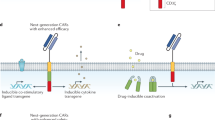
Abstract
Therapeutic gene manipulation has been at the forefront of popular scientific discussion and basic and clinical research for decades. Basic and clinical research applications of CRISPR–Cas9-based technologies and ongoing clinical trials in this area have demonstrated the potential of genome editing to cure human disease. Evaluation of research and clinical trials in gene therapy reveals a concentration of activity in prostate cancer research and practice. Multiple aspects of prostate cancer care — including anatomical considerations that enable direct tumour injections and sampling, the availability of preclinical immune-competent models and the delineation of tumour-related antigens that might provide targets for an induced immune system — make gene therapy an appealing treatment option for this common malignancy. Vaccine-based therapies that induce an immune response and new technologies exploiting CRISPR–Cas9-assisted approaches, including chimeric antigen receptor (CAR) T cell therapies, are very promising and are currently under investigation both in the laboratory and in the clinic. Although laboratory and preclinical advances have, thus far, not led to oncologically relevant outcomes in the clinic, future studies offer great promise for gene therapy to become established in prostate cancer care.
Key points
-
Prostate cancer gene therapy research and clinical trials established a foundation and path forwards for future advances in the field.
-
Gene therapy strategies with potential applications in patients with prostate cancer include direct injection into a prostate tumour or systemic administration (vaccination).
-
Preclinical data on both strategies are promising, but large clinical trials have failed to demonstrate oncological benefit in patients.
-
Further work, for example, modified vaccination strategies and the use of the CRISPR–Cas9 system, offers hope for future applications of gene therapy approaches in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hacein-Bey-Abina, S. et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 (2003).
High, K. A. & Roncarolo, M. G. Gene therapy. N. Engl. J. Med. 381, 455–464 (2019).
Stratton, M. R., Campbell, P. J. & Futreal, P. A. The cancer genome. Nature 458, 719–724 (2009).
Catalona, W. J. et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N. Engl. J. Med. 324, 1156–1161 (1991).
Cooperberg, M. R., Broering, J. M. & Carroll, P. R. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J. Natl Cancer Inst. 101, 878–887 (2009).
Herman, J. R. et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum. Gene Ther. 10, 1239–1249 (1999).
Miles, B. J. et al. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum. Gene Ther. 12, 1955–1967 (2001).
Shalev, M. et al. Gene therapy for prostate cancer. Urology 57, 8–16 (2001).
Thompson, T. C. et al. In situ gene therapy for prostate cancer: immunomodulatory approaches. Expert. Opin. Biol. Ther. 1, 481–495 (2001).
Stewart, R. A., Pilié, P. G. & Yap, T. A. Development of PARP and immune-checkpoint inhibitor combinations. Cancer Res. 78, 6717–6725 (2018).
Pilié, P. G. et al. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 16, 81–104 (2019).
Pilié, P. G. et al. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin. Cancer Res. 25, 3759–3771 (2019).
Akagi, K. et al. RTCGD: retroviral tagged cancer gene database. Nucleic Acids Res. 32, D523–D527 (2004).
Sadelain, M., Papapetrou, E. P. & Bushman, F. D. Safe harbours for the integration of new DNA in the human genome. Nat. Rev. Cancer 12, 51–58 (2011).
Huber, P. E. & Pfisterer, P. In vitro and in vivo transfection of plasmid DNA in the Dunning prostate tumor R3327-AT1 is enhanced by focused ultrasound. Gene Ther. 7, 1516–1525 (2000).
Wolff, J. A. & Budker, V. The mechanism of naked DNA uptake and expression. Adv. Genet. 54, 3–20 (2005).
Yin, H. et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555 (2014).
Kotterman, M. A., Chalberg, T. W. & Schaffer, D. V. Viral vectors for gene therapy: translational and clinical outlook. Annu. Rev. Biomed. Eng. 17, 63–89 (2015).
Peng, W. et al. Nanoparticulate delivery of suicide DNA to murine prostate and prostate tumors. Prostate 67, 855–862 (2007).
Felgner, P. L. et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl Acad. Sci. USA 84, 7413–7417 (1987).
Hattori, Y. & Maitani, Y. Folate-linked nanoparticle-mediated suicide gene therapy in human prostate cancer and nasopharyngeal cancer with herpes simplex virus thymidine kinase. Cancer Gene Ther. 12, 796–809 (2005).
Samad, A., Sultana, Y. & Aqil, M. Liposomal drug delivery systems: an update review. Curr. Drug. Deliv. 4, 297–305 (2007).
Xu, L. et al. Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum. Gene Ther. 13, 469–481 (2002).
Samad, A., Sultana, Y. & Aqil, M. Liposomal drug delivery systems: an update review. Curr. Drug. Deliv. 4, 297–305 (2007).
Dorraj, G. et al. Lipid nanoparticles as potential gene therapeutic delivery systems for oral administration. Curr. Gene Ther. 17, 89–104 (2017).
Tila, D. et al. Functional liposomes in the cancer-targeted drug delivery. J. Biomater. Appl. 30, 3–16 (2015).
Galanis, E. et al. Immunotherapy of advanced malignancy by direct gene transfer of an interleukin-2 DNA/DMRIE/DOPE lipid complex: phase I/II experience. J. Clin. Oncol. 17, 3313–3323 (1999).
Lu, Y. et al. Peptide T7-modified polypeptide with disulfide bonds for targeted delivery of plasmid DNA for gene therapy of prostate cancer. Int. J. Nanomed. 13, 6913–6927 (2018).
Fournier, P. G. J. et al. The TGF-β signaling regulator PMEPA1 suppresses prostate cancer metastases to bone. Cancer Cell 27, 809–821 (2015).
Darquet, A. M. et al. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther. 4, 1341–1349 (1997).
Wang, T., Chen, Y. & Ronald, J. A. A novel approach for assessment of prostate cancer aggressiveness using survivin-driven tumour-activatable minicircles. Gene Ther. 26, 177–186 (2019).
Chen, Z.-Y. et al. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol. Ther. 8, 495–500 (2003).
Chen, Z.-Y. et al. Silencing of episomal transgene expression in liver by plasmid bacterial backbone DNA is independent of CpG methylation. Mol. Ther. 16, 548–556 (2008).
Ambrosini, G., Adida, C. & Altieri, D. C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 3, 917–921 (1997).
Eslami, M. et al. Evaluation of survivin expression in prostate specimens of patients with prostate adenocarcinoma and benign prostate hyperplasia underwent transurethral resection of the prostate or prostatectomy. SpringerPlus 5, 621 (2016).
Shariat, S. F. et al. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer 100, 751–757 (2004).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Su, Y.-L. et al. STAT3 in tumor-associated myeloid cells: multitasking to disrupt immunity. Int. J. Mol. Sci. 19, 1803 (2018).
Chen, Y. H., Keiser, M. S. & Davidson, B. L. Viral vectors for gene transfer. Curr. Protoc. Mouse Biol. 8, e58 (2018).
Warnock, J. N., Daigre, C. & Al-Rubeai, M. Introduction to viral vectors. Methods Mol. Biol. 737, 1–25 (2011).
Harrington, K. J. et al. Gene therapy for prostate cancer: current status and future prospects. J. Urol. 166, 1220–1233 (2001).
Howe, S. J. et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 118, 3143–3150 (2008).
Braun, C. J. et al. Gene therapy for Wiskott-Aldrich syndrome — long-term efficacy and genotoxicity. Sci. Transl. Med. 6, 227ra33 (2014).
Ott, M. G. et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 12, 401–409 (2006).
Hay, R. T. The origin of adenovirus DNA replication: minimal DNA sequence requirement in vivo. EMBO J. 4, 421–426 (1985).
Berkner, K. L. Development of adenovirus vectors for the expression of heterologous genes. BioTechniques 6, 616–629 (1988).
Kovesdi, I. et al. Adenoviral vectors for gene transfer. Curr. Opin. Biotechnol. 8, 583–589 (1997).
Gonçalves, M. A. F. V. Adeno-associated virus: from defective virus to effective vector. Virol. J. 2, 43 (2005).
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 (2006).
Vieweg, J. et al. Efficient gene transfer with adeno-associated virus-based plasmids complexed to cationic liposomes for gene therapy of human prostate cancer. Cancer Res. 55, 2366–2372 (1995).
Martuza, R. L. et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 252, 854–856 (1991).
Mineta, T. et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1, 938–943 (1995).
Oyama, M. et al. Intravesical and intravenous therapy of human bladder cancer by the herpes vector G207. Hum. Gene Ther. 11, 1683–G1693 (2000).
Walker, J. R. et al. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum. Gene Ther. 10, 2237–G2243 (1999).
Puhlmann, M. et al. Thymidine kinase-deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor-directed gene therapy. Hum. Gene Ther. 10, 649–657 (1999).
Kawakita, M. et al. Effect of canarypox virus (ALVAC)-mediated cytokine expression on murine prostate tumor growth. J. Natl Cancer Inst. 89, 428–436 (1997).
Gnant, M. F. et al. Systemic administration of a recombinant vaccinia virus expressing the cytosine deaminase gene and subsequent treatment with 5-fluorocytosine leads to tumor-specific gene expression and prolongation of survival in mice. Cancer Res. 59, 3396–3403 (1999).
Gulley, J. L. et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. 37, 1051–1061 (2019).
Belldegrun, A. et al. Interleukin 2 gene therapy for prostate cancer: phase I clinical trial and basic biology. Hum. Gene Ther. 12, 883–892 (2001).
Hong, D. et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci. Transl. Med. 7, 314ra185 (2015).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03421353 (2021).
Pisters, L. L. et al. Evidence that transfer of functional p53 protein results in increased apoptosis in prostate cancer. Clin. Cancer Res. 10, 2587–2593 (2004).
Trudel, S. et al. A phase I trial of adenovector-mediated delivery of interleukin-2 (AdIL-2) in high-risk localized prostate cancer. Cancer Gene Ther. 10, 755–763 (2003).
Kumon, H. et al. Adenovirus vector carrying REIC/DKK-3 gene: neoadjuvant intraprostatic injection for high-risk localized prostate cancer undergoing radical prostatectomy. Cancer Gene Ther. 23, 400–409 (2016).
Reid, T., Warren, R. & Kirn, D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 9, 979–986 (2002).
Larson, C. et al. Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget 6, 19976–19989 (2015).
Xia, Z.-J. et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng 23, 1666–1670 (2004).
Garber, K. China approves world’s first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 98, 298–300 (2006).
Heise, C. et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 6, 1134–1139 (2000).
Dong, W. et al. ORCA-010, a novel potency-enhanced oncolytic adenovirus, exerts strong antitumor activity in preclinical models. Hum. Gene Ther. 25, 897–904 (2014).
Yu, D. C., Sakamoto, G. T. & Henderson, D. R. Identification of the transcriptional regulatory sequences of human kallikrein 2 and their use in the construction of calydon virus 764, an attenuated replication competent adenovirus for prostate cancer therapy. Cancer Res. 59, 1498–1504 (1999).
Yu, D. C. et al. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res. 59, 4200–4203 (1999).
Yu, D. C. et al. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 61, 517–525 (2001).
Rodriguez, R. et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 57, 2559–2563 (1997).
DeWeese, T. L. et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 61, 7464–7472 (2001).
Ren, C. et al. mRTVP-1, a novel p53 target gene with proapoptotic activities. Mol. Cell. Biol. 22, 3345–3357 (2002).
Sonpavde, G. et al. GLIPR1 tumor suppressor gene expressed by adenoviral vector as neoadjuvant intraprostatic injection for localized intermediate or highrisk prostate cancer preceding radical prostatectomy. Clin. Cancer Res. 17, 7174–7182 (2011).
Freytag, S. O. et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose threedimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 63, 7497–7506 (2003).
Freytag, S. O. et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 89, 268–276 (2014).
Nasu, Y. et al. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 6, 338–349 (1999).
Satoh, T. et al. Macrophages transduced with an adenoviral vector expressing interleukin 12 suppress tumor growth and metastasis in a preclinical metastatic prostate cancer model. Cancer Res. 63, 7853–7860 (2003).
Freytag, S. O., Zhang, Y. & Siddiqui, F. Preclinical toxicology of oncolytic adenovirus-mediated cytotoxic and interleukin-12 gene therapy for prostate cancer. Mol. Ther. Oncolytics 2, 15006 (2015).
Sasaki, K. et al. A phase I/II study of adenovirus-mediated interleukin-12 gene therapy for hormone refractory prostate cancer: an interim report. J. Clin. Oncol. 29, 148–148 (2011).
Zahm, C. D., Colluru, V. T. & McNeel, D. G. DNA vaccines for prostate cancer. Pharmacol. Ther. 174, 27–42 (2017).
Weber, J. S. et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J. Immunother. 34, 556–567 (2011).
Smith, K. A. et al. Enhancing DNA vaccination by sequential injection of lymph nodes with plasmid vectors and peptides. Vaccine 27, 2603–2615 (2009).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
McNeel, D. G. et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J. Clin. Oncol. 27, 4047–4054 (2009).
McNeel, D. G. et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J. Clin. Oncol. 37, 3507–3517 (2019).
Becker, J. T. et al. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J. Immunother. 33, 639–647 (2010).
Rekoske, B. T., Olson, B. M. & McNeel, D. G. Antitumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. OncoImmunology 5, e1165377 (2016).
Zahm, C. D., Colluru, V. T. & McNeel, D. G. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8+ T cells. Cancer Immunol. Res. 5, 630–641 (2017).
Rekoske, B. T. et al. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol. Res. 3, 946–955 (2015).
McNeel, D. G. et al. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget 9, 25586–25596 (2018).
Pavlenko, M. et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br. J. Cancer 91, 688–694 (2004).
Mincheff, M. et al. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur. Urol. 38, 208–217 (2000).
Todorova, K. et al. Biochemical nature and mapping of PSMA epitopes recognized by human antibodies induced after immunization with gene-based vaccines. Anticancer. Res. 25, 4727–4732 (2005).
Chudley, L. et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8+ T-cell responses and increases PSA doubling time. Cancer Immunol. Immunother. 61, 2161–2170 (2012).
Lubaroff, D. M. et al. Phase I clinical trial of an adenovirus/prostate-specific antigen vaccine for prostate cancer: safety and immunologic results. Clin. Cancer Res. 15, 7375–7380 (2009).
Pantuck, A. J. et al. Phase I trial of antigen-specific gene therapy using a recombinant vaccinia virus encoding MUC-1 and IL-2 in MUC-1-positive patients with advanced prostate cancer. J. Immunother. 27, 240–253 (2004).
Barnd, D. L. et al. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc. Natl Acad. Sci. USA 86, 7159–7163 (1989).
Eder, J. P. et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin. Cancer Res. 6, 1632–1638 (2000).
Kaufman, H. L. et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 22, 2122–2132 (2004).
DiPaola, R. S. et al. A national multicenter phase 2 study of prostate-specific antigen (PSA) pox virus vaccine with sequential androgen ablation therapy in patients with PSA progression: ECOG 9802. Eur. Urol. 68, 365–371 (2015).
Paschalis, A. et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur. Urol. 76, 469–478 (2019).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014).
Sánchez-Rivera, F. J. & Jacks, T. Applications of the CRISPR-Cas9 system in cancer biology. Nat. Rev. Cancer 15, 387–395 (2015).
Friedemann, M. et al. Diverse effects of phospholipase A2 receptor expression on LNCaP and PC-3 prostate cancer cell growth in vitro and in vivo. Oncotarget 9, 35983–35996 (2018).
Wei, C. et al. CRISPR/Cas9 targeting of the androgen receptor suppresses the growth of LNCaP human prostate cancer cells. Mol. Med. Rep. 17, 2901–2906 (2018).
Yu, G. et al. Organelle-derived acetyl-CoA promotes prostate cancer cell survival, migration, and metastasis via activation of calmodulin kinase II. Cancer Res. 78, 2490–2502 (2018).
Takao, A. et al. Generation of PTEN-knockout (−/−) murine prostate cancer cells using the CRISPR/Cas9 system and comprehensive gene expression profiling. Oncol. Rep. 40, 2455–2466 (2018).
Guo, H. et al. Modulation of long noncoding RNAs by risk SNPs underlying genetic predispositions to prostate cancer. Nat. Genet. 48, 1142–1150 (2016).
Gao, P. et al. Biology and clinical implications of the 19q13 aggressive prostate cancer susceptibility locus. Cell 174, 576–589.e18 (2018).
Kardooni, H. et al. CRISPR-mediated reactivation of DKK3 expression attenuates TGF-β signaling in prostate cancer. Cancers 10, 165 (2018).
Zhen, S. et al. Targeted delivery of CRISPR/Cas9 to prostate cancer by modified gRNA using a flexible aptamer-cationic liposome. Oncotarget 8, 9375–9387 (2017).
Wang, T. et al. Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84 (2014).
Shalem, O. et al. Genome-Scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Chen, S. et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell 160, 1246–1260 (2015).
Parnas, O. et al. A genome-wide CRISPR screen in primary immune cells to dissect regulatory networks. Cell 162, 675–686 (2015).
Fei, T. et al. Genome-wide CRISPR screen identifies HNRNPL as a prostate cancer dependency regulating RNA splicing. Proc. Natl Acad. Sci. USA 114, E5207–E5215 (2017).
Zimmermann, M. et al. CRISPR screens identify genomic ribonucleotides as a source of PARP-trapping lesions. Nature 559, 285–289 (2018).
Sadelain, M., Brentjens, R. & Rivière, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 3, 388–398 (2013).
Gill, S. & June, C. H. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol. Rev. 263, 68–89 (2015).
Yu, H. et al. CART cell therapy for prostate cancer: status and promise. OncoTargets Ther. 12, 391–395 (2019).
Ma, Q. et al. Advanced generation anti-prostate specific membrane antigen designer T cells for prostate cancer immunotherapy. The Prostate 74, 286–296 (2014).
Liu, X. et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 76, 1578–1590 (2016).
Priceman, S. J. et al. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. OncoImmunology 7, e1380764 (2018).
Zhang, Q. et al. Efficacy against human prostate cancer by prostate-specific membrane antigen-specific, transforming growth factor-β insensitive genetically targeted CD8+ T-cells derived from patients with metastatic castrate-resistant disease. Eur. Urol. 73, 648–652 (2018).
Hillerdal, V. & Essand, M. Chimeric antigen receptor-engineered T cells for the treatment of metastatic prostate cancer. BioDrugs 29, 75–89 (2015).
Grigor, E. J. M. et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus. Med. Rev. 33, 98–110 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01341652 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01706458 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02499835 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02555397 (2018).
Parsons, J. K. et al. A randomized, double-blind, phase II trial of PSA-TRICOM (PROSTVAC) in patients with localized prostate cancer: the immunotherapy to prevent progression on active surveillance study. Eur. Urol. Focus. 4, 636–638 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01140373 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03089203 (2021).
Kloss, C. C. et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Valerio, M. et al. New and established technology in focal ablation of the prostate: a systematic review. Eur. Urol. 71, 17–34 (2017).
Boutros, P. C. et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat. Genet. 47, 736–745 (2015).
Bill-Axelson, A. et al. Radical prostatectomy or watchful waiting in prostate cancer — 29-year follow-up. N. Engl. J. Med. 379, 2319–2329 (2018).
Ko, S. C. et al. Molecular therapy with recombinant p53 adenovirus in an androgen-independent, metastatic human prostate cancer model. Hum. Gene Ther. 7, 1683–1691 (1996).
Eastham, J. A. et al. Suppression of primary tumor growth and the progression to metastasis with p53 adenovirus in human prostate cancer. J. Urol. 164, 814–819 (2000).
Gurnani, M. et al. Adenovirus-mediated p53 gene therapy has greater efficacy when combined with chemotherapy against human head and neck, ovarian, prostate, and breast cancer. Cancer Chemother. Pharmacol. 44, 143–151 (1999).
Radhakrishnan, S. et al. Efficacy of oncolytic mutants targeting pRb and p53 pathways is synergistically enhanced when combined with cytotoxic drugs in prostate cancer cells and tumor xenografts. Hum. Gene Ther. 21, 1311–1325 (2010).
Mishel, S. et al. Delivery of the gene encoding the tumor suppressor Sef into prostate tumors by therapeutic-ultrasound inhibits both tumor angiogenesis and growth. Sci. Rep. 7, 15060 (2017).
Spitzweg, C. et al. Prostate-specific antigen (PSA) promoter-driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res. 59, 2136–2141 (1999).
Spitzweg, C. et al. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 60, 6526–6530 (2000).
Shalev, M. et al. Suicide gene therapy toxicity after multiple and repeat injections in patients with localized prostate cancer. J. Urol. 163, 1747–1750 (2000).
Eastham, J. A. et al. Prostate cancer gene therapy: herpes simplex virus thymidine kinase gene transduction followed by ganciclovir in mouse and human prostate cancer models. Hum. Gene Ther. 7, 515–523 (1996).
Burton, J. B. et al. Adenovirus-mediated gene expression imaging to directly detect sentinel lymph node metastasis of prostate cancer. Nat. Med. 14, 882–888 (2008).
Rice, J., Ottensmeier, C. H. & Stevenson, F. K. DNA vaccines: precision tools for activating effective immunity against cancer. Nat. Rev. Cancer 8, 108–120 (2008).
Moreira, D. et al. STAT3 inhibition combined with CpG immunostimulation activates antitumor immunity to eradicate genetically distinct castration-resistant prostate cancers. Clin. Cancer Res. 24, 5948–5962 (2018).
Acknowledgements
This work was supported by MD Anderson NCI Prostate Cancer SPORE Grant P50 CA140388 and the NCI Cancer Center Support Grant P30 CA16672.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, made substantial contributions to the discussion of the content and wrote, reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
Parts of the discussion in this article involve data relevant to intellectual property that has been licensed by Baylor College of Medicine to Progression Therapeutics, Inc., a private biotechnology start-up company. T.C.T. is an inventor of record on patents that are included in this licensing agreement. J.R.G. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Gene Therapy Net: http://www.genetherapynet.com/viral-vectors.html
Rights and permissions
About this article
Cite this article
Gregg, J.R., Thompson, T.C. Considering the potential for gene-based therapy in prostate cancer. Nat Rev Urol 18, 170–184 (2021). https://doi.org/10.1038/s41585-021-00431-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00431-x
This article is cited by
-
Comprehensive review of CRISPR-based gene editing: mechanisms, challenges, and applications in cancer therapy
Molecular Cancer (2024)
-
Exosome-derived lncRNA A1BG-AS1 attenuates the progression of prostate cancer depending on ZC3H13-mediated m6A modification
Cell Division (2024)
-
Inhibition of orthotopic castration-resistant prostate cancer growth and metastasis in mice by JC VLPs carrying a suicide gene driven by the PSA promoter
Cancer Gene Therapy (2024)
-
The use of RNA-based treatments in the field of cancer immunotherapy
Molecular Cancer (2023)