura, L. J. et al. SARS-CoV-2 RNA shedding in semen and oligozoospermia of patient with severe coronavirus disease 11 weeks after infection. Emerg. Infect. Dis. 28, 196–200 (2022).
Tur-Kaspa, I., Tur-Kaspa, T., Hildebrand, G. & Cohen, D. COVID-19 may affect male fertility but is not sexually transmitted: a systematic review. F. S Rev. 2, 140–149 (2021).
Mumm, J. N. et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur. Urol. 78, 624–628 (2020).
Dhar, N. et al. De novo urinary symptoms associated with COVID-19: COVID-19-associated cystitis. J. Clin. Med. Res. 12, 681–682 (2020).
Barry, M. J. et al. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. J. Urol. 197, S189–s197 (2017).
Can, O., Erkoç, M., Ozer, M., Umeyir Karakanli, M. & Otunctemur, A. The effect of COVID-19 on lower urinary tract symptoms in elderly men. Int. J. Clin. Pract. https://doi.org/10.1111/ijcp.14110 (2021).
Sighinolfi, M. C., Rocco, B. & Mussini, C. COVID-19: importance of the awareness of the clinical syndrome by urologists. Eur. Urol. 78, e40–e41 (2020).
Lamb, L. E. et al. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC). Med. Hypotheses 145, 110375 (2020).
Hedges, S. & Svanborg, C. The mucosal cytokine response to urinary tract infections. Int. J. Antimicrob. Agents 4, 89–93 (1994).
Liu, W., Han, R., Wu, H. & Han, D. Viral threat to male fertility. Andrologia 50, e13140 (2018).
Dejucq, N. & Jégou, B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol. Mol. Biol. Rev. 65, 208–231 (2001).
Luppi, F., Arpino, B. & Rosina, A. The impact of COVID-19 on fertility plans in Italy, Germany, France, Spain and UK. Demogr. Res. 43, 1399–1412 (2020).
Zhang, J. et al. Bioinformatic and mouse model reveal the potential high vulnerability of Leydig cells on SARS-CoV-2. Ann. Transl. Med. 9, 678 (2021).
Liu, X. et al. Single-cell transcriptome analysis of the novel coronavirus (SARS-CoV-2) associated gene ACE2 expression in normal and non-obstructive azoospermia (NOA) human male testes. Sci. China Life Sci. 63, 1006–1015 (2020).
Li, H. et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 28, 100604 (2020).
Achua, J. K. et al. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J. Mens. Health 39, 65–74 (2021).
Flaifel, A. et al. Testicular changes associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Arch. Pathol. Lab. Med. 145, 8–9 (2021).
Carneiro, F. et al. Radiological patterns of incidental epididymitis in mild-to-moderate COVID-19 patients revealed by colour Doppler ultrasound. Andrologia 53, e13973 (2021).
Ediz, C. et al. Is there any association of COVID-19 with testicular pain and epididymo-orchitis? Int. J. Clin. Pract. 75, e13753 (2020).
Carlsen, E., Andersson, A. M., Petersen, J. H. & Skakkebaek, N. E. History of febrile illness and variation in semen quality. Hum. Reprod. 18, 2089–2092 (2003).
Sergerie, M., Mieusset, R., Croute, F., Daudin, M. & Bujan, L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil. Steril. 88, 970.e971–977 (2007).
Mieusset, R. & Bujan, L. The potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Int. J. Androl. 17, 186–191 (1994).
Donders, G. G. G. et al. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil. Steril. 117, 287–296 (2022).
Erbay, G. et al. Short-term effects of COVID-19 on semen parameters: a multicenter study of 69 cases. Andrology https://doi.org/10.1111/andr.13019 (2021).
Best, J. C. et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J. Mens. Health 39, 489–495 (2021).
Ruan, Y. et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology 9, 99–106 (2021).
Cooper, T. G. et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 16, 231–245 (2010).
Salonia, A. et al. Severely low testosterone in males with COVID-19: a case-control study. Andrology https://doi.org/10.1111/andr.12993 (2021).
Kadihasanoglu, M., Aktas, S., Yardimci, E., Aral, H. & Kadioglu, A. SARS-CoV-2 pneumonia affects male reproductive hormone levels: a prospective, cohort study. J. Sex. Med. 18, 256–264 (2021).
Seftel, A. Male hypogonadism. Part II: etiology, pathophysiology, and diagnosis. Int. J. Impot. Res. 18, 223–228 (2006).
Spratt, D. I. Altered gonadal steroidogenesis in critical illness: is treatment with anabolic steroids indicated? Best. Pract. Res. Clin. Endocrinol. Metab. 15, 479–494 (2001).
Lifshitz, E. & Kramer, L. Outpatient urine culture: does collection technique matter? Arch. Intern. Med. 160, 2537–2540 (2000).
Salonia, A. et al. Testosterone in males with COVID-19: a 7-month cohort study. Andrology 10, 34–41 (2022).
Temiz, M. Z. et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia 53, e13912 (2021).
Kelada, M., Anto, A., Dave, K. & Saleh, S. N. The role of sex in the risk of mortality from COVID-19 amongst adult patients: a systematic review. Cureus 12, e10114 (2020).
Vahidy, F. S. et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLoS ONE 16, e0245556 (2021).
Alwani, M. et al. Sex-based differences in severity and mortality in COVID-19. Rev. Med. Virol. https://doi.org/10.1002/rmv.2223 (2021).
Tharakan, T. et al. Are sex disparities in COVID-19 a predictable outcome of failing men’s health provision? Nat. Rev. Urol. 19, 47–63 (2022).
Samuel, R. M. et al. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell 27, 876–889.e812 (2020).
Qiao, Y. et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and MPRSS2. Proc. Natl Acad. Sci. USA 118, e2021450118 (2021).
Mjaess, G., Karam, A., Aoun, F., Albisinni, S. & Roumeguère, T. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog. Urol. 30, 484–487 (2020).
Bhowmick, N. A. et al. COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr. Relat. Cancer 27, R281–r292 (2020).
Montopoli, M. et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N=4532). Ann. Oncol. 31, 1040–1045 (2020).
Shaw, G. L. et al. The early effects of rapid androgen deprivation on human prostate cancer. Eur. Urol. 70, 214–218 (2016).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Klein, E. A. et al. Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2. J. Urol. 205, 441–443 (2021).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020).
Baek, M. S., Lee, M.-T., Kim, W.-Y., Choi, J. C. & Jung, S.-Y. COVID-19-related outcomes in immunocompromised patients: a nationwide study in Korea. PLoS ONE 16, e0257641 (2021).
Traish, A., Bolanos, J., Nair, S., Saad, F. & Morgentaler, A. Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. J. Clin. Med. https://doi.org/10.3390/jcm7120549 (2018).
Foo, Y. Z., Nakagawa, S., Rhodes, G. & Simmons, L. W. The effects of sex hormones on immune function: a meta-analysis. Biol. Rev. Camb. Philos. Soc. 92, 551–571 (2017).
Salciccia, S. et al. Modeling the contribution of male testosterone levels to the duration of positive COVID testing among hospitalized male COVID-19 patients. Diagnostics https://doi.org/10.3390/diagnostics11040581 (2021).
Camici, M. et al. Role of testosterone in SARS-CoV-2 infection: a key pathogenic factor and a biomarker for severe pneumonia. Int. J. Infect. Dis. 108, 244–251 (2021).
Welén, K. et al. A phase 2 trial of the effect of antiandrogen therapy on COVID-19 outcome: no evidence of benefit, supported by epidemiology and in vitro data. Eur. Urol. https://doi.org/10.1016/j.eururo.2021.12.013 (2021).
Kollias, A. et al. Venous thromboembolism in COVID-19: a systematic review and meta-analysis. Vasc. Med. 26, 415–425 (2021).
Klok, F. A. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 191, 145–147 (2020).
Jiménez, D. et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest 159, 1182–1196 (2021).
Mei, F. et al. Comparison of venous thromboembolism risks between COVID-19 pneumonia and community-acquired pneumonia patients. Arterioscler. Thromb. Vasc. Biol. 40, 2332–2337 (2020).
Gorog, D. A. et al. Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-021-00665-7 (2022).
Iba, T., Connors, J. M. & Levy, J. H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 69, 1181–1189 (2020).
Levi, M., Thachil, J., Iba, T. & Levy, J. H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 7, e438–e440 (2020).
Krychtiuk, K. A. et al. Biomarkers of coagulation and fibrinolysis in acute myocardial infarction: a joint position paper of the Association for Acute CardioVascular Care and the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J. Acute Cardiovasc. Care 10, 343–355 (2020).
Connors, J. M. & Levy, J. H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 135, 2033–2040 (2020).
Duarte, S. A. C., Pereira, J. G., Iscaife, A., Leite, K. R. M. & Antunes, A. A. Is prostate infarction and acute urinary retention a possible complication of severe COVID-19 infection? Pathology 52, 818–821 (2020).
Post, A. et al. Kidney infarction in patients with COVID-19. Am. J. Kidney Dis. 76, 431–435 (2020).
Mukherjee, A., Ghosh, R. & Furment, M. M. Case report: COVID-19 associated renal infarction and ascending aortic thrombosis. Am. J. Trop. Med. Hyg. 103, 1989–1992 (2020).
Ammous, A., Ghaffar, M. A., El-Charabaty, E. & El-Sayegh, S. Renal infarction in COVID-19 patient. J. Nephrol. 34, 267–268 (2021).
Añazco, P. H., Balta, F. M. & Córdova-Cueva, L. Bilateral renal infarction in a patient with severe COVID-19 infection. J. Bras. Nefrol. 43, 127–131 (2021).
Lamamri, M. et al. Priapism in a patient with coronavirus disease 2019 (COVID-19). Am. J. Emerg. Med. 39, 251.e255–251.e257 (2021).
Fisman, D. N. & Tuite, A. R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. CMAJ 193, E1619–e1625 (2021).
Kozlov, M. Omicron’s feeble attack on the lungs could make it less dangerous. Nature 601, 177 (2022).
Sigal, A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat. Rev. Immunol. 22, 69–71 (2022).
Tregoning, J. S. et al. Vaccines for COVID-19. Clin. Exp. Immunol. 202, 162–192 (2020).
Yigit, M., Ozkaya-Parlakay, A. & Senel, E. Evaluation of COVID-19 vaccine refusal in parents. Pediatr. Infect. Dis. J. 40, e134–e136 (2021).
Troiano, G. & Nardi, A. Vaccine hesitancy in the era of COVID-19. Public Health 194, 245–251 (2021).
Iacobucci, G. Covid-19: no evidence that vaccines can affect fertility, says new guidance. BMJ 372, n509 (2021).
Zhao, H., Souders, C., Carmel, M. & Anger, J. T. Low rates of urologic side effects following COVID vaccination: an analysis of the FDA vaccine adverse event reporting system. Urology https://doi.org/10.1016/j.urology.2021.04.002 (2021).
Gonzalez, D. C. et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA https://doi.org/10.1001/jama.2021.9976 (2021).
Tentori, K., Passerini, A., Timberlake, B. & Pighin, S. The misunderstanding of vaccine efficacy. Soc. Sci. Med. 289, 114273 (2021).
Wong, L. E. et al. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catal. Innov. Care Deliv. https://doi.org/10.1056/CAT.1020.0193 (2020).
Sánchez-González, J. V. et al. The invisible impact of COVID-19: indirect mortality in urology. Minerva Urol. Nephrol. 73, 132–133 (2021).
The Lancet Oncology. COVID-19 and cancer: 1 year on. Lancet Oncol. 22, 411 (2021).
Quinn-Scoggins, H. D. et al. Cancer symptom experience and help-seeking behaviour during the COVID-19 pandemic in the UK: a cross-sectional population survey. BMJ Open 11, e053095 (2021).
Ferrari, A., Sanchis-Gomar, F., Mattiuzzi, C., Henry, B. M. & Lippi, G. Is COVID-19 impacting prostate cancer screening? A survey of prostate-specific antigen test requests during a local outbreak. EJIFCC 32, 69–77 (2021).
De Vincentiis, L., Carr, R. A., Mariani, M. P. & Ferrara, G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018–2019: an audit study from cellular pathology. J. Clin. Pathol. 74, 187–189 (2021).
Bakouny, Z. et al. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 7, 458–460 (2021).
Fallara, G. et al. Prostate cancer diagnosis, staging, and treatment in Sweden during the first phase of the COVID-19 pandemic. Scand. J. Urol. 55, 184–191 (2021).
Maringe, C. et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 21, 1023–1034 (2020).
Charumilind, S. et al. When will the COVID-19 Pandemic End? McKinsey & Company https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/when-will-the-covid-19-pandemic-end (2021).
Aschwanden, C. Five reasons why COVID herd immunity is probably impossible. Nature 591, 520–522 (2021).
Adam, D. Will Omicron end the pandemic? Here’s what experts say. Nature 602, 20–21 (2022).
Author information
Authors and Affiliations
Contributions
B.E., G.M., Y.V. and J.-N.M. researched data for the article. B.E. and G.M. wrote the article. Y.V., G.M. and C.G.S. made a substantial contribution to discussion of the content. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Nature Reviews Urology thanks A. Kadioglu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebner, B., Volz, Y., Mumm, JN. et al. The COVID-19 pandemic — what have urologists learned?. Nat Rev Urol 19, 344–356 (2022). https://doi.org/10.1038/s41585-022-00586-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00586-1
This article is cited by
-
Rapid assays of SARS-CoV-2 virus and noble biosensors by nanomaterials
Nano Convergence (2024)
-
COVID-associated cystitis: the culprit behind the bladder woes post-COVID infection? A review
International Urology and Nephrology (2023)
-
A cross-sectional study on the mental health of patients with COVID-19 1 year after discharge in Huanggang, China
European Archives of Psychiatry and Clinical Neuroscience (2023)